Abstract
PAK5 is a member of the group B family of PAK serine/threonine kinases and is an effector for the Rho GTPase Cdc42. PAK5 is highly expressed in the brain and is expressed at lower levels in several other tissues. In cell lines, PAK5 has been shown to play a role in filopodia formation and neurite outgrowth. To examine the biological function of PAK5, we deleted the PAK5 gene in mice. The phenotypes of the PAK5-null mice are completely different from those of mice null for PAK4, another member of the group B PAK family. Unlike PAK4-null mice, which are embryonic lethal, PAK5-null mice develop normally and are fertile. The nervous system appears normal in the absence of PAK5, as do other tissues in which PAK5 is normally expressed. Our results suggest functional redundancy between PAK5 and other Rho GTPase targets.
The PAK kinases are a family of serine/threonine kinases that are targets for the Rho GTPases Cdc42 and Rac (3, 6, 7, 15, 25). The Rho GTPases have important roles in regulating cytoskeletal organization, cell motility, and signaling (28), and the PAK kinases are thought to be important effector proteins in these processes (3). In mammals there are six different PAKs, which fall into two categories based on their amino acid sequences and their functions. The first family, group A, consists of PAKs 1, 2, and 3, whereas the second family, group B, consists of PAKs 4, 5, and 6 (14). The group A and B PAKs are similar to each other only within a short amino-terminal region known as a GTPase-binding domain and within a carboxyl-terminal kinase domain. Even within these regions, however, they share only approximately 50% sequence identity. Outside of these regions the two families are completely different from each other (14). The different PAKs also differ from each other in function. Unlike the group A PAKs, for example, which bind to both activated Cdc42 and Rac, the group B PAKs bind more efficiently to activated Cdc42 (1, 5). Furthermore, overexpression of members of the group B PAKs leads to the formation of filopodia, actin-rich microspikes at the periphery of the cell (1, 5). Filopodia formation is an important function of Cdc42, and several studies have suggested that it is the group B PAKs that specifically mediate the formation of filopodia in response to Cdc42 (1, 5, 18, 26). PAK4, which has been used as a model for studying the group B PAKs, has also been shown to have other functions. These include regulation of cell adhesion, regulation of cell growth, and activation of cell survival pathways that lead to protection from apoptosis (9, 23).
The different PAK family members have different tissue specific expression patterns. While PAK4 is expressed ubiquitously in all tissues (1, 23a), PAK5 and PAK6 have restricted tissue-specific expression patterns (5, 16, 22, 29). PAK6 was identified based on its expression in the prostate and testes, but later it was also shown to be highly expressed in the brain (16, 29). Likewise, PAK5 is also highly expressed in the brain (5, 22). The expression of all of these proteins in the brain is significant because cytoskeletal changes triggered by Rho GTPases are thought to have important functions in the developing nervous system. For example, filopodia and lamellipodia play key roles in the guidance of neuronal growth cones and consequently play important roles in the outgrowth of neurons (17, 21). Consistent with this, Rho GTPases have been implicated in all aspects of neuronal development, including growth cone guidance and the extension of axons (17). Likewise, the PAK kinase have also been shown to have important roles in neuronal development in Drosophila melanogaster (11, 12, 19, 24) but less is known about its functions in mammals. In neuroblastoma cell lines, PAK5 was shown to promote the outgrowth of neurons, whereas dominant-negative PAK5 inhibited neurite outgrowth, suggesting an important role for mammalian PAK5 in neuronal development (5).
Although the mammalian PAKs have been shown to have important functions in cell lines, relatively little is known about their biological and developmental functions. Here we report the generation of PAK5 knockout mice, as well as expression pattern analysis of PAK5, to determine what functions it may have during development. We have found that mice lacking PAK5 develop normally and are fertile. Interestingly, this phenotype is completely different from PAK4 knockouts, which are embryonic lethal and have defects in the nervous system (23a). Our results suggest the possibility of functional redundancy between PAK5 and other brain enriched PAK kinases, such as PAK6.
MATERIALS AND METHODS
Cloning mouse PAK5 cDNA.
Mouse PAK5 cDNA was cloned by PCR by using the following primers corresponding to the 5′ and 3′ ends of human PAK5, respectively: 5′-ATGTTTGGGAAGAAAAAG and 3′-TCAGTGATGCCTGTATTG. Mouse brain cDNA was used as the template. The product was subcloned into TOPO-TA cloning vector and sequenced. The 2.1-kb product was found to be the full-length mouse PAK5 cDNA, which was 89% identical to the human sequence.
Northern blot.
A 1-kb fragment of the mouse or human PAK5 cDNA corresponding to the regulatory domain was radioactive labeled by using random primer labeling kit (Stratagene). The fragment was then used, according to the protocol provided by the manufacturer, to probe a mouse RNA master blot or a human multiple tissue expression array (Clontech).
