Abstract
The transcriptional activator Ime1 is a key regulator of meiosis and sporulation in budding yeast. Ime1 is controlled at different levels by nutrients and cell-type signals. Previously, we have proposed that G1 cyclins would transmit nutritional signals to the Ime1 pathway by preventing the accumulation of Ime1 within the nucleus. We show here that nutritional signals regulate the subcellular localization of Ime1 through the TOR pathway. The inactivation of TOR with rapamycin promotes the nuclear accumulation and stabilization of Ime1, with consequent induction of early meiotic genes. On the contrary, the activation of TOR by glutamine induces the relocalization of Ime1 to the cytoplasm. Thus, TOR may sense optimal nitrogen- and carbon-limiting conditions to modulate Ime1 function. Besides TOR, ammonia induces an independent mechanism that prevents the accumulation of Ime1 in the nucleus. Both TOR and ammonia regulate Ime1 localization in the absence of Cdk1 activity and therefore use mechanisms different from those exerted by G1 cyclins. Integration of independent mechanisms into a single early controlling step, such as the nuclear accumulation of Ime1, may help explain why yeast cells execute the meiotic program only when the appropriate internal and external conditions are met together.
Diploid cells of budding yeast are able to proceed through different developmental pathways, such as pseudohyphal growth and sporulation, in response to nutrient availability. The transcriptional factor Ime1 is the upstream activator of a transcriptional cascade of sporulation-specific genes involved in different steps of meiosis and spore formation (7, 22, 33, 35). Ime1 only induces the meiotic program in a narrow range of genetic and nutritional conditions: i.e., diploidy, starvation of an essential nutrient, and the presence of nonfermentable carbon sources (20). Nitrogen starvation and acetate as the sole carbon source are the most efficient and prevalent nutritional conditions to induce meiosis in Saccharomyces cerevisiae (12). The IME1 gene shows a complex pattern of transcriptional regulation (see references 18 and 47 for review). The IME1 transcript is undetectable when glucose is present in the medium, but IME1 expression is induced at low levels in complete medium with acetate, which is further increased to maximal levels by nitrogen starvation (17, 20, 41, 43). However, because ectopic expression at high levels of Ime1 is not sufficient for meiosis and spore formation in the presence of glucose and nitrogen (20, 24, 44), other mechanisms must exist to control Ime1 function by nutrients.
Ime1 is a nuclear protein under sporulation conditions (45) and is regulated by a negative feedback loop mechanism that restricts its synthesis to a transient period during initiation of meiosis (14). The activation of meiotic expression requires the interaction of Ime1 with the DNA binding protein Ume6, and this interaction is prevented by glucose and stimulated by nitrogen starvation (40, 48). The GSK3β homologous kinases Rim11 and Mck1 phosphorylate Ume6 in response to nitrogen limitation (49). Rim11 also phosphorylates Ime1, and phosphorylation of both Ime1 and Ume6 is required for formation of an active transcriptional complex (5, 30, 31). Thus, besides transcriptional regulation, nitrogen starvation and glucose regulate Ime1 function by controlling the Ime1-Ume6 interaction.
G1 cyclins negatively regulate the initiation of meiosis by downregulating IME1 expression (8, 36). Furthermore, Cln-Cdc28 activity prevents the accumulation of Ime1 in the nucleus of mitotic cells, and ectopic expression of IME1 in cells depleted of G1 cyclins is sufficient to promote meiosis and sporulation in rich medium (8). G1 cyclins are rapidly downregulated in yeast cells deprived of nitrogen (8, 13, 29). Because depletion of G1 cyclins mimics nitrogen starvation, we proposed that G1 cyclins would transmit nutritional signals to Ime1 function (8).
The TOR pathway is involved in cellular responses to the changes in nitrogen and carbon sources (9, 23, 37, 38). There are two functionally distinct TOR complexes in yeast. One of them, TOR complex 1 (TORC1), is the target of the immunosupressor rapamycin (25). In response to nutrient availability, TORC1 regulates a broad spectrum of cellular responses, and among them, the subcellular localization of several transcriptional activators (2, 19, 21). The inactivation of TORC1 with rapamycin allows cells to enter a meiotic cycle and sporulate in rich medium (50). However, transcriptome changes induced by rapamycin (15) differ from those obtained during entry into meiosis (7). Thus, it is still uncertain whether the TOR pathway has a direct effect on the initiation of meiosis. To gain insight in the regulatory mechanisms induced by nitrogen starvation, we have analyzed the effects of high levels of rich nitrogen sources on the regulation of Ime1. Here we report that TOR and ammonium availability regulate the initiation of meiosis by controlling the subcellular localization of Ime1. These mechanisms would act independently of G1 cyclins increasing the competence of yeast cells to prevent the initiation of meiosis under low-proliferation or intermediate-nutrient-limiting situations.
MATERIALS AND METHODS
Yeast strains and plasmids.
