Abstract
Daxx is a nuclear protein involved in apoptosis and transcriptional repression, and it interacts with the death receptor Fas, promyelocytic leukemia protein (PML), and several transcriptional repressors. The function of Daxx in apoptosis is controversial because opposite results were obtained in transient overexpression and genetic knockout studies. Furthermore, the roles of PML and transcriptional repression in Daxx-regulated apoptosis are currently unknown. In this study, we investigated the role of Daxx in Fas- and stress-induced apoptosis by small interfering RNA-mediated Daxx silencing in mammalian cells. Daxx silencing had no apparent cytotoxic effects on mammalian cells within 72 h. Intriguingly, Daxx silencing strongly sensitized cells to Fas- and stress-induced apoptosis, which was accompanied by caspase activation, cytochrome c release, and Jun N-terminal kinase activation. Consistently, endogenous Daxx was degraded rapidly upon induction of apoptosis by stress or anti-Fas antibody. Finally, PML silencing had no effect on Daxx silencing-mediated apoptotic events, while caspase gene expression was upregulated in the absence of Daxx. These data strongly suggest that Daxx may inhibit Fas and stress-mediated apoptosis by suppressing proapoptotic gene expression outside of PML domains.
Daxx is a highly conserved nuclear protein that contains a Ser/Pro/Thr-rich domain, an acidic domain, a coiled-coil region, and two paired amphipathic helices (27, 32, 36, 46). Daxx has been isolated several times from yeast two-hybrid screens by using baits involved in various signaling pathways, such as acute promyelocytic leukemia (36), apoptosis (4, 9, 45, 54, 63), chromosome segregation (46), transcription (27, 37), tumor suppression (44), heat shock response (9), and viral infection (23, 38). Daxx also interacts with the nucleolar protein MSP58 (40), sentrin/SUMO-1 and Ubc9 (48), human papillomavirus L2 (18), and Ask1 (5, 30, 34). In addition, Daxx knockout mice die at early stages during development (41), suggesting that Daxx may be involved in multiple cellular processes and embryonic development.
An interesting function of Daxx is its ability to regulate apoptosis, which serves to remove excess or damaged cells. Apoptosis can be activated through cell surface receptors, cytotoxic stress, or DNA-damaging agents. It is characterized by morphological changes, DNA fragmentation, caspase activation, and cytochrome c release (for reviews, see references 20, 22, and 50).
Several reports have suggested that Daxx is a proapoptotic protein. First, Daxx reportedly binds to the cytosolic domain of Fas to transmit a Fas-associated death domain (FADD)-independent apoptotic signal by activating Jun N-terminal kinase (JNK) (63). Daxx was also reported to mediate UV-induced JNK activation and cell death (61). Daxx was shown to bind directly to and activate the apoptosis signal-regulating kinase 1 (Ask1), a mitogen-activated protein kinase kinase kinase that activates JNK (5, 7, 34). Ask1 is capable of translocating Daxx to the cytoplasm, and binding of Daxx may release an intramolecular interaction within Ask1 to activate its kinase activity (5, 34). However, Daxx was also reported to promote Ask1-mediated apoptosis in a kinase- and caspase-independent manner (7).
Recent data further suggest that certain p53 mutants may support cell survival by inhibiting the Daxx-Ask1 pathway (44). Similarly, the antiapoptotic protein HSP27 may inhibit cytosolic translocation of Daxx and block the Daxx-Ask1 cascade (8, 9, 56). Finally, Daxx was also reported to bind to the transforming growth factor beta receptor to mediate apoptosis and JNK activation (45). These studies indicate an important role of Daxx in regulating apoptotic signaling in various pathways.
Despite the many reports suggesting a proapoptosis function of Daxx, several studies are in disagreement. First, binding of Fas is not completely reproducible, and overexpression of Daxx does not always enhance apoptosis (54). Second, FADD−/− and caspase 8-deficient cells are resistant to Fas-mediated apoptosis (29, 65, 66), and a FasΔ mutant defective in binding of FADD but not Daxx is also resistant to Fas-mediated apoptosis (6). These data suggest that Daxx is not sufficient for Fas-mediated apoptosis in the absence of FADD or caspase 8. Moreover, JNK is also dispensable for Fas-induced apoptosis (55), and the involvement of Daxx in Fas-induced JNK activation and cell death has been challenged (24, 27, 57).
Since disruption of the Daxx gene in mice resulted in early embryonic death and elevated apoptosis (41), it is possible that Daxx may be essential for cell survival. Indeed, overexpression of Daxx inhibited both CD43-mediated apoptosis in hematopoietic progenitor cells (4) and UV-induced apoptosis in 293 cells (61). Furthermore, Daxx expression is downregulated by histone deacetylase inhibitors that induce apoptosis (1). Human immunodeficiency virus-induced-apoptosis in CD4+ T lymphocytes is also accompanied by downregulation of Daxx (19). However, Daxx expression is upregulated in the brains of persons with Alzheimer's disease with an active proapoptotic process (10). Daxx is also upregulated in prostate tumor cells (58), while mantel cell lymphoma shows downregulation of Daxx (25). These studies indicate that Daxx expression may be sensitive to cellular transformation and apoptosis; however, a precise relationship between Daxx expression and apoptosis remains unclear.
We and others have previously shown that Daxx interacts and colocalizes with promyelocytic leukemia protein (PML) in PML-oncogenic domains (PODs) (28, 36, 54, 67). Localization to the PODs appears to correlate with Daxx's proapoptotic activity because Daxx mutants that failed to localize to PODs are defective in promoting apoptosis (54). In addition, apoptosis of splenocytes is impaired in the absence of PML (67), and PML overexpression greatly enhances apoptosis (47, 60), indicating that PML is proapoptotic. These studies suggest that Daxx may act together with PML through the POD environment to influence apoptosis.
In addition to apoptosis, Daxx is also involved in transcriptional control by interacting directly with several transcriptional repressors. Daxx reportedly forms a ternary complex with homeodomain proteins Pax-3 and Pax-7 to inhibit transcription, while the Pax-3/FKHR oncogenic fusion protein in alveolar rhabdomyosarcoma cells is resistant to Daxx inhibition (27). Similarly, Daxx also interacts with Pax-5 and functions as a corepressor (16) and with ETS1 to inhibit target gene expression (37).
We have previously shown that Daxx inhibits basal transcription through recruitment of histone deacetylases, and PML overexpression may antagonize Daxx-mediated transcriptional repression by sequestrating Daxx to PODs (36). Similarly, inhibition of Pax-3 transactivation by Daxx could be released by PML overexpression (35). The transcriptional repression activity of Daxx is also inhibited by MSP58 and Ask1, which target Daxx to the nucleolar and cytoplasmic compartment, respectively (40). Recent studies have confirmed the association of Daxx with histone deacetylase II, core histones, and a chromatin-associated protein, Dek (26), suggesting that Daxx may repress basal transcription through chromatin modification. However, the relationship between Daxx-mediated transcriptional repression and apoptosis remains unknown.
In this study, we investigated the role of Daxx in Fas- and stress-induced apoptosis by silencing Daxx expression in mammalian cells with small interfering RNA (siRNA) (2, 15, 17). While Daxx silencing alone has little effect on cell survival and proliferation, it dramatically enhances both Fas- and stress-induced apoptosis. This enhanced apoptosis can be blocked by caspase inhibitors and is accompanied by proteolytic cleavage of caspases and poly(ADP-ribose) polymerase (PARP). Daxx silencing also causes synergistic enhancement of cytochrome c release and a slight increase in JNK activation. Consistently, induction of apoptosis accelerates degradation of endogenous Daxx. In addition, PML silencing does not affect Daxx silencing-mediated apoptosis, and a search for potential Daxx target genes revealed upregulation of three caspases in the absence of Daxx. Our data suggest that Daxx may play an inhibitory role in Fas- and stress-mediated apoptosis, possibly by inhibiting expression of apoptosis regulatory genes in a nucleoplasmic environment outside of PODs.
MATERIALS AND METHODS
Cell culture and reagents.
HeLa cells were grown at 37°C with 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 5 μg of gentamicin per ml (Life Technologies, Grand Island, N.Y.). The general caspase inhibitor z-VAD-fmk, the caspase 1 inhibitor z-YVAD-fmk, the caspase 8 inhibitor z-IETD-fmk, and the caspase 9 inhibitor z-LEHD-fmk were purchased from BD Biosciences Clontech (Palo Alto, Calif.). Actinomycin D, cycloheximide, and 4′,6′-diamidino-2-phenylindole dihydrochloride hydrate (DAPI) were purchased from Sigma (St. Louis, Mo.). Human tumor necrosis factor alpha (TNF-α) was purchased from Clontech. The anti-human Fas antibody CH11 was purchased from Upstate Biotechnology (Lake Placid, N.Y.).
Transfection of siRNA.