Total RNA from different tissues of wild-type and PAK5-null mice was prepared by using RNAzol B (Tel-Test, Inc.). The isolated total RNA was separated and transferred to a positively charged nylon membrane (Amersham Bioscience) as described elsewhere (8). A 500 PAK6 cDNA fragment from the regulatory domain was radioactive labeled by using random primer labeling kit (Stratagene) and used to probe the membrane. The hybridization was performed in ExpressHyb solution from Clontech according to the manufacturer's protocol, and the membrane was exposed to Kodak film at −80°C for 5 days. The same membrane was probed with radiolabeled α-actin cDNA probe as loading control.
In situ hybridization.
A 1-kb mouse PAK5 cDNA fragment corresponding to the regulatory domain of PAK5 was subcloned into TOPO-PCR-4 vector (Invitrogen) between T3 and T7 promoters. Sense and antisense RNA probes were generated by using an in vitro transcription kit (Promega) and a digoxigenin (DIG) RNA labeling kit from Roche.
An 8-week-old female mouse (C57BL/6) was fixed by perfusion with 4% paraformaldehyde plus 0.25% gluteraldehyde in phosphate-buffered saline (PBS). The mouse was sacrificed, and the brain was removed and fixed in 4% paraformaldehyde overnight. The brain was then embedded in paraffin and cut into 10-μm-thick sections. The sections were dried at 60°C for 30 min, dewaxed by using xylene and ethanol, and then treated with proteinase K for 30 min. The hybridization was performed at 42°C overnight in hybridization buffer (50% formamide, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 10% dextran sulfate, 2.25× SSPE [1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA; pH 7.7], 2.5× Denhardt solution, 100 μg of herring sperm DNA/ml, 5 mM dithiothreitol, 40 U of RNase inhibitor/ml), followed by three 30-min washes (5× SSC, 2× SSC, and 0.1× SSC) at 55°C. The DIG was detected according to the protocol from the DIG detection kit (Roche).
Construction of the targeting vector and generation of PAK5 knockout mice.
A radioactive-labeled 1-kb fragment of mouse PAK5 cDNA corresponding to the regulatory domain (exon 1, exon 2, and part of exon 3) was used to screen a murine S129 genomic library (Genome Systems). Four positive bacterial artificial chromosome (BAC) clones were identified, two of them were partially mapped by restriction enzymes and partially sequenced. One of the BAC clones was 58 kb and contained only exon1 and part of the surrounding sequence. A second clone was 75 kb and contained the rest of the exons (i.e., exons 2 to 8). The total size of the PAK5 locus is 105 kb.
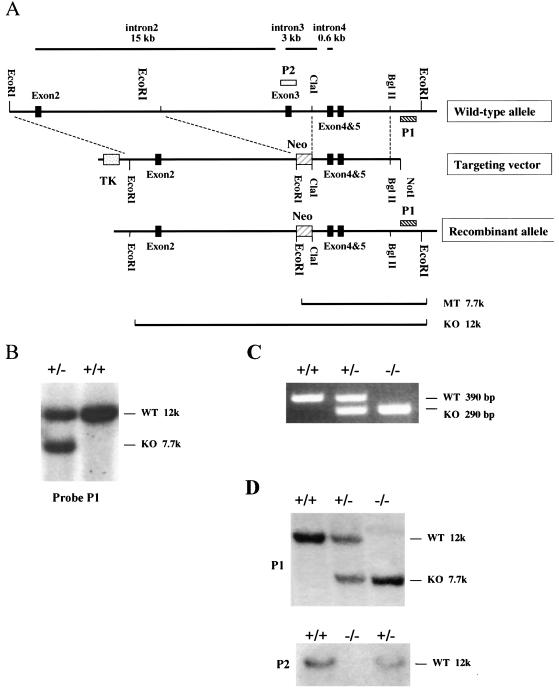
Sequencing of the BAC clones revealed that exon 3 contained several conserved amino acids, including the lysine within subdomain II, known to be important for the kinase activity of serine/threonine kinases, including the other PAKs (1, 5, 10). Therefore, a targeting vector was generated in which exon 3 is deleted, as follows. A 6.5-kb EcoRI fragment upstream of exon 3 and a 4.5-kb ClaI-BglII fragment downstream of exon 3 were sequentially subcloned into pPNT targeting vector (27) flanking the PGK-Neo gene. The PGK-Neo gene contains stop codons in all three reading frames. The resulting targeting vector has the PGK-Neo gene for positive selection and the PGK-thymidine kinase (TK) gene for negative selection. Homologus recombination between mouse genomic DNA and targeting vector results in the replacement of a 13-kb fragment, including exon 3 and surrounding intron sequence, by the 1.8-kb neomycin gene.
The targeting vector was linearized by cutting at a unique NotI site and electroporated into E14 embryonic stem (ES) cells. A total of 200 clones resistant to both G418 (400 μg/ml) and ganciclovir (2 μM) were picked 7 days after transfection and expanded. Genomic DNA was isolated from the clones, and the genotypes of the clones were assessed by Southern blotting. Three PAK5 heterozygous (+/−) ES clones were identified and expanded.