Our parental diploid strain 1788 and haploid strain CML128 (MATa leu3-2,112 ura3-52 trp1-1 his4 canIr) have been described by Gallego et al. (13). The derivative strains CML268 (MATa/MATα Δime1::KanMX4/Δime1::KanMX4), CML200 (MATa cdc28-13), and CML344 (MATa cdc34-2) have been described previously (8). The parental strain JK9-3da (MATa leu2-3,112 ura3-52 trp1 his4 rme1) and the rapamycin-resistant mutant strain JH11-1c (JK9-3da TOR1-1)—from M. N. Hall laboratory—have been described by Heitman et al. (16).
All plasmids used in this work are derivatives from pCM279 (adhp-IME1-3xHA), which contains the three-hemagglutinin (3HA)-tagged IME1 open reading frame (ORF) expressed under the control of the Schizosaccharomyces pombe adh promoter in the YCPlac22 (CEN TRP1) vector (8). Two nuclear localization signals (NLS) from simian virus 40 (SV40) were fused to the 3HA-tagged IME1 ORF, obtaining pCM388 (adhp-IME1-2xNLS-3xHA). In pCYC129 (GAL1-IME1-3xHA) the adh1 promoter was substituted for by the GAL1 promoter. Details on plasmid construction are available upon request.
Growth and sporulation conditions.
The media and sporulation conditions were as described in reference 8 with only slight modifications. Sporulation medium (0.3% potassium acetate) was modified by lowering the initial pH of the medium to 6.0 with 5 mM 2-(N-morpholino)ethanesulfonic acid (MES). Also, the acetate-based rich medium YPA was modified by addition of 60 mM MES to obtain an initial pH of 5.8. Briefly, cells grown on minimal media with 2% glucose (SD) for 24 h to an optical density at 600 nm (OD600) of 5 were washed, resuspended in YPA at an OD600 of 0.3, and incubated at 30°C for 20 h with vigorous agitation to reach an OD600 of 6 to 7. To initiate meiosis, cells were then washed, resuspended in sporulation medium at an OD600 of 1, and incubated at 30°C. To obtain samples from cells growing exponentially in YPA medium, cells grown in SD as described above were washed, resuspended at an OD600 of 0.03 in YPA, and incubated for 16 to 20 h at 30°C. Ammonium sulfate, glutamine, and proline were added at a final concentration of 40 mM unless otherwise indicated. l-Methionine-sulfoximine from Sigma was used at a final concentration of 2 mM. Rapamycin from Sigma was added at a final concentration of 200 ng/ml (200 nM).
Immunofluorescence.
The intracellular localization of the 3HA-tagged proteins was determined by indirect immunofluorescence techniques essentially as described by Rose et al. (39). The rat anti-HA antibody (clone 3F10) was used at 1 μg/ml, and Alexa 488 goat anti-rat antibody from Molecular Probes was used at 10 μg/ml. Nuclei were visualized by staining the DNA with 4′,6-diamidino-2-phenylindole (DAPI) at 1 μg/ml. Images were obtained with a Nikon fluorescence microscope and LSR Ultra software.
Flow cytometry and morphological determinations.
Distributions of DNA content were obtained by propidium iodide staining as described by Nash et al. (34) with an Epics XL cytometer (Coulter). Budding and sporulation percentages were obtained under a phase-contrast microscope by inspecting a minimum of 200 cells that had been fixed with 1% formaldehyde in 1× SSC (1× SSC is 0.15 M NaCl plus 0.0015 M sodium citrate) and sonicated for 5 s.
Northern blotting.
Total RNA samples were analyzed by Northern blotting as described previously (13). DNA fragments containing only ORF sequences were used to synthesize probes by random PCR with a digoxigenin-dUTP labeling mixture as directed by Roche. RNA samples were blotted and probed in the same membrane for accurate comparison.
Western blot and pulse-chase analyses.
Whole-cell extracts and Western blot analysis with the mouse anti-HA antibody (clone 12CA5) were performed as described by Gallego et al. (13). To evaluate the total Ime1 protein decay, cells harboring pCYC129 (GAL1-IME1-3xHA) were grown exponentially on raffinose-based rich medium (YPRaf), and then 2% galactose (YPRafhal) was added to induce the GAL1 promoter. Two hours later, cells were filtered and resuspended in YPA or YPA with 200 ng of rapamycin per ml or YPA with 200 ng of rapamycin per ml and 40 mM ammonium sulfate. Samples were harvested and immediately boiled for 2 min at the indicated time points before protein extraction.