The siRNAs used in this study were siDaxx, 5′-GGAGUUGGAUCUCUCAGAAdTdT-3′ (sense), 5′-UUCUGAGAGAUCCAACUCCdTdT-3′ (antisense); siPML, 5′-CGTCTTTTTCGAGAGTCTGdTdT-3′ (sense), 5′-CAGACTCTCGAAAAAGACGdTdT-3′ (antisense); and siRAC3, 5′-AGACUCCUUAGGACCGCUUdTdT-3′ (sense), 5′-AAGCGGUCCUAAGGAGUCUdTdT-3′ (antisense). All siRNAs were synthesized by 2′ amplification of cDNA end protection chemistry from Dharmacon Research (Lafayette, Colo.). The sense and antisense siRNAs were deprotected and annealed according to the manufacturer's instructions. The siRNA duplexes were precipitated by ethanol and prepared in diethyl pyrocarbonate-treated water at a 20 μM concentration. For siRNA transfection, confluent cells in 10-cm plates were trypsinized and seeded in 6- or 12-well plates 18 to 24 h before transfection. Transfection of siRNAs was carried out with Oligofectamine following the manufacturer's instructions (Gibco-Invitrogen, Rockville, Md.). The standard siRNA duplex concentration used in this study was 50 nM. Transfected cells were rinsed with fresh medium 8 h after transfection and maintained in culture medium until treatments. The normal transfection efficiency was about 85 to 95% based on immunofluorescence staining of Daxx in siDaxx-treated cells.
Western blotting.
Cells were lysed in SDS sample buffer and resolved by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred onto a polyvinylidene difluoride membrane by semidry electroblotter (Bio-Rad). Western blot was conducted with the enhanced chemiluminescence (ECL) reagents according to the manufacturer's recommendation (Amersham). The primary antibodies used were antibodies against ACTR/RAC3 (AX15, Upstate Biotech.), caspase 9 (96-2-22; Upstate Biotech.), tubulin (Sigma), caspase 3 (65906E), caspase 8 (3-1-9), PARP (4C10-5; BD Bioscience), JNK1 (sc-571, Santa Cruz), and the affinity-purified rabbit anti-Daxx polyclonal antibody (36).
Immunofluorescence microscopy.
Cells were grown on cover glasses in 24-well plate and fixed in a methanol-acetone (1:1) mixture on dry ice for 1 min. The fixed cells were processed for immunofluorescence staining as previously described (12). The primary antibodies used here were the mouse anti-cytochrome c monoclonal antibody 6H2.B4 (BD Biosciences), the affinity-purified Daxx polyclonal antibody (36), and the PML monoclonal antibody 5E10 (52). After washing, cells were stained with rhodamine- or fluorescein-conjugated goat anti-rabbit or anti-mouse immunoglobulin secondary antibodies, and cell nuclei were stained with DAPI. The cover glasses were mounted on slides with Pro-Long antifade reagent (Molecular Probes) and visualized with a Zeiss Axiovert 200 inverted epifluorescence microscope. The images were captured with Axiocam and analyzed with Axiovision software.
Apoptosis assay.
Cells were cultured on cover glasses in 24-well plates and treated with monoclonal anti-Fas antibody (200 ng/ml), TNF-α (5 ng/ml) in the absence or presence of cycloheximide (5 μg/ml), actinomycin D (100 nM), or UV (50 J/m2) irradiation to induce apoptosis. After treatment, cells were fixed with 2% paraformaldehyde for 45 min and subjected to terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay following the manufacturer's instructions (Roche). The samples were examined by epifluorescence microscopy, and percent apoptosis determined by counting cells with positive TUNEL staining. On average, 300 to 500 cells in three different fields were scored in every sample.
JNK kinase assay.
HeLa cells were cultured in a 10-cm dish and solubilized with 1 ml of lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1× protease inhibitor cocktail) in Eppendorf tubes at 4°C for 30 min. The cell lysates were centrifuged at 14,000 × g for 15 min at 4°C and preabsorbed with 10 μg of rabbit immunoglobulin G in the presence of 50 μl of protein A-Sepharose for 1 h. The protein concentration was determined with the BCA protein assay kit (Pierce). Equal amounts of protein from each sample were subjected to immunoprecipitation with rabbit anti-JNK polyclonal antibody (sc-571, Santa Cruz Biotechnology, Santa Cruz, Calif.) at 4°C overnight, followed by a 1-h incubation with protein A-Sepharose. Kinase reactions were carried out at 30°C for 30 min by mixing the immunoprecipitates with 50 μl of reaction mix (25 mM Tris-HCl, 5 mM β-glycerophosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM MgCl2, 20 μM ATP, 5 μg of purified glutathione S-transferase [GST]-c-Jun[1-79], 3 μl of [γ-32P]ATP [3,000 Ci/mmol]). The reactions were stopped by adding 25 μl of 2× SDS sample buffer and boiled for 5 min. The reaction products were separated by electrophoresis on SDS-12% polyacrylamide gels, and the 32P-labeled proteins were visualized by autoradiography.
RT-PCR.
Total cellular RNAs were purified by Trizol reagents (Invitrogen). Then 3 μg of DNase-digested total RNA was subjected to reverse transcription in 20 μl of reaction volume with the Super Script First Strand Synthesis System from Invitrogen. The products of reverse transcription were digested with RNase H and diluted to a final volume of 60 μl. Then 2 μl of the reverse transcription sample was used for PCR. The primers used in the PCRs were GAPDH, 5′-GACCACAGTCCATGCCATCAC-3′ and 5′-CATACCAGGAAATGAGCTTGAC-3′; Daxx, 5′-TCTCCTTGGACCCCACAAATG-3′ and 5′-TCAGGCCCTGGCTTGTTGATG-3′; caspase 3, 5′-ATGACATCTCGGTCTGGTACA-3′ and 5′-TGTCTCAATGCCACAGTCCAG-3′; caspase 8, 5′-CTACCAACTCATGGACCACAG-3′ and 5′-GTGACTGGATGTACCAGGTTC-3′; caspase 9, 5′-CAGAAAGACCATGGGTTTGAG-3′ and 5′-ACTGCAGGTCTTCAGAGTGAG-3′; caspase 10, 5′-AGAAGCAGAAGTGCAATCCAG-3′ and 5′-CTGAATATACCAGCTGCCTTC-3′; BAD, 5′-AAGGGACTTCCTCGCCCGAAG-3′ and 5′-TCAGCCCTCCCTCCAAAGGAG-3′; BAX, 5′-TTCATCCAGGATCGAGCAGG-3′ and 5′-CTCTGCAGCTCCATGTTACT-3′. After PCR, the products were separated on a 2% agarose gel and visualized by ethidium bromide staining. The image was captured with an EDAS 290 system and analyzed by one-dimensional image analysis software (Kodak).
RESULTS
Silencing of Daxx expression in mammalian cells by siRNA.
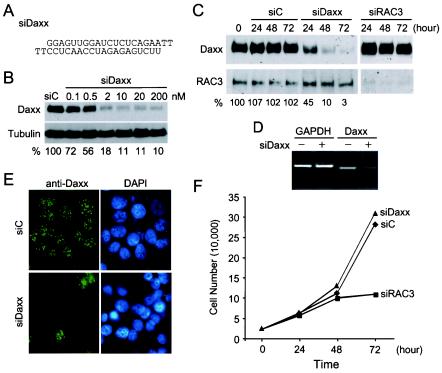
An siRNA duplex against human Daxx mRNA termed siDaxx was designed and synthesized (Fig. 1A). The effects of this siDaxx on Daxx expression in mammalian cells were analyzed by Western blot, RT-PCR, and immunofluorescence staining. The concentration of siDaxx required for optimal silencing of Daxx expression was determined by Western blot analysis of endogenous Daxx at 48 h after transfection with increasing concentrations of siDaxx. We found that siDaxx silenced Daxx expression in a dose-dependent manner and that optimal inhibition occurred between 2 and 10 nM (Fig. 1B). As a control, tubulin remained unchanged at all concentrations of siDaxx tested. Normalization of Daxx to tubulin protein levels indicated a 28% inhibition at 0.1 nM siDaxx and 82 to 90% inhibition at 2 nM and higher concentrations of siDaxx (Fig. 1B).
FIG. 1.
Silencing of Daxx expression in mammalian cells by siRNA. (A) Nucleotide sequence of the double-stranded siRNA against Daxx mRNA (siDaxx). (B) Dose-dependent inhibition of Daxx expression by siDaxx. HeLa cells were transfected with control reagent (siC) or increasing concentrations of siDaxx. Total cell lysate were prepared 48 h after transfection and analyzed by SDS-PAGE and Western blot detection of Daxx and tubulin. The relative Daxx protein levels after normalization with tubulin are shown at bottom. (C) Time course-dependent silencing of Daxx by siDaxx. HeLa cells were transfected with siC, siDaxx, or the siRNA against coactivator RAC3 (siRAC3) at 50 nM concentration. Cells were harvested at indicated hours after transfection and analyzed for Daxx and RAC3 expression by Western blot. Relative Daxx levels in comparison with untransfected cells are shown at bottom. (D) RT-PCR analysis of Daxx mRNA in untransfected cells (−) and cells transfected with siDaxx (+). Total RNA was isolated 48 h after transfection and analyzed by RT-PCR for GAPDH and Daxx mRNAs. PCR products were analyzed on a 1% agarose gel followed by ethidium bromide staining and imaging with the Kodak EDAS system. (E) Immunofluorescence staining of Daxx in siDaxx-transfected cells. MCF-7 cells transfected with siC or siDaxx for 48 h were analyzed by immunofluorescence staining for Daxx with affinity-purified rabbit anti-Daxx polyclonal antibodies. Cell nuclei were visualized with 4′,6′-diamidino-2-phenylindole (DAPI) staining. (F) Effect of Daxx silencing on cell proliferation. HeLa cells were transfected with 50 nM siC (♦), siDaxx (▴), or siRAC3 (▪). Total cell numbers were counted on a hemacytometer at the indicated hours after transfection. Daxx silencing had no effect on cell growth and proliferation.