The PAK5+/− ES clones were injected into C57BL/6 blastocysts. Seven chimeric male mice with >50% ES cell contribution to the coat color were bred with C57BL/6 females. Both Southern blotting and competitive PCR on tail DNA were used to genotype the F1 generation. Heterozygous male and female pups were intercrossed to generate the homozygous mice.
Southern blots.
Genomic DNA was isolated from the ES clones or tails by treating the cells with proteinase K in ETS buffer (0.1 M Tris-HCl, 0.2 M NaCl, 0.5 mM EDTA, 0.4% sodium dodecyl sulfate [SDS]), followed by isopropanol precipitation. The purified DNA was digested with EcoRI. The resulting bands were separated on 0.8% agarose gel and transferred to a positively charged nylon membrane (Amersham Bioscience). Two probes were used, as shown in Fig. 1. P1 is a 700-bp BamHI-BglII fragment 3′ to the homologous region, which is used to determine the genotype of ES clones and pups from intercross. P2 is a 500-bp KpnI-BglII fragment within exon 3, which was used to confirm the deletion of exon 3 in homozygous mice. To generate the probes, the DNA fragments were purified by gel purification (Qiagen) and radioactively labeled by using a random primer labeling kit (Stratagene). Hybridization was performed in FBI buffer (10% PEG 8000, 1.5× SSPE, 7% SDS) at 65°C in a rotation oven, followed by three washes of 20 min each (3× SSC-0.1% SDS, 1× SSC-0.1% SDS, and 0.1× SSC-0.1% SDS) at the same temperature. The membrane was then exposed to X-ray film (Kodak) at −80°C overnight.
FIG. 1.
Generation of PAK5 knockout mice. (A) Generation of the targeting vector. A partial restriction map of the wild-type allele of PAK5 (exons 2, 3, 4, and 5) is shown. The lengths of introns 2, 3, and 4 are indicated above. To disrupt exon 3 in the mouse genome, a 6.5-kb EcoRI fragment, including exon 2, and a 4.5-kb ClaI-BglII fragment, including exons 4 and 5, were sequentially subcloned into pPNT targeting vector (27) flanking the PGK-Neo gene. The Neo gene contains a stop codon in all three reading frames. A PGK-TK gene was included in the targeting vector for negative selection. Homologous recombination between genomic DNA and the targeting vector results in the replacement of a 13-kb genomic fragment, including exon 3 by a 1.8-kb Neo gene. (B) Southern blot analysis of PAK5+/− ES cells. Southern blot analysis was carried out on EcoRI-digested genomic DNA from G418-resistant ES clones with probe P1, a 700-bp BamHI-BglII fragment 3′ of the homologous region (see panel A). The wild-type (WT) allele results in a 12-kb band and the knockout (KO) allele results in a 7.7-kb band. (C) PCR Genotyping of PAK5 knockout mice. Competitive PCR was done on genomic tail DNA with three primers: one common 3′ primer and 5′ PAK5- and Neo-specific primers. The wild-type (WT) allele results in a 390-bp band, and the knockout (KO) allele results in a 290-bp band. (D) Southern blot analysis of PAK5+/+, PAK5+/−, and PAK5−/− mice. Southern blot analysis was carried out on EcoRI-digested genomic DNA. Blots were probed with probe P1 (top) or probe P2 (bottom) (see panel A). For P1, the wild-type (WT) allele results in a 12-kb band, and the knockout (KO) allele results in a 7.7-kb band. P2 is a 500-bp sequence within exon 3. A 12-kb band is seen in the wild type. There is no band in the knockout, indicating that exon 3 is deleted.
Genotyping by PCR.
Competitive PCR was done by using genomic tail DNA as a template and three primers: a common 3′ primer (AGATGCATTGAGTGCTGGGGAA), a 5′ PAK5-specific primer (GCTTCCTCAGATCCATCCAAGGT), and a 5′ Neo-specific primer (CTTCCTGACTAGGGGAGGAGTA). The template, primers, deoxynucleoside triphosphates, and Taq polymerase (Invitrogen) were mixed together, and the following PCR cycles were used: 1 cycle of 94°C for 5 min; 30 cycles of 94°C for 30 s, 62°C for 30 s, and 72°C for 1 min; and a final cycle of 72°C for 10 min.
Antibodies.
Rabbit polyclonal anti-peptide PAK5 antibody was generated by Covance, Inc. The peptide LDLYYKSSHAAKQN, corresponding to part of the PAK5 regulatory domain, was used as the antigen. The antibody was affinity purified. The PAK1 antibody was from Santa Cruz Biochemicals, the Ste20 antibody was from Upstate Biotechnology, the PAK4 antibody was from Pharmingen, and the anti-α-actin antibody was from Sigma.
Western blots.
Tissue extracts were made from 8-week-old mice by using a homogenizer in tissue lysis buffer (1% SDS, 1 mM dithiothreitol, 1.0 mM sodium orthovanadate, 10 mM Tris-HCl [pH 7.5]). The resulting lysate was centrifuged at 13,000 rpm at 4°C for 10 min. The concentration of the protein was determined by protein assay (Bio-Rad). Equal amounts of protein were subjected to SDS-polyacrylamide gel electrophoresis on an 8% gel and then transferred to a polyvinylidene difluoride membrane. The membrane was blocked by 4% bovine serum albumin in PBS containing 0.2% Tween 20. The desired antibody was diluted as recommended in blocking buffer. The membrane was incubated in the antibody solution at 4°C overnight, followed by three washes in PBS plus 0.2% Tween 20 (once for 15 min and twice for 5 min). The membrane was then incubated with a horseradish peroxidase-linked second antibody at room temperature for 1 h, followed by three washes as described above. The bands were detected by using an enhanced chemiluminescence detection system (Amersham Bioscience).
Histology.
Organs were isolated from 8-week-old wild-type and knockout littermates. The tissues were fixed in 10% buffered formalin and then embedded in paraffin and sectioned. The sections were stained with hematoxylin and eosin (H&E) and analyzed by microscopy.
RESULTS
PAK5 knockout mice are viable and fertile.
The mouse PAK5 locus was found to be 105 kb long and to have eight exons. Interestingly, the intron/exon structure of PAK5 was exactly the same as what has been found previously for PAK4 (23a), where the GTPase-binding domain was encompassed by exon 1, and the kinase domain was encompassed by exons 3 to 8. The PAK5 genomic sequence was much bigger than PAK4, however, because of the very large introns. In particular, intron 1 (57 kb) and intron 2 (15 kb) were very large. The construction of exons 2, 3, 4 and 5 is shown schematically in Fig. 1A. The kinase domain of PAK5 begins in exon 3. This exon contains the lysine within kinase subdomain II that is known to be essential for the kinase activity of serine/threonine kinases in general, including the other PAKs (1, 5, 10). A targeting vector designed to delete exon 3 of mouse PAK5 gene was generated as shown in Fig. 1A. In this vector exon 3 was replaced with a neomycin gene, which also contains stop codons in all three reading frames. The targeting vector was linearized and electroporated into E14 ES cells, which were selected by both G418 and ganciclovir resistance. Three ES cells clones harboring the correct integration of the targeting sequence were identified by Southern blotting with a probe outside of the homologous recombination regions. A Southern blot analysis of one clone is shown in Fig. 1B. Heterozygous ES clones were injected into C57BL/6 blastocysts. Seven chimeric male mice from one of the ES cell lines were bred with C57BL/6 females to generate heterozygous mice. The resulting heterozygotes were intercrossed to generate the homozygous mice. Genotyping of the progeny revealed that PAK5-null mice were born at Mendelian frequencies (Table 1). Genotyping of the mice was verified by PCR (Fig. 1C), and the absence of exon 3 was also confirmed by Southern blot analysis (Fig. 1D). PAK5 knockout mice were backcrossed with each other and were found to be fertile and to produce litters of normal size (data not shown).
TABLE 1.
Genotype analysis of progeny resulting from crosses of PAK5+/− mice
| Mouse group | No. of mice with genotype (% total):
|
Total mice (% total) | ||
|---|---|---|---|---|
| +/+ | +/− | −/− | ||
| Female | 14 | 51 | 26 | 91 (44) |
| Male | 38 | 52 | 27 | 117 (56) |
| Total | 52 (25) | 103 (50) | 53 (25) | 208 |
Expression pattern of PAK5.
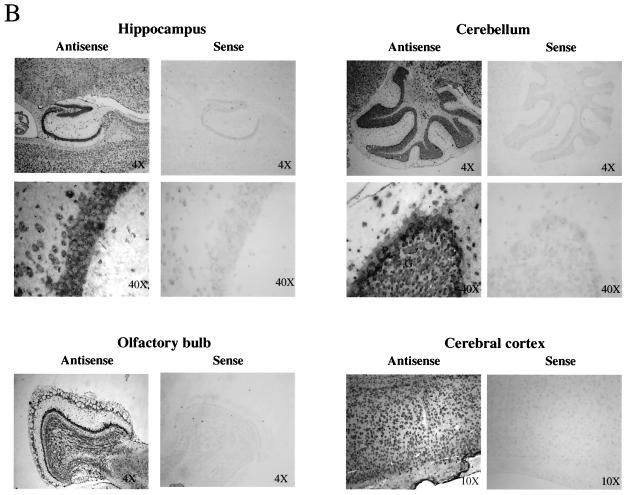
Northern blot analysis indicates that PAK5 was most highly expressed in the brain and the eye (Fig. 2A). PAK5 was found at high levels in every part of the brain that was tested and in both adult and embryonic brains. PAK5 was also expressed in several other tissues, although at significantly lower levels. These tissues include the adrenal gland, pancreas, prostate, and testes. To further study PAK5 expression in the brain, in situ hybridization was carried out on sagittal sections of wild-type adult brain (Fig. 2B). The results indicate that PAK5 was expressed specifically in neurons, in every part of the brain that was tested. For example, in the cerebellum PAK5 could be seen in Purkinje and granule cells but not in surrounding glial cells.
FIG. 2.