The incubation time required for optimal silencing of Daxx expression was then determined at saturating concentrations of siDaxx (50 nM). As expected, the control reagent siC had no effect on the Daxx or RAC3 level within 72 h after transfection, and siDaxx had no effect on RAC3 expression either (Fig. 1C). In contrast, Daxx levels decreased to 45% at 24 h after transfection with siDaxx and continued to decline to 10 and 3% at 48 and 72 h, respectively (Fig. 1C). Similarly, the siRNA against RAC3 (siRAC3) inhibited RAC3 expression but had no effect on Daxx expression. These data suggest that siDaxx inhibits Daxx expression specifically and that Daxx expression is repressed by siDaxx but not other siRNAs.
To confirm that siDaxx indeed causes degradation of Daxx mRNA, RT-PCR was performed to measure Daxx mRNA level in siDaxx-transfected cells. As expected, siDaxx had no effect on the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA level, while it decreased the Daxx mRNA level about sixfold (Fig. 1D). Finally, the effect of siDaxx on Daxx expression was visualized directly by immunofluorescence staining of the transfected cells. As expected, siDaxx reduced Daxx immunostaining to background levels in about 90% of transfected cells (Fig. 1E). These data indicate that siDaxx can specifically and efficiently silence Daxx expression in mammalian cells.
Daxx silencing had no effect on cell proliferation.
To determine if siDaxx has any adverse effects on cell growth, we measured the proliferation of siDaxx-treated cells. Since the transfection efficiency of siDaxx is around 90% (Fig. 1E), cell proliferation was determined by counting total cell numbers every 24 h after transfection. No significant differences between proliferation of siDaxx- and siC-treated cells were observed (Fig. 1F). In contrast, siRAC3 transfection had a threefold reduction in total cell number at 72 h after transfection. As expected, no alteration of cell cycle distribution was observed in siDaxx-treated cells (data not shown). These data suggest that Daxx silencing in mammalian cells may not cause significant cytotoxic effects within 72 h. In contrast, RAC3 silencing has a strong effect on cell proliferation, consistent with the RAC3 knockout phenotype (62). These data suggest that Daxx silencing by siDaxx may be suitable for analyzing the role of Daxx in various signaling pathways.
Daxx silencing sensitizes cells to Fas and stress-induced apoptosis.
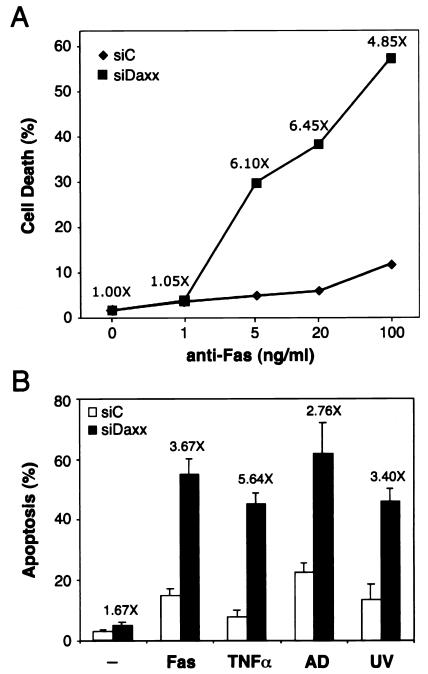
To investigate the role of Daxx in apoptosis, we first analyzed the response of siDaxx-treated cells to anti-Fas antibody-induced cell death (Fig. 2A). In control cells (siC), anti-Fas antibody triggered concentration-dependent cell death, reaching approximately 12% dead cells at 100 ng/ml within 24 h. Strikingly, this apoptotic response was greatly enhanced by 4.8-fold in siDaxx-treated cells, reaching 57% cell death. In this experiment, siDaxx transfection alone without anti-Fas antibody treatment did not cause any additional cell death above that of the siC control, suggesting a synergistic effect between siDaxx and anti-Fas antibody in inducing cell death. This synergistic effect was even more prominent at lower concentrations of the anti-Fas antibody. At 5 and 20 ng/ml, the anti-Fas antibody-mediated cell death was above sixfold higher in siDaxx- versus siC-treated cells. These data strongly suggest that siDaxx may sensitize cells to Fas-induced apoptosis.
FIG. 2.
Daxx silencing sensitizes cells to Fas and stress-induced apoptosis. (A) Effect of siDaxx on Fas-induced cell death. HeLa cells transfected with control reagent siC (♦) or siDaxx (▪) for 36 h were treated with increasing concentrations of the agonistic anti-Fas antibody CH11. Percent cell death was determined at 24 h after the addition of antibody by trypan blue staining. The antibody concentrations are shown on a log scale, with 0 ng/ml placed at an arbitrary position. The increases in cell death are shown above the siDaxx data points at each antibody concentration, and the actual increases were, from left to right, 0, 0.2, 25, 33, and 46%, respectively. (B) Effect of siDaxx on stress-induced apoptosis. HeLa cells transfected with siC (open bars) or siDaxx (black bars) for 48 h were treated with the anti-Fas antibody (CH11, 200 ng/ml) for 12 h, TNF-α (10 ng/ml) or actinomycin D (AD, 100 nM) for 8 h, or UV (50 J/m2), followed by an 8-h incubation. Apoptosis was determined by TUNEL assay as described in Materials and Methods. Enhancement of apoptosis between siDaxx- and siC-treated cells were shown above the black bars.
To determine if Daxx silencing could also sensitize cells to stress-induced apoptosis, we analyzed the effect of siDaxx on apoptosis caused by TNF-α, actinomycin D, and UV irradiation. The TUNEL assay was used in this experiment to ensure that cell death was caused by apoptosis. siC-treated sample had 3% background apoptosis, while siDaxx-treated cells displayed 5% apoptosis (1.67-fold) (Fig. 2B), which varied from experiment to experiment and was not statistically significant. Consistent with Fig. 2A, the anti-Fas antibody at 200 ng/ml for 12 h induced 15% apoptosis in the siC sample. Again, this Fas-induced apoptosis increased to 55% in siDaxx-treated cells, equivalent to a 3.67-fold enhancement. When siC- and siDaxx-treated cells were challenged with stress-inducing agents, significant increases of apoptosis in siDaxx-treated cells were also observed compared to siC samples. At 10 ng/ml for 8 h, TNF-α caused 8% apoptosis in siC-treated cells, which increased to 45% in siDaxx-treated cells (a 5.64-fold enhancement). Similarly, actinomycin D at 100 nM for 8 h and UV irradiation at 50 J/m2 followed by an 8-h recovery induced much higher levels of apoptosis in siDaxx-treated cells than in the siC-treated sample (2.76- and 3.4-fold, respectively). These data strongly suggest that Daxx silencing can sensitize cells to both Fas- and stress-induced apoptosis.
Involvement of caspases in Daxx silencing-mediated apoptosis.
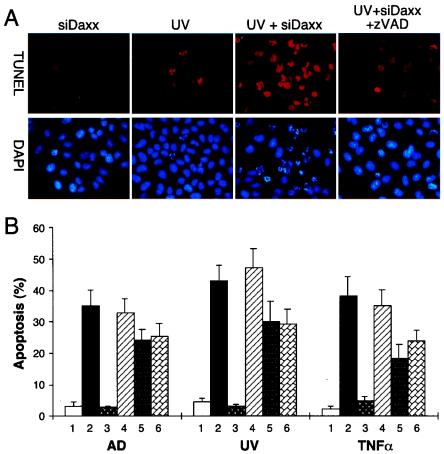
To determine the mechanism(s) by which Daxx silencing enhances Fas- and stress-induced apoptosis, we analyzed the involvement of caspases by using caspase inhibitors. As shown by TUNEL staining in Fig. 3A, siDaxx or UV alone caused only minimal levels of apoptosis, while siDaxx plus UV synergistically induced apoptosis. Intriguingly, this siDaxx/UV-induced apoptosis was completely blocked by the general caspase inhibitor z-VAD-fmk, indicating the involvement of caspase in Daxx silencing-enhanced apoptosis.
FIG. 3.
Involvement of caspases in Daxx silencing-mediated apoptosis. (A) TUNEL assay showing synergistic induction of apoptosis by siDaxx and UV. HeLa cells were transfected with siDaxx or control reagent for 48 h. The caspase inhibitor z-VAD-fmk (z-VAD, 20 μM) was added 1 h prior to UV irradiation (50 J/m2). Apoptosis was determined by TUNEL (red) and DAPI (blue) staining 8 h after UV irradiation. (B) Effect of various caspase inhibitors on siDaxx-mediated apoptosis. HeLa cells were transfected with siC (lanes 1) or siDaxx (lanes 2 to 6), followed by treatment with actinomycin D (AD, 100 nM), UV irradiation (50 J/m2), or TNF-α (10 ng/ml) in the absence (lanes 2) or presence (lanes 3 to 6) of various caspase inhibitors. Cells were treated with the general caspase inhibitor z-VAD-fmk (lanes 3), the caspase 1 inhibitor z-YVAD-fmk (lanes 4), the caspase 8 inhibitor z-IETD-fmk (lanes 5), or the caspase 9 inhibitor z-LEHD-fmk (lanes 6). Apoptotic cells were detected by TUNEL assay and counting at least 200 cells in three different fields. The general caspase inhibitor z-VAD-fmk completely blocked siDaxx-enhanced apoptosis, while z-IETD-fmk and z-LEHD-fmk partially suppressed siDaxx-enhanced apoptosis.
To identify specific caspases that may be involved in siDaxx-enhanced apoptosis, siDaxx-transfected cells were treated with various caspase inhibitors, and percent apoptosis was determined after actinomycin D, UV, or TNF-α treatment (Fig. 3B). Under the experimental conditions, actinomycin D, UV, or TNF-α alone (Fig. 3B, lanes 1) induced low levels of apoptosis in siC-treated control cells. In contrast, siDaxx transfection greatly enhanced the apoptosis induced by actinomycin D, UV, and TNF-α (Fig. 3B, lanes 2). The siDaxx-enhanced apoptosis was completely blocked by the general caspase inhibitor z-VAD-fmk (Fig. 3B, lanes 3) but not by the caspase 1-specific inhibitor z-YVAD-fmk (Fig. 3B, lanes 4). Interestingly, the caspase 8 inhibitor z-IETD-fmk (Fig. 3B, lanes 5) and the caspase 9 inhibitor z-LEHD-fmk (lanes 6) partially inhibited apoptosis by 20 to 40%. These data suggest that Daxx silencing-mediated apoptosis may involve activation of caspases such as caspase 8 and caspase 9 but not caspase 1.
To confirm the activation of caspases in Daxx silencing-enhanced apoptosis, we analyzed proteolytic cleavages of endogenous caspase 8, caspase 9, and the caspase substrate poly(ADP-ribose) polymerase (PARP) by Western blotting (Fig. 4A). Under conditions in which either anti-Fas antibodies or siDaxx alone did not induce significant cleavage of caspase 8, caspase 9, and PARP, prominent cleavages of the three proteins were observed in cells treated with both anti-Fas antibodies and siDaxx. As expected, the proteolytic cleavage of caspase 8, caspase 9, and PARP was completely blocked by the general caspase inhibitor z-VAD-fmk but not by the caspase 1 inhibitor z-YVAD-fmk. We also analyzed proteolytic cleavage of caspase 8, caspase 9, and PARP after UV and actinomycin D treatment with and without siDaxx transfection. Again, proteolytic cleavage of caspase 8, caspase 9, and PARP was synergistically induced in siDaxx-treated cells, and z-VAD-fmk but not z-YVAD-fmk blocked the proteolytic cleavages. These data are consistent with the TUNEL assay results (Fig. 3) and clearly indicate that activations of caspase 8 and caspase 9 are significantly enhanced in the absence of Daxx.
FIG. 4.
Synergistic activation of caspases by Daxx silencing. (A) Effects of siDaxx on cleavage of caspase 8, caspase 9, and PARP. HeLa cells with (+) or without (−) siDaxx transfection were treated with anti-Fas antibody (200 ng/ml), UV (50 J/m2), or actinomycin D (AD, 100 nM). Proteolytic cleavage of caspase 8 (Casp-8), caspase 9 (Casp-9), and PARP was determined by Western blotting 6 h after the treatment. Arrows at the right mark the proteolytic fragments. Protein molecular size markers are shown at left (in kilodaltons). Daxx silencing synergistically activated caspase 8, caspase 9, and PARP cleavage with anti-Fas antibody, UV, and actinomycin D. These proteolytic cleavages were inhibited by the general caspase inhibitor z-VAD-fmk (z-VAD, 20 μM), but not by the caspase 1 inhibitor z-YVAD-fmk (z-YVAD, 20 μM). (B) Kinetics of caspase activation in siDaxx-treated cells. Control siC or siDaxx-transfected HeLa cells were treated with anti-Fas antibody (200 ng/ml), TNF-α (10 ng/ml), TNF-α (2 ng/ml) plus cycloheximide (Chx, 2 μg/ml), actinomycin D (100 nM), or UV (50 J/m2), and cells were harvested at the time points shown at the bottom. The harvested cells were analyzed by Western blotting for proteolytic processing of caspase 8 and caspase 9 and by TUNEL assay for percent apoptosis. Boxes at the bottom mark the apparent time points of caspase activation and apoptosis.
The kinetics of caspase activation was then analyzed by comparing siC- and siDaxx-treated cells at a series of time points after induction of apoptosis (Fig. 4B). In siC-treated cells, the anti-Fas antibody did not activate caspase 8 or caspase 9 much until 24 h after addition of the antibody. At that time, there were 20% apoptotic cells, as determined by the TUNEL assay. In contrast, cleavage of caspase 8 and caspase 9 was clearly visible in siDaxx-treated cells at 5 h after the addition of antibody, when there were already 30% apoptotic cells. This result suggests that Daxx silencing can lead to caspase activation at least 19 h earlier in the case of Fas-mediated apoptosis. In the case of TNF-α treatment, caspase 8 was not significantly activated even after 24 h of continuous treatment in siC-transfected cells. However, caspase 9 was slightly activated at 24 h, when there were 10% apoptotic cells. In contrast, caspase 8 and caspase 9 were activated much earlier in siDaxx-treated cells at 5 h after the treatment, when there were 15% apoptotic cells. When the TNF-α-induced apoptosis was accelerated by cycloheximide, as observed previously (43, 53, 59), proteolytic cleavage of caspase 8 and caspase 9 started at 3 h in siC control cells with 12% apoptosis. Again, cleavage of both caspase 8 and caspase 9 occurred earlier, at 2 h, in siDaxx-transfected cells, which was accompanied by 15% apoptosis.
We also analyzed the kinetics of caspase activation by actinomycin D and UV treatment in siC- versus siDaxx-transfected cells. Activation of both caspase 8 and caspase 9 started at around 12 h in siC control cells after actinomycin D treatment, with 16% apoptosis. Similarly, activation of both caspase 8 and caspase 9 occurred much earlier in siDaxx-transfected cells, at 5 h after actinomycin D treatment, when there was 27% apoptosis. Finally, UV caused activation of both caspase 8 and caspase 9 at 12 h in siC control cells after irradiation, with 16% apoptosis. In contrast, both caspase 8 and caspase 9 were activated at 5 h in siDaxx-treated cells, when there was 35% apoptosis. Together, these data clearly indicate earlier onset of caspase activation and apoptosis by Daxx silencing upon anti-Fas antibody or stress treatment.
Daxx silencing stimulates early cytochrome c release.
Cytochrome c release from mitochondria is an essential early step for apoptosome formation and caspase activation (for reviews, see references 33 and 39). To determine if Daxx silencing affects cytochrome c release, we analyzed the intracellular distribution of cytochrome c by immunofluorescence staining of siDaxx-transfected cells. As a control, siC and siDaxx alone did not cause cytochrome c release from mitochondria (Fig. 5A and B), consistent with the apoptosis (Fig. 2) and caspase activation (Fig. 4) data. Similarly, short-term (1 h) treatment with anti-Fas antibody, UV irradiation, and actinomycin D also did not significantly increase the number of cells with cytochrome c release in siC-treated control cells (Fig. 5B, open bars). In contrast, cytochrome c release was dramatically enhanced in siDaxx-treated cells upon anti-Fas antibody, UV irradiation, or actinomycin D treatment (Fig. 5A, arrows; 5B, black bars). These results indicate that Daxx silencing may synergize with Fas, UV, and actinomycin D to induce cytochrome c release from mitochondria.
FIG. 5.
Daxx silencing stimulates early cytochrome c release. HeLa cells transfected with the siC control or siDaxx were treated with UV irradiation (50 J/m2), anti-Fas antibody (200 ng/ml), or actinomycin D (AD, 100 nM) for 1 h. Daxx (green) and cytochrome c (red) were double stained with the respective polyclonal and monoclonal antibodies along with DAPI staining for nuclei (blue). (A) Representative immunofluorescence images for mitochondrial cytochrome c release at 1 h after the indicated treatments. Release of cytochrome c from mitochondria caused diffuse cytochrome c staining in cells marked by arrows. (B) Quantitation of cytochrome c release after various treatments. The data represent three independent experiments with at least 300 cells scored from three different fields. Under the experimental conditions, siC, siDaxx, and the treatment alone did not cause significant cytochrome c release. However, siDaxx transfection in combination with anti-Fas antibody, UV, or actinomycin D synergistically enhanced cytochrome c release.
Daxx silencing affects JNK activation.
The Jun N-terminal kinase (JNK) can be activated by stresses such as UV and TNF-α treatments, which plays an important role in regulating cell proliferation and apoptosis (11). Intriguingly, Daxx reportedly activates JNK through Ask1 (5, 7, 34, 63). To determine if Daxx is indeed required for JNK activation and whether Daxx silencing affects stress-mediated JNK activation, we analyzed JNK activation in siC- versus siDaxx-transfected cells upon treatment with anti-Fas antibody, UV irradiation, or TNF-α (Fig. 6). Total JNK proteins were immunoprecipitated and an in vitro kinase assay was performed with purified GST-Jun (amino acids 1 to 79) fusion protein as the substrate. The JNK proteins isolated from siC control cells did not significantly phosphorylate Jun upon anti-Fas antibody treatment for over 240 min (Fig. 6A, siC). In contrast, JNK proteins isolated from siDaxx-transfected cells were able to phosphorylate Jun slightly upon Fas antibody treatment for 120 to 240 min (Fig. 6A, siDaxx). Western blot analysis showed approximately equal amounts of immunoprecipitated JNK1 and JNK2 proteins in the reactions (Fig. 6A). These data suggest that Daxx may not be required for Fas-mediated JNK activation. In contrast, Daxx silencing slightly enhances JNK activation.