Expression patterns of PAK5. (A) Northern blot analysis of PAK5. Each Northern blot was probed with a 1-kb radiolabeled mouse or human PAK5 cDNA probe corresponding to the regulatory domain. Equal amounts of poly(A)+ RNA were dotted on the membrane as follows. Top panel (mouse RNA master blot): A1, brain; A2, eye; A3, liver; A4, lung; A5, kidney; B1, heart; B2, skeletal muscle; B3, smooth muscle; C1, pancreas; C2, thyroid; C3, thymus; C4, submaxillary gland; C5, spleen; D1, testis; D2, ovary; D3, prostate; D4, epididymis; D5, uterus; E1, embryo (7 days); E2, embryo (11 days); E3, embryo (15 days); E4, embryo (17 days). Bottom panel (human multiple tissue expression array): A1, whole brain; B1, cerebral cortex; C1, frontal lobe; D1, parietal lobe; E1, occipital lobe; F1, temporal lobe; G1, paracentral gyrus of cerebral cortex; H1, pons; A2, cerebellum (left); B2, cerebellum (right); C2, corpus callosum; D2, amygdala; E2, caudate nucleus; F2, hippocampus; G2, medulla oblongata; H2, putamen; B3, nucleus accumbens; C3, thalamus; A4, heart; B4, aorta; C4, atrium (left); D4, atrium (right); E4, ventricle (left); F4, ventricle (right); G4, interventricular septum; H4, apex of the heart; A5, esophagus; B5, stomach; C5, duodenum; D5, jejunum; E5, ileum; F5, ileocecum; G5, appendix; H5, ascending colon; A6, transverse colon; B6, descending colon; C6, rectum; A7, kidney; B7, skeleton muscle; C7, spleen; D7, thymus; E7, peripheral blood leukocyte; F7, lymph node; G7, bone morrow; H7, trachea; A8, lung; B8, placenta; C8, bladder; D8, uterus; E8, prostate; F8, testis; G8, ovary; A9, liver; B9, pancreas; C9, adrenal; D9, thyroid gland; E9, salivary gland; F9, mammary gland; A10, leukemia HL-60; B10, HeLa S3; C10, leukemia K-562; D10, leukemia MOLT-4; E10, Burkitt's lymphoma Raji; F10, Burkitt's lymphoma Daudi; G10, colorectal adenocarcinoma (SW480); H10, lung carcinoma, A549; A11, fetal brain; B11, fetal heart; C11, fetal kidney; D11, fetal liver; E11, fetal spleen; F11, fetal thymus; G11, fetal lung. (B) In situ hybridization on sagittal sections of wild-type adult brain. A 1-kb DIG-labeled mouse PAK5 antisense RNA sequence corresponding to the regulatory domain of PAK5 was used as a probe. Mouse PAK5 mRNA is expressed in neurons in all parts of brain. The fold objective is indicated for each panel. In the cerebellum ×40 picture, Purkinje cells (P) and granule cells (G) are labeled.
PAK5 protein is found in the brain and is absent in PAK5 knockouts.
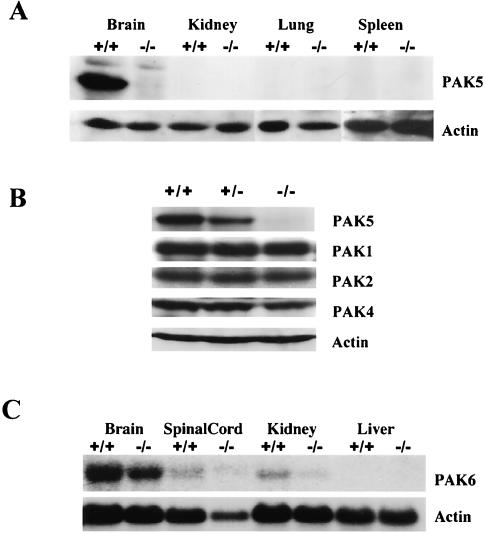
Western blot analysis indicates that PAK5 protein was also found at a high level in the adult brain but was not detectable in several other tissues that were tested (Fig. 3A). No PAK5 protein was detectable in the brains of the PAK5 knockout mice (Fig. 3A), and there was no evidence of a truncated PAK5 protein in the knockouts (data not shown). To determine whether other PAK family members may be abnormally upregulated in the absence of PAK5, lysates from PAK5+/+, PAK5+/−, and PAK5−/− brains were probed with anti-Ste20 antibody (which recognizes PAK2 in mouse), anti-PAK1 antibody, and anti-PAK4 antibody. The results indicated that the expression levels of PAK1, PAK2, and PAK4 in the brain were not affected by the absence of PAK5 protein (Fig. 3B). Likewise, PAK6 mRNA levels were not altered in the PAK5 knockouts, although PAK6 was clearly expressed in the brain (Fig. 3C).
FIG. 3.