FIG. 6.
Daxx silencing affects JNK activation. (A) Effect of Daxx silencing on Fas-mediated JNK activation. HeLa cells were transfected with siC or siDaxx for 48 h, followed by anti-Fas antibody (200 ng/ml) treatment for the indicated periods of time. The JNK kinase assay was performed as described in Materials and Methods. Phosphorylated Jun (p-Jun) was analyzed by SDS-PAGE and autoradiography. The immunoprecipitated JNK1 and -2 proteins were detected by Western blot (W.B.). Daxx silencing slightly enhanced JNK activation at 120 and 240 min of anti-Fas antibody treatment. (B) Effect of Daxx silencing on UV-induced JNK activation. The JNK kinase assay (K.A.) and Western blot (W.B.) were performed as described above. Cells were harvested at the indicated time (in minutes) after UV irradiation (50 J/m2). A threefold-higher level of phosphorylated Jun remained in siDaxx- versus siC-treated cells at 120 min after UV irradiation. (C) Effect of Daxx silencing on JNK activation induced by TNF-α. The JNK kinase assay (K.A.) and Western blot (W.B.) are described above. There was a 33% increase in JNK kinase activity at 15 min after TNF-α treatment (10 ng/ml).
Next, we analyzed the effects of Daxx silencing on JNK activation induced by UV and TNF-α. As expected, UV irradiation induced a rapid activation of JNK, within 30 min after irradiation in siC-treated control cells (Fig. 6B). As expected, this JNK activity declined gradually, within 120 min. Interestingly, Daxx silencing not only did not inhibit UV-induced JNK activation, it caused a significant delay in the decay of JNK activity. An approximately threefold higher JNK activity remained at 120 min after UV irradiation in siDaxx- versus siC-treated cells, suggesting that Daxx may be involved in regulating JNK activity. Similar to UV, TNF-α also caused a rapid activation of JNK, within 15 min in siC-treated control cells. As expected, this JNK activity disappeared gradually, within 60 min. Interestingly, Daxx silencing also did not inhibit TNF-α-induced JNK activation; instead, it enhanced JNK activation by 33% at 15 min compared with the siC-treated control cells. Together, these data suggest that Daxx may not be essential for JNK activation and that Daxx silencing may affect JNK function by enhancing or prolonging its kinase activity.
Degradation of endogenous Daxx during apoptosis.
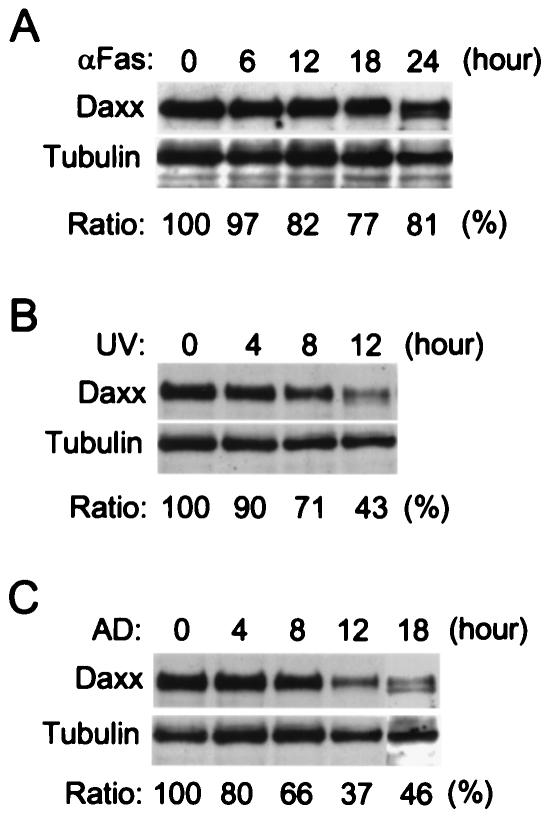
Since Daxx silencing synergizes with Fas and stress to induce apoptosis, it would be interesting to know if endogenous Daxx levels are affected during apoptosis. Western blotting was used to analyze Daxx protein levels in HeLa cells after anti-Fas antibody or stress treatment. Tubulin was used as an internal reference for protein loading and normalization. Daxx protein levels declined gradually to about 80% within 12 h after the addition of anti-Fas antibody (Fig. 7A). A smaller form of Daxx was observed at 24 h, suggesting that Daxx may be subjected to proteolysis during apoptosis. Interestingly, Daxx protein levels declined much more rapidly following UV irradiation (Fig. 7B). The relative Daxx protein levels were reduced to 71% at 8 h and 43% at 12 h after the treatment. Similarly, Daxx protein levels also decreased rapidly upon actinomycin D treatment, reducing to 66% at 8 h and 37% at 12 h (Fig. 7C). Both UV and actinomycin D treatments also resulted in the appearance of the smaller form of Daxx as seen in the anti-Fas antibody-treated cells. These data suggest that the endogenous Daxx protein may rapidly degrade upon induction of apoptosis.
FIG. 7.
Reduction of Daxx protein levels during induction of apoptosis. The endogenous Daxx and tubulin protein levels in HeLa cells were analyzed by Western blot upon treatment of the cells with (A) anti-Fas antibody (200 ng/ml), (B) UV irradiation (50 J/m2), or (C) actinomycin D (AD, 100 nM). Total cell lysates from approximately equal numbers of cells were prepared at the indicated time points after treatment and analyzed by SDS-PAGE followed by Western blot detection for Daxx and tubulin. The relative Daxx protein levels are shown at the bottom as a ratio over the tubulin protein levels, which remained relatively unchanged during the treatment. The Daxx protein level declined to 82, 43, and 37% at 12 h after treatment with anti-Fas antibody, UV, and actinomycin D, respectively.
Role of PML in Daxx silencing-mediated apoptosis.
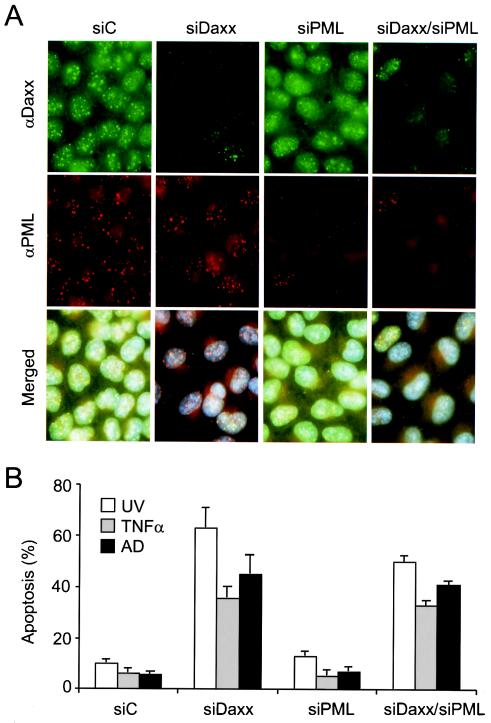
PML has been implicated in regulating Daxx-mediated apoptosis and transcriptional repression activities (54, 36). To investigate the role of PML in Daxx silencing-mediated apoptosis, we designed and synthesized an siRNA against PML mRNA (siPML) and analyzed its effects on Daxx's localization and silencing-mediated apoptosis by immunofluorescence staining and TUNEL assays. As expected, Daxx colocalized with PML at 10 to 20 nuclear dots in control cells (Fig. 8A, siC). Interestingly, depletion of Daxx did not affect PML staining in the PODs (Fig. 8A, siDaxx), consistent with previous observations (28, 36, 67). Similar to siDaxx, siPML was also capable of depleting PML staining in about 90% of transfected cells (Fig. 8A, siPML). Depletion of PML apparently altered the distribution pattern of Daxx in the nucleus, resulting in stronger nucleoplasmic staining and fewer dots. Cotransfection of siDaxx and siPML resulted in depletion of both Daxx and PML staining in transfected cells (Fig. 8A, siDaxx/siPML).
FIG. 8.
Role of PML in Daxx silencing-mediated apoptosis. (A) HeLa cells were transfected with siC, siDaxx, siPML, or siDaxx plus siPML, followed by coimmunofluorescence staining for Daxx (green) and PML (red) with anti-Daxx polyclonal and anti-PML monoclonal antibodies. (B) Apoptotic response of Daxx- and/or PML-depleted cells to UV, TNF-α, and actinomycin D treatment. Apoptosis was determined by the TUNEL assay, with at least 300 cells scored in three different fields.
We then analyzed the apoptotic responses of Daxx- and/or PML-depleted cells to stress treatments. Again, Daxx silencing strongly enhanced UV, TNF-α, and actinomycin D-induced apoptosis (Fig. 8B, siC versus siDaxx). However, PML silencing did not result in significant changes in apoptosis induced by UV, TNF-α, or actinomycin D (Fig. 8B, siPML), consistent with prior genetic studies (60). Finally, we found that the Daxx and PML double-silenced cells also displayed enhanced apoptotic responses to UV, TNF-α, and actinomycin D treatments, in a manner similar to the Daxx-silenced cells (Fig. 8B, siDaxx/siPML), suggesting that Daxx silencing-enhanced apoptosis may not require PML.
Daxx silencing affects expression of apoptosis-regulatory genes.
Finally, we decided to investigate if Daxx silencing may affect the expression of apoptosis-regulatory genes. Total RNAs from HeLa cells were isolated 48 h after initiation of Daxx silencing by siDaxx transfection. RT-PCRs were conducted to measure the expression levels of several apoptosis-regulatory genes (Fig. 9). As expected, expression of GAPDH remained unchanged in siDaxx-transfected cells, and Daxx ex-pression decreased 5.9-fold to 17% of the control sample value. Similar to GAPDH, Daxx silencing did not affect the expression of several apoptosis-regulatory genes, including BAD, BAX, and caspase 9. However, in siDaxx- versus siC-transfected cells, the expression of caspase 3, caspase 8, and caspase 10 was 2.5-, 1.5-, and 2.8-fold higher, respectively (Fig. 9), suggesting that Daxx silencing may directly or indirectly stimulate the expression of these apoptosis-regulatory genes.
FIG. 9.
Daxx silencing affects expression of apoptosis-regulatory genes. HeLa cells were transfected with the siC control (−) or siDaxx (+) for 48 h, and total RNAs were isolated. Three micrograms of total RNAs was analyzed by RT-PCR for the indicated genes. The PCR products were analyzed on a 2% agarose gel and ethidium bromide staining. The relative intensities of expected PCR bands were measured by the Kodak ID image analysis software. The increase or decrease (in parentheses) in expression for each gene is shown at the bottom. The upper band in the caspase 10 reaction was the expected PCR product, while the lower band may represent an alternatively spliced form.
DISCUSSION
In this study, we investigated the role of Daxx in Fas- and stress-mediated apoptosis by silencing its expression in mammalian cells with siRNA. In contrast to the proposed proapoptotic function, both Fas- and stress-mediated apoptosis was significantly enhanced in Daxx-deficient cells. Enhancement of apoptosis by Daxx silencing involved earlier activation of caspases, earlier release of cytochrome c from mitochondria, and stronger or prolonged activation of JNK kinases. Conversely, induction of apoptosis by Fas and stresses caused accelerated degradation of endogenous Daxx. Furthermore, Daxx silencing-mediated apoptosis appears to be independent of PML, while the expressions of several caspase genes were stimulated in Daxx-silenced cells. These data strongly suggest that Daxx may have an antiapoptotic function outside of PML nuclear domains, where it may contribute to repression of apoptotic gene expression.
siRNA has been used successfully to silence gene expression in mammalian cells (13-15). As expected, siDaxx acted specifically and efficiently in reducing Daxx protein and mRNA levels in mammalian cells. We were able to reduce Daxx protein level to 10% of that in control cells, which coincides with a 90% transfection efficiently, suggesting that siDaxx may be able to deplete endogenous Daxx protein in transfected cells. siDaxx had no effect on the expression of unrelated proteins even at a concentration 100-fold higher than optimal, confirming the specificity of the siRNA. We believe that silencing by siRNA may have advantages over the mouse gene knockout strategy because it may avoid long-term compensatory effects. Indeed, Daxx silencing had no effect on cell growth and proliferation within 72 h, while Daxx knockout mice die early during embryonic development (41). Therefore, Daxx silencing by siRNA may be useful for analyzing the role of Daxx in various signaling pathways.
In contrast to several prior reports implicating Daxx as a proapoptotic protein (5, 45, 54, 61, 64, 67), it is interesting that Daxx silencing caused enhancement of Fas- and stress-induced apoptosis. While our data are consistent with the reported increase in spontaneous apoptosis in Daxx−/− mouse cells (41) and the recent report of Daxx-silenced mammalian cells (42), we observed little or no change in the basal apoptotic rate without treatment with apoptosis-inducing agents. The ability of Fas and stress to induce apoptosis in Daxx-silenced cells strongly suggests that Daxx is not essential for initiation of programmed cell death. In fact, an inhibitory role for endogenous Daxx in apoptosis may be possible.
It is unclear why overexpression and depletion of Daxx can both enhance apoptosis. However, similar to Daxx, overexpression and deletion of the death domain kinase RIP both induce apoptosis (21, 31, 51). One possibility is that Daxx might have dual functions depending on its cellular concentration. For instance, at higher levels, Daxx might interact with lower-affinity receptors that promote apoptosis, while at lower levels, Daxx might preferentially bind to higher-affinity targets to inhibit apoptosis. It is also possible that overexpression of an antiapoptotic protein might result in a dominant negative effect due to disruption of antiapoptotic complexes. Finally, Daxx might act as a proapoptotic protein in the cytoplasm but as an antiapoptotic protein in the nucleus. Overexpression might result in accumulation of Daxx in the cytoplasm, where it might interact with Fas and other death receptors to promote apoptosis (34).
The fact that Daxx-deficient cells are sensitive to several apoptotic agents suggests that Daxx may be involved in multiple apoptotic pathways. Indeed, caspases, JNK kinase, and cytochrome c are all activated earlier in the absence of Daxx in response to Fas or stress. In the Daxx-silenced cells, the caspase inhibitor z-VAD-fmk blocked apoptosis, suggesting an involvement of caspase in Daxx silencing-mediated apoptosis. Indeed, cleavage of caspase 8, caspase 9, and their substrate PARP was also observed earlier in the absence of Daxx. Kinetic studies further support a synergistic effect on caspase activation by siDaxx and apoptotic agents. These data suggest that Daxx may be dispensable for Fas- and stress-induced caspase activation. Interestingly, stable overexpression of Daxx in HT1080 cells also caused a slight increase in caspase activation in response to Fas antibody treatment (54), further suggesting that Daxx may directly or indirectly regulate caspase activation.
The role of Daxx in Fas-mediated JNK activation has been controversial (5, 24, 45, 54, 57, 63). Recently, Daxx was reported to mediate JNK activation and apoptosis induced by transforming growth factor beta (45) and UV irradiation (61). Surprisingly, our data show that JNK activation does not require Daxx. In contrast, Daxx depletion caused earlier and/or sustained JNK activation in response to various treatments. In contrast to previous observations showing strong JNK activation in 293 cells overexpressing Fas (5, 63), little activation of JNK was observed in HeLa cells without Fas overexpression. Indeed, a role for JNK in Fas-mediated apoptosis is also controversial because Jnk is dispensable for Fas-induced apoptosis (11, 55). Together, these data suggest that not only is Daxx not essential for JNK activation in HeLa cells, but that endogenous Daxx may be inhibitory to JNK activation.
The genetic pathway of stress-induced apoptosis is thought to be mediated by JNK activation (11), which subsequently induces the release of mitochondrial cytochrome c via unknown mechanisms. Cytochrome c then acts with Apaf-1 to activate caspase 9, which subsequently activates caspase 3 to cleave a wide spectrum of death substrates. Because Daxx silencing caused early activations of caspase 9 and JNK, it is not surprising to see early cytochrome c release in the absence of Daxx. This result is consistent with a recent finding that Bcl-2 can also prevent Daxx silencing-mediated apoptosis (42). Since the nature of JNK activation is unknown, it is difficult to speculate how Daxx might influence JNK activation. Because the processing of caspase 8 was also accelerated in the absence of Daxx and caspase 8 is recruited to the activated Fas through FADD, it is possible that Daxx may inhibit a proximal step(s) in the apoptosis signaling pathway induced by Fas or stress. Perhaps the binding of Daxx to Fas is inhibitory to FADD-mediated apoptosis as well as JNK activation. Additional studies are needed in order to understand how Daxx regulates the Fas-FADD-caspase 8 pathway and stress-induced JNK activation.
The localization of Daxx in the nucleus and its association with PODs suggest a potentially nuclear route to regulate apoptosis by Daxx. In fact, overexpression of Daxx has been shown to promote Fas-induced apoptosis through PODs (54, 67). PML was also shown to be essential for multiple apoptotic pathways (60), and overexpression of PML promotes apoptosis (3, 47). In contrast, PML-retinoic acid receptor alpha stimulates cell survival (3, 47, 49). Previously, we showed that PML inhibits the transcriptional repression activity of Daxx by sequestering Daxx to the PODs (36). By using siRNA against PML, we confirmed that depletion of PML altered Daxx's localization (Fig. 8), presumably by releasing Daxx from PODs into the nucleoplasm. Interestingly, Daxx silencing still caused remarkable enhancement of apoptosis in the absence of PML, suggesting that PML and perhaps PODs may not be essential for Daxx to inhibit apoptosis. Based on these observations, we speculate that Daxx may exert its antiapoptotic function outside of PODs, while PML may sequester Daxx to PODs to block this antiapoptosis function (Fig. 10), reminiscent of its effect on the transcriptional repression activity of Daxx (36). In contrast, PML- retinoic acid receptor alpha may promote survival by disrupting PODs and releasing Daxx to the nucleoplasm to protect cells against apoptosis.