Western blot analysis of PAK5 expression. (A) Western analysis of different tissue extracts from 8-week-old wild-type and knockout mice shows that the PAK5 protein is highly expressed in the brain. No PAK5 is found in the brains of the knockouts. The same blot was also probed with an actin antibody. (B) Western analysis of brain extracts from 8-week-old PAK5+/+, PAK5+/−, and PAK5−/− mice shows that PAK1, PAK2, and PAK4 protein are not abnormally upregulated in the absence of PAK5. The same blot was also probed with anti-actin antibody. (C) Northern analysis of total RNA from different tissues of 8-week-old wild-type and knockout mice. The blot was probed with a portion of PAK6 cDNA that is specific to PAK6. The same blot was also probed with actin as control. The results indicate that the PAK6 mRNA level is not changed in the absence of PAK5.
The nervous system develops normally in PAK5-null mice.
Since PAK5 is highly expressed in the brain, we were interested in determining whether there were abnormalities in the brains of the PAK5-null mice. Histological analysis indicated that the PAK5-null brains appeared to be similar to those of wild-type controls. For example, in Fig. 4 it is clear that the size and structure of the hippocampus, cerebral cortex, cerebellum, spinal cord, and olfactory bulb appeared normal in the knockouts. In addition, we found that primary culture of hippocampal neurons from PAK5 knockouts appeared normal, and no defects in cell spreading, attachment to the substrate, or neurite outgrowth were seen (data not shown).
FIG. 4.
H&E-stained coronal brain sections from the caudal diencephalons (I), rostral mesencephalon (II), and rostral cerebellum (III) sections from wild-type (WT) and PAK5 knockout mutant (KO) mice. The results indicate that the disruption of PAK5 does not affect the normal brain structure. All pictures were obtained with a 2× objective lens. All structures of the brain appear normal in the knockouts. The cerebral cortex (C), hippocampus (H), thalamus (T), cerebellum (CM), and spinal cord (S) are indicated.
The pancreas develops normally in PAK5-null mice.
Since PAK5 is also expressed in the pancreas, we examined the pancreases of PAK5-null mice and wild-type controls. Histological analysis of the pancreas of PAK5-null mice indicates that three major structures in the adult pancreas—the endocrine islets of Langerhans, the exocrine pancreatic acinus, and the pancreatic ducts—were normal as shown in Fig. 5. Consistent with this, the PAK5-null mice had normal growth rates and were not obese. The chemical blood analysis of the wild-type and knockout mice showed no obvious difference (data not shown).
FIG. 5.
H&E-stained pancreas sections from wild-type (WT) and PAK5 knockout mutant (KO) mice show that the pancreas is normally developed in PAK5-null mice. The top images were obtained with a 10× objective lens; the islets of Langerhans (I) and the pancreatic ducts (D) are shown. The 40× objective pictures on the bottom show the detailed structure of the islets (I) and pancreatic acinar cells (AC) with zymogen granules.
Normal development of the eyes of PAK5 knockouts.
PAK5 mRNA was also found in the eye. However, we found that all parts of the eye, including the cornea, lens, ciliary body, iris, and retina, developed normally in PAK5-null mice (Fig. 6).
FIG. 6.
Morphology of the eye in wild-type and PAK5 knockout mice as observed in H&E-stained sections of the eye. The cornea (C), lens (L), iris (I), and retina (R) are indicated; no major differences between wild-type (WT) and PAK5 knockout mutant (KO) mice are seen. The images were obtained with a 4× objective lens.
Normal development of the adrenal gland in PAK5 knockouts.
Since PAK5 is expressed in the adrenal gland, we also examined the morphology of the adrenal gland. We found that the histological structures of adrenal glands of the knockout mice were normal. As shown in Fig. 7, the medulla and the three concentric zones (zona glomerulosa, zona fasiculata, and zona reticularis) in the cortex were well organized.
FIG. 7.
Morphology of the adrenal gland in wild-type and PAK5 knockout mice as observed in H&E-stained sections of the adrenal gland. The medulla (M) and the three layers (zona glomerulosa [g], zona fasiculata [f], and zona reticularis [r]) of cortex (C) are labeled. No major difference between wild-type (WT) and PAK5 knockout (KO) mice are seen. The images were obtained with a 4× objective lens.
DISCUSSION
To study the biological function of PAK5, we generated PAK5 knockout mice. In addition, we have examined the expression pattern of PAK5 in detail. PAK5 was highly expressed in every part of the brain, but it was poorly expressed in most other tissues. In situ hybridization experiments indicated that within the brain, PAK5 was expressed specifically in neurons. Interestingly, “mushroom body tiny” (MBT), the Drosophila group B PAK, has been shown to be important for development of neurons within the brain and the eye (19, 24). Furthermore, our previous work indicates that at least in cell lines, PAK5 has a role in filopodia formation and neurite outgrowth (5). We were therefore interested in determining whether PAK5 plays an important role in neuronal development in the mouse. However, we found that the morphology of the brain appeared normal in mice that lack PAK5. Flies with mutations in MBT have a small mushroom body, a structure that corresponds to the mammalian hippocampus (19). However, the size and structure of the hippocampus appeared normal in the PAK5 knockouts. Likewise, the eyes also appear normal in PAK5 knockout mice. One possible explanation for this is that abnormalities in the nervous systems of these mice were too subtle to be detected. There is precedent for this in humans where mutations in PAK3 are associated with mental retardation with no gross abnormalities in the brain (2). Another possibility is that there is functional redundancy between PAK5 and another group B PAK family member. The most likely candidate for this is PAK6. We have found that the expression pattern of PAK5 is very similar to what has been previously reported for PAK6 (Fig. 2 and (16), so that the presence of PAK6 could potentially compensate for the lack of PAK5. It should also be noted that the PAK5 knockout mice were of a mixed genetic background (C57BL/6 × S129). Since phenotypes can vary with different genetic backgrounds (20), it will also be interesting in future studies to study the PAK5 knockout mice in different backgrounds.