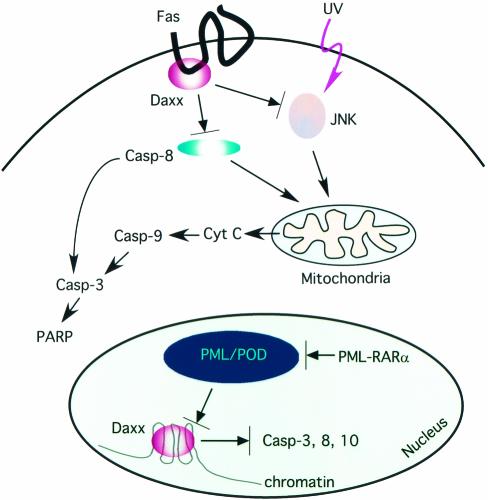
FIG. 10.
Potential mechanisms of the antiapoptotic function of Daxx. Daxx might inhibit Fas-mediated caspase 8 activation and UV- or stress-induced JNK activation. This ability might be due to association of Daxx with the cytoplasmic domain of Fas. Consequently, depletion of Daxx stimulated caspase 8 and JNK activation, causing early mitochondrial cytochrome c release and activation of caspase 9 and caspase 3. In addition, PML may sequester Daxx to PODs, inhibiting its transcriptional repressor function. The oncogenic fusion protein PML-retinoic acid receptor alpha (RARα) may disrupt PODs, causing the release of Daxx from PODs and its association with condensed chromatin. We hypothesize that the association of Daxx with condensed chromatin might cause repression of selected caspase genes. Consequently, depletion of Daxx may increase the expression of apoptotic proteins, resulting in sensitization of cells to induction of apoptosis.
The fact that Daxx may be antiapoptotic in the nucleoplasm, where it may also repress transcription, suggests a possible connection between transcriptional repression and antiapoptosis by Daxx. Indeed, Daxx silencing stimulated the expression of several caspase genes (Fig. 9). Since Daxx silencing alone had little effect on apoptosis without cotreatment with other agents, higher expression of caspases may simply sensitize cells to apoptotic stimuli. Interestingly, most of the apoptotic genes involved in Fas signaling are not altered in Daxx-overexpressing cells (54). We speculate that overexpression of Daxx might have minimal effects on gene expression because Daxx seems to be abundant in cells. Alternatively, Daxx may regulate gene expression in a cell type-specific manner. Recently, depletion of Daxx has been shown to increase expression of NF-κB- and E2F1-regulated reporter genes (42), implying that Daxx might also repress NF-κB- and/or E2F1-activated proapoptotic genes to inhibit apoptosis. Together, these data suggest that Daxx may play an important role in regulating apoptotic gene expression, suggesting a potential pathway for the antiapoptotic function of Daxx.
Acknowledgments
We are grateful to members of the Chen laboratory and faculty in the Pharmacology Department for discussion, and we thank Roger Davis for the GST-Jun construct.
J.D.C. is a Research Scholar of the Leukemia and Lymphoma Society. This work was supported by a grant (CA87074) to J.D.C. from the National Cancer Institute.
REFERENCES
- 1.Amin, H. M., S. Saeed, and S. Alkan. 2001. Histone deacetylase inhibitors induce caspase-dependent apoptosis and downregulation of daxx in acute promyelocytic leukaemia with t(15;17). Br. J. Haematol. 115:287-297. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein, E., A. M. Denli, and G. J. Hannon. 2001. The rest is silence. RNA 7:1509-1521. [PMC free article] [PubMed] [Google Scholar]
- 3.Borden, K. L., E. J. CampbellDwyer, and M. S. Salvato. 1997. The promyelocytic leukemia protein PML has a proapoptotic activity mediated through its RING domain. FEBS Lett. 418:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cermak, L., a. imova, A. Pintzas, V. Horeji, and L. Andera. 2002. Molecular mechanisms involved in CD43-mediated apoptosis of TF-1 cells: roles of transcription, Daxx expression and adhesion molecules. J. Biol. Chem. 277:7955-7961. [DOI] [PubMed] [Google Scholar]
- 5.Chang, H. Y., H. Nishitoh, X. Yang, H. Ichijo, and D. Baltimore. 1998. Activation of apoptosis signal- regulating kinase 1 (ASK1) by the adapter protein daxx. Science 281:1860-1863. [DOI] [PubMed] [Google Scholar]
- 6.Chang, H. Y., X. Yang, and D. Baltimore. 1999. Dissecting Fas signaling with an altered-specificity death-domain mutant: requirement of FADD binding for apoptosis but not Jun N- terminal kinase activation. Proc. Natl. Acad. Sci. USA 96:1252-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charette, S. J., H. Lambert, and J. Landry. 2001. A kinase-independent function of Ask1 in caspase-independent cell death. J. Biol. Chem. 276:36071-36074. [DOI] [PubMed] [Google Scholar]
- 8.Charette, S. J., and J. Landry. 2000. The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann. N. Y. Acad. Sci. 926:126-131. [DOI] [PubMed] [Google Scholar]
- 9.Charette, S. J., J. N. Lavoie, H. Lambert, and J. Landry. 2000. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol. Cell. Biol. 20:7602-7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colangelo, V., J. Schurr, M. J. Ball, R. P. Pelaez, N. G. Bazan, and W. J. Lukiw. 2002. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J. Neurosci. Res. 70:462-473. [DOI] [PubMed] [Google Scholar]
- 11.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 12.Dyck, J. A., G. G. Maul, W. H. Miller, Jr., J. D. Chen, A. Kakizuka, and R. M. Evans. 1994. A novel macromolecular structure is a target of the promyelocyte- retinoic acid receptor oncoprotein. Cell 76:333-343. [DOI] [PubMed] [Google Scholar]
- 13.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 14.Elbashir, S. M., J. Harborth, K. Weber, and T. Tuschl. 2002. Analysis of gene function in somatic mammalian cells with small interfering RNAs. Methods 26:199-213. [DOI] [PubMed] [Google Scholar]
- 15.Elbashir, S. M., W. Lendeckel, and T. Tuschl. 2001. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15:188-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emelyanov, A. V., C. R. Kovac, M. A. Sepulveda, and B. K. Birshtein. 2002. The interaction of Pax5 (BSAP) with Daxx can result in transcriptional activation in B cells. J. Biol. Chem. 277:11156-11164. [DOI] [PubMed] [Google Scholar]
- 17.Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver, and C. C. Mello. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806-811. [DOI] [PubMed] [Google Scholar]
- 18.Florin, L., F. Schafer, K. Sotlar, R. E. Streeck, and M. Sapp. 2002. Reorganization of nuclear domain 10 induced by papillomavirus capsid protein l2. Virology 295:97-107. [DOI] [PubMed] [Google Scholar]
- 19.Genini, D., D. Sheeter, S. Rought, J. J. Zaunders, S. A. Susin, G. Kroemer, D. D. Richman, D. A. Carson, J. Corbeil, and L. M. Leoni. 2001. HIV induces lymphocyte apoptosis by a p53-initiated, mitochondrial-mediated mechanism. FASEB J. 15:5-6. [DOI] [PubMed] [Google Scholar]
- 20.Green, D. R. 2000. Apoptotic pathways: paper wraps stone blunts scissors. Cell 102:1-4. [DOI] [PubMed] [Google Scholar]
- 21.Grimm, S., B. Z. Stanger, and P. Leder. 1996. RIP and FADD: two “death domain”-containing proteins can induce apoptosis by convergent, but dissociable, pathways. Proc. Natl. Acad. Sci. USA 93:10923-10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann, H., H. Sindre, and T. Stamminger. 2002. Functional interaction between the pp71 protein of human cytomegalovirus and the PML-interacting protein human Daxx. J. Virol. 76:5769-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofmann, T. G., A. Moller, S. P. Hehner, D. Welsch, W. Droge, and M. L. Schmitz. 2001. CD95-induced JNK activation signals are transmitted by the death-inducing signaling complex (DISC), but not by Daxx. Int. J. Cancer 93:185-191. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann, W. K., S. de Vos, K. Tsukasaki, W. Wachsman, G. S. Pinkus, J. W. Said, and H. P. Koeffler. 2001. Altered apoptosis pathways in mantle cell lymphoma detected by oligonucleotide microarray. Blood 98:787-794. [DOI] [PubMed] [Google Scholar]
- 26.Hollenbach, A. D., C. J. McPherson, E. J. Mientjes, R. Iyengar, and G. Grosveld. 2002. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J. Cell Sci. 115:3319-3330. [DOI] [PubMed] [Google Scholar]
- 27.Hollenbach, A. D., J. E. Sublett, C. J. McPherson, and G. Grosveld. 1999. The Pax3-FKHR oncoprotein is unresponsive to the Pax3-associated repressor hDaxx. EMBO J. 18:3702-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishov, A. M., A. G. Sotnikov, D. Negorev, O. V. Vladimirova, N. Neff, T. Kamitani, E. T. Yeh, J. F. Strauss 3rd, and G. G. Maul. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juo, P., C. J. Kuo, J. Yuan, and J. Blenis. 1998. Essential requirement for caspase-8/FLICE in the initiation of the Fas- induced apoptotic cascade. Curr. Biol. 8:1001-1008. [DOI] [PubMed] [Google Scholar]
- 30.Kadosh, D., and K. Struhl. 1997. Repression by Ume6 involves recruitment of a complex containing sin3 corepressor and Rpd3 Histone deacetylase to target promoters. Cell 89:365-371. [DOI] [PubMed] [Google Scholar]
- 31.Kelliher, M. A., S. Grimm, Y. Ishida, F. Kuo, B. Z. Stanger, and P. Leder. 1998. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 8:297-303. [DOI] [PubMed] [Google Scholar]
- 32.Kiriakidou, M., D. A. Driscoll, J. M. Lopez-Guisa, and J. F. Strauss 3rd. 1997. Cloning and expression of primate Daxx cDNAs and mapping of the human gene to chromosome 6p21.3 in the MHC region. DNA Cell Biol. 16:1289-1298. [DOI] [PubMed] [Google Scholar]
- 33.Kluck, R. M., E. Bossy-Wetzel, D. R. Green, and D. D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132-1136. [DOI] [PubMed] [Google Scholar]
- 34.Ko, Y. G., Y. S. Kang, H. Park, W. Seol, J. Kim, T. Kim, H. S. Park, E. J. Choi, and S. Kim. 2001. Apoptosis signal-regulating kinase 1 controls the proapoptotic function of death-associated protein (Daxx) in the cytoplasm. J. Biol. Chem. 276:39103-39106. [DOI] [PubMed] [Google Scholar]
- 35.Lehembre, F., S. Muller, P. P. Pandolfi, and A. Dejean. 2001. Regulation of Pax3 transcriptional activity by SUMO-1-modified PML. Oncogene 20:1-9. [DOI] [PubMed] [Google Scholar]
- 36.Li, H., C. Leo, J. Zhu, X. Wu, J. O'Neil, E. J. Park, and J. D. Chen. 2000. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 20:1784-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, R., H. Pei, D. K. Watson, and T. S. Papas. 2000. EAP1/Daxx interacts with ETS1 and represses transcriptional activation of ETS1 target genes. Oncogene 19:745-753. [DOI] [PubMed] [Google Scholar]
- 38.Li, X. D., T. P. Makela, D. Guo, R. Soliymani, V. Koistinen, O. Vapalahti, A. Vaheri, and H. Lankinen. 2002. Hantavirus nucleocapsid protein interacts with the Fas-mediated apoptosis enhancer Daxx. J. Gen. Virol. 83:759-766. [DOI] [PubMed] [Google Scholar]
- 39.Lim, M. L., M. G. Lum, T. M. Hansen, X. Roucou, and P. Nagley. 2002. On the release of cytochrome c from mitochondria during cell death signaling. J. Biomed. Sci. 9:488-506. [DOI] [PubMed] [Google Scholar]
- 40.Lin, D.-Y., and H.-M. Shih. 2002. Essential role of the 58-kDa microspherule protein in the modulation of Daxx-dependent transcriptional repression as revealed by nucleolar sequestration. J. Biol. Chem. 277:25446-25456. [DOI] [PubMed] [Google Scholar]
- 41.Michaelson, J. S., D. Bader, F. Kuo, C. Kozak, and P. Leder. 1999. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev. 13:1918-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michaelson, J. S., and P. Leder. 2003. RNAi reveals antiapoptotic and transcriptionally repressive activities of DAXX. J. Cell Sci. 116:345-352. [DOI] [PubMed] [Google Scholar]
- 43.Miura, M., R. M. Friedlander, and J. Yuan. 1995. Tumor necrosis factor-induced apoptosis is mediated by a CrmA-sensitive cell death pathway. Proc. Natl. Acad. Sci. USA 92:8318-8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohiro, Y., A. Usheva, S. Kobayashi, S. L. Duffy, R. Nantz, D. Gius, and N. Horikoshi. 2003. Inhibition of stress-inducible kinase pathways by tumorigenic mutant p53. Mol. Cell. Biol. 23:322-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perlman, R., W. P. Schiemann, M. W. Brooks, H. F. Lodish, and R. A. Weinberg. 2001. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat. Cell Biol. 3:708-714. [DOI] [PubMed] [Google Scholar]
- 46.Pluta, A. F., W. C. Earnshaw, and I. G. Goldberg. 1998. Interphase-specific association of intrinsic centromere protein CENP-C with HDaxx, a death domain-binding protein implicated in Fas-mediated cell death. J. Cell Sci. 111:2029-2041. [DOI] [PubMed] [Google Scholar]
- 47.Quignon, F., F. De Bels, M. Koken, J. Feunteun, J. C. Ameisen, and H. de The. 1998. PML induces a novel caspase-independent death process. Nat. Genet. 20:259-265. [DOI] [PubMed] [Google Scholar]
- 48.Ryu, S. W., S. K. Chae, and E. Kim. 2000. Interaction of Daxx, a Fas binding protein, with sentrin and Ubc9. Biochem. Biophys. Res. Commun. 279:6-10. [DOI] [PubMed] [Google Scholar]
- 49.Salomoni, P., and P. P. Pandolfi. 2002. The role of PML in tumor suppression. Cell 108:165-170. [DOI] [PubMed] [Google Scholar]
- 50.Shi, Y. 2002. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 9:459-470. [DOI] [PubMed] [Google Scholar]
- 51.Stanger, B. Z., P. Leder, T. H. Lee, E. Kim, and B. Seed. 1995. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81:513-523. [DOI] [PubMed] [Google Scholar]
- 52.Stuurman, N., A. de Graaf, A. Floore, A. Josso, B. Humbel, L. de Jong, and R. van Driel. 1992. A monoclonal antibody recognizing nuclear matrix-associated nuclear bodies. J. Cell Sci. 101:773-784. [DOI] [PubMed] [Google Scholar]
- 53.Tang, G., J. Yang, Y. Minemoto, and A. Lin. 2001. Blocking caspase-3-mediated proteolysis of IKKbeta suppresses TNF-alpha-induced apoptosis. Mol. Cell 8:1005-1016. [DOI] [PubMed] [Google Scholar]
- 54.Torii, S., D. A. Egan, R. A. Evans, and J. C. Reed. 1999. Hum. Daxx regulates Fas-induced apoptosis from nuclear PML oncogenic domains (PODs). EMBO J. 18:6037-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tournier, C., P. Hess, D. D. Yang, J. Xu, T. K. Turner, A. Nimnual, D. Bar-Sagi, S. N. Jones, R. A. Flavell, and R. J. Davis. 2000. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288:870-874. [DOI] [PubMed] [Google Scholar]
- 56.Vautier, D., D. Besombes, D. Chassoux, F. Aubry, and P. Debey. 1994. Redistribution of nuclear antigens linked to cell proliferation and RNA processing in mouse oocytes and early embryos. Mol. Reprod. Dev. 38:119-130. [DOI] [PubMed] [Google Scholar]
- 57.Villunger, A., D. C. Huang, N. Holler, J. Tschopp, and A. Strasser. 2000. Fas ligand-induced c-Jun kinase activation in lymphoid cells requires extensive receptor aggregation but is independent of DAXX, and Fas-mediated cell death does not involve DAXX, RIP, or RAIDD. J. Immunol. 165:1337-1343. [DOI] [PubMed] [Google Scholar]
- 58.Waghray, A., M. Schober, F. Feroze, F. Yao, J. Virgin, and Y. Q. Chen. 2001. Identification of differentially expressed genes by serial analysis of gene expression in human prostate cancer. Cancer Res. 61:4283-4286. [PubMed] [Google Scholar]
- 59.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 60.Wang, Z. G., D. Ruggero, S. Ronchetti, S. Zhong, M. Gaboli, R. Rivi, and P. P. Pandolfi. 1998. PML is essential for multiple apoptotic pathways. Nat. Genet. 20:266-272. [DOI] [PubMed] [Google Scholar]
- 61.Wu, S., H. N. Loke, and A. Rehemtulla. 2002. Ultraviolet radiation-induced apoptosis is mediated by Daxx. Neoplasia 4:486-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu, J., L. Liao, G. Ning, H. Yoshida-Komiya, C. Deng, and B. W. O'Malley. 2000. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc. Natl. Acad. Sci. USA 97:6379-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang, X., R. Khosravi-Far, H. Y. Chang, and D. Baltimore. 1997. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 89:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang, X. J., V. V. Ogryzko, J. Nishikawa, B. H. Howard, and Y. Nakatani. 1996. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382:319-324. [DOI] [PubMed] [Google Scholar]
- 65.Yeh, W. C., J. L. Pompa, M. E. McCurrach, H. B. Shu, A. J. Elia, A. Shahinian, M. Ng, A. Wakeham, W. Khoo, K. Mitchell, W. S. El-Deiry, S. W. Lowe, D. V. Goeddel, and T. W. Mak. 1998. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279:1954-1958. [DOI] [PubMed] [Google Scholar]
- 66.Zhang, J., D. Cado, A. Chen, N. H. Kabra, and A. Winoto. 1998. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392:296-300. [DOI] [PubMed] [Google Scholar]
- 67.Zhong, S., P. Salomoni, S. Ronchetti, A. Guo, D. Ruggero, and P. P. Pandolfi. 2000. Promyelocytic leukemia protein (PML) and Daxx participate in a novel nuclear pathway for apoptosis. J. Exp. Med. 191:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]