Although PAK5 is most highly expressed in the brain, it is also expressed in several other tissues. For example, relatively high levels of PAK5 are found in the pancreas. There are a number of other examples of genes that are expressed in both the brain and the pancreas. One example is NeuroD, which is found in the brain, pancreas, and intestine. Interestingly, mouse knockouts of NeuroD do not have abnormalities in the nervous system but die due to severe diabetes (13). The pancreases of the NeuroD knockout mice have severe abnormalities, including a reduction in the amount of insulin producing beta cells and failure to form mature islets (13). We therefore examined the pancreases of PAK5 knockout mice. However, we found no abnormalities in the pancreas of PAK5 knockout mice, and mice did not die early due to diabetes. Similarly, other tissues in which PAK5 is expressed, such as the testes, prostate, epididymis, and adrenal gland, appeared normal in the PAK5 knockouts (Fig. 5 and data not shown).
It is interesting that the phenotypes of the PAK5 knockout mice were completely different from the knockouts of PAK4, another member of the group B PAK family. Unlike deletion of PAK5, deletion of PAK4 led to lethality prior to E11.5. The most likely cause of lethality in the PAK4 knockout embryos is a heart defect, but they also had severe abnormalities in the brain and nervous system, including defects in neurite outgrowth and differentiation. According to these results, PAK4 seems to be more functionally similar to Drosophila MBT than does PAK5.
Taken together, these results suggest that the different group B PAK family members—PAK4, PAK5, and PAK6—may have different but overlapping functions. PAK4, for example, is expressed very early in development. Large amounts of PAK4 can be seen in ES cells (23a), and PAK4 mRNA is found in embryos as early as E7. In contrast, PAK5 expression begins later in development. Very little PAK5 mRNA is detected in ES cells (data not shown) or before E11 (Fig. 2) and, unlike PAK4, PAK5 expression increases as development proceeds (Fig. 2) (23a). Although PAK6 mRNA cannot be detected in ES cells (23a), in the adult brain PAK6 mRNA was shown to be expressed at a significantly higher level than PAK4 (4), and we have also found that PAK5 protein levels are higher than PAK4 in the adult brain (data not shown). We propose that PAK4 is required for early embryonic development, and it is specifically required for the early development of several tissues, including the brain and the heart. In contrast, PAK5 and PAK6 may have functionally redundant roles later in development and in the adult, especially the brain. Functional redundancy between PAK5 and PAK6, however, may ensure that mutations in one gene do not lead to detrimental effects late in development and in adults. The future development of PAK6 and PAK5/PAK6 double knockout mice will be important for determining whether these two kinases have functionally redundant roles and at what stage in development they are important.
Acknowledgments
We thank C. Yang of Rockefeller University for help with the ES cell culture and injection, A. Beg for providing the S129 genomic library, R. G. Russell for helping with the histology analysis, and J. Qu for help in developing the knockouts.
This work was supported by NIH grant CA76342 to A.M.
REFERENCES
- 1.Abo, A., J. Qu, M. S. Cammarano, C. Dan, A. Fritsch, V. Baud, B. Belisle, and A. Minden. 1998. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 17:6527-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, K. M., J. G. Gleeson, S. Bagrodia, M. W. Partington, J. C. MacMillan, R. A. Cerione, J. C. Mulley, and C. A. Walsh. 1998. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat. Genet. 20:25-30. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, A. L., and A. Hall. 2000. Rho GTPase and their effector proteins. Biochem. J. 348:241-255. [PMC free article] [PubMed] [Google Scholar]
- 4.Callow, M. G., F. Clairvoyant, S. Zhu, B. Schryver, D. B. Whyte, J. R. Bischoff, B. Jallal, and T. Smeal. 2002. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J. Biol. Chem. 277:550-558. [DOI] [PubMed] [Google Scholar]
- 5.Dan, C., N. Nath, M. Liberto, and A. Minden. 2002. PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol. Cell. Biol. 22:567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dan, I., N. M. Watanabe, and A. Kusumi. 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11:220-230. [DOI] [PubMed] [Google Scholar]
- 7.Daniels, R. H., and G. M. Bokoch. 1999. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem. Sci. 24:350-355. [DOI] [PubMed] [Google Scholar]
- 8.Fourney, R. M., J. Miyakoshi, R. S. Day III, and M. C. Patterson. 1988. Northern blotting: efficient RNA staining and transfer. Focus 10:5-7. [Google Scholar]
- 9.Gnesutta, N., J. Qu, and A. G. Minden. 2001. The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. J. Biol. Chem. 276:14414-14419. [DOI] [PubMed] [Google Scholar]
- 10.Hanks, S. K., A. M. Quinn, and T. Hunter. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42-52. [DOI] [PubMed] [Google Scholar]
- 11.Harden, N., J. Lee, H. Y. Loh, Y. M. Ong, I. Tan, T. Leung, E. Manser, and L. Lim. 1996. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol. Cell. Biol. 16:1896-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hing, H., J. Xiao, N. Harden, L. Lim, and S. L. Zipursky. 1999. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell 97:853-863. [DOI] [PubMed] [Google Scholar]
- 13.Huang, H. P., K. Chu, E. Nemoz-Gaillard, D. Elberg, and M. J. Tsai. 2002. Neogenesis of beta-cells in adult BETA2/NeuroD-deficient mice. Mol. Endocrinol. 16:541-551. [DOI] [PubMed] [Google Scholar]
- 14.Jaffer, Z. M., and J. Chernoff. 2002. p21-Activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 34:713-717. [DOI] [PubMed] [Google Scholar]
- 15.Knaus, U. G., and G. M. Bokoch. 1998. The p21Rac/Cdc42-activated kinases (PAKs). Int. J. Biochem. Cell Biol. 30:857-862. [DOI] [PubMed] [Google Scholar]
- 16.Lee, S. R., S. M. Ramos, A. Ko, D. Masiello, K. D. Swanson, M. L. Lu, and S. P. Balk. 2002. AR and ER interaction with a p21-activated kinase (PAK6). Mol. Endocrinol. 16:85-99. [DOI] [PubMed] [Google Scholar]
- 17.Luo, L. 2000. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1:173-180. [DOI] [PubMed] [Google Scholar]
- 18.Manser, E., H. Y. Huang, T. H. Loo, X. Q. Chen, J. M. Dong, T. Leung, and L. Lim. 1997. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol. Cell. Biol. 17:1129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melzig, J., K. H. Rein, U. Schafer, H. Pfister, H. Jackle, M. Heisenberg, and T. Raabe. 1998. A protein related to p21-activated kinase (PAK) that is involved in neurogenesis in the Drosophila adult central nervous system. Curr. Biol. 8:1223-1226. [DOI] [PubMed] [Google Scholar]
- 20.Montagutelli, X. 2000. Effect of the genetic background on the phenotype of mouse mutations. J. Am. Soc. Nephrol. 16(Suppl. 11):S101-S105. [PubMed] [Google Scholar]
- 21.Mueller, B. K. 1999. Growth cone guidance: first steps toward a deeper understanding. Annu. Rev. Neurosci. 22:351-388. [DOI] [PubMed] [Google Scholar]
- 22.Pandey, A., I. Dan, T. Z. Kristiansen, N. M. Watanabe, J. Voldby, E. Kajikawa, R. Khosravi-Far, B. Blagoev, and M. Mann. 2002. Cloning and characterization of PAK5, a novel member of mammalian p21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene 21:3939-3948. [DOI] [PubMed] [Google Scholar]
- 23.Qu, J., M. S. Cammarano, Q. Shi, K. C. Ha, P. de Lanerolle, and A. Minden. 2001. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol. Cell. Biol. 21:3523-3533.11313478 [Google Scholar]
- 23a.Qu, J., X. Li, B. G. Novitch, Y. Zheng, M. Kohn, J.-M. Xie, S. Kozinn, R. Bronson, A. A. Beg, and A. Minden. 2003. PAK4 kinase is essential for embryonic lethality and for proper neuronal development. Mol. Cell. Biol. 23:7122-7133. [DOI] [PMC free article] [PubMed]
- 24.Schneeberger, D., and T. Raabe. 2003. Mbt, a Drosophila PAK protein, combines with Cdc42 to regulate photoreceptor cell morphogenesis. Development 130:427-437. [DOI] [PubMed] [Google Scholar]
- 25.Sells, M. A., and J. Chernoff. 1997. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 7:162-167. [DOI] [PubMed] [Google Scholar]
- 26.Sells, M. A., U. G. Knaus, S. Bagrodia, D. M. Ambrose, G. M. Bokoch, and J. Chernoff. 1997. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 7:202-210. [DOI] [PubMed] [Google Scholar]
- 27.Tybulewicz, V. L., C. E. Crawford, P. K. Jackson, R. T. Bronson, and R. C. Mulligan. 1991. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65:1153-1163. [DOI] [PubMed] [Google Scholar]
- 28.Van Aelst, L., and C. D'Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295-2322. [DOI] [PubMed] [Google Scholar]
- 29.Yang, F., X. Li, M. Sharma, M. Zarnegar, B. Lim, and Z. Sun. 2001. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J. Biol. Chem. 276:15345-15353. [DOI] [PubMed] [Google Scholar]