The Ime1 degradation rate was measured by pulse-chase analysis as previously described (13). 1788(pCM279) cells growing exponentially in YPA were transferred to sporulation medium with or without ammonium sulfate. Two hours after transfer, 60 ml of culture was added to 3 mCi of Tran35S-label (ICN), and this mixture was incubated for 15 min at 30°C. Samples of 10 ml were taken at the indicated points after addition of unlabeled methionine and cysteine to a final concentration of 30 μM. Cells were then rapidly filtered, washed in cold water, and quickly frozen in liquid nitrogen. Cell pellets were processed for double immunoprecipitation in BC buffer (250 mM NaCl, 50 mM Tris-HCl [pH7.5], 5 mM EDTA, 0.1% Triton X-100, protease, phosphatase inhibitors) with rat anti-HA antibody (clone 3F10) from Boehringer Manheim, essentially as described by Blondel and Mann (4). Immunoprecipitated samples were split and loaded onto two sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels for Western blot analysis or radioactivity detection in a BAS-1000 phosphorimager (Fuji).
RESULTS
Nitrogen availability regulates the subcellular localization of Ime1.
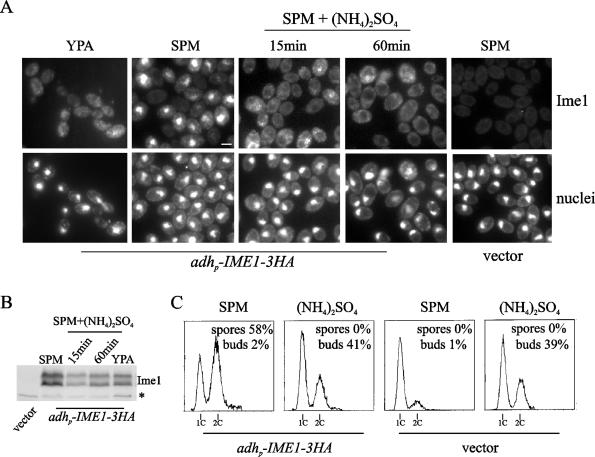
Yeast cells incubated in sporulation medium show Ime1 localized in the nucleus, as expected for a transcriptional activator (45). However, Ime1 is localized in the cytoplasm of cells growing in acetate-based rich medium and accumulates within the nucleus after G1 cyclin depletion (8). In order to characterize further the regulation of Ime1 localization exerted by nutritional signals, we determined the effects of nitrogen refeeding of cells growing in sporulation medium. A 3HA-tagged IME1 gene was constitutively expressed from the S. pombe adh promoter in an ime1-null mutant. Cultures growing in acetate-based rich medium were transferred to sporulation medium and incubated at 30°C to allow initiation of meiosis. One hour after transfer, ammonium sulfate was added to the culture at a final concentration of 40 mM, and samples were taken at different time points. Figure 1A shows that Ime1 was rapidly relocalized to the cytoplasm after addition of ammonia, whereas the untreated culture showed Ime1 accumulated in the nucleus. The same result was observed at concentrations of ammonia as low as 5 mM (data not shown). Ime1 is a phosphoprotein that shows a characteristic electrophoretic mobility pattern with two major bands: a high-mobility form and a low-mobility form (5, 14, 45) (Fig. 1B). These modified forms of Ime1 are mainly due to differences in the phosphorylation level (our unpublished results). While the relative levels of these phosphorylated forms remained almost unaffected, the overall Ime1 protein levels showed a transient decrease after ammonia addition (Fig. 1B). Nonetheless, the presence of ammonia in the sporulation medium did not significantly change the half-life of Ime1 (see Fig. 5A and B). Figure 1C shows that IME1-expressing untreated cells normally proceeded into a premeiotic S phase—with no signs of budding—and finally sporulated with high efficiency. However, after the addition of ammonia, these cells arrested the meiotic cycle and resumed proliferation by mitosis as deduced from budding indices (Fig. 1C) and increases in cell number (data not shown). These results would indicate that ammonium refeeding inhibits meiosis initiation, at least in part by relocalizing Ime1 to the cytoplasm.
FIG. 1.
Ammonium refeeding prevents Ime1 accumulation within the nucleus. (A) Diploid CML268 (Δime1/Δime1) cells transformed with either pCM279 (adhp-IME1-3HA) or YCplac22 (vector) were transferred to sporulation medium and incubated at 30°C. One sample was removed 1 h after transfer to sporulation medium (SPM). Then ammonium sulfate ([NH4]2SO4) was added to the cell culture, and samples were taken at the indicated time points. Ime1-3HA was visualized by immunofluorescence (Ime1). Logarithmic-phase cells growing in acetate-based rich medium (YPA) are shown as a control of the localization of Ime1 in the cytoplasm. Untreated cells harboring the vector are shown for background comparison. DNA was stained with DAPI (nuclei). Bar, 5 μm. (B) Ime1 levels were determined by Western blotting in samples taken as described for panel A. A 12CA5 cross-reactive band (*) serves as a control for loading. (C) DNA content distributions from cultures described for panel A. Samples for analyzing DNA content were collected after 6 h under sporulation conditions. Sporulation and budding indices obtained at 24 h are indicated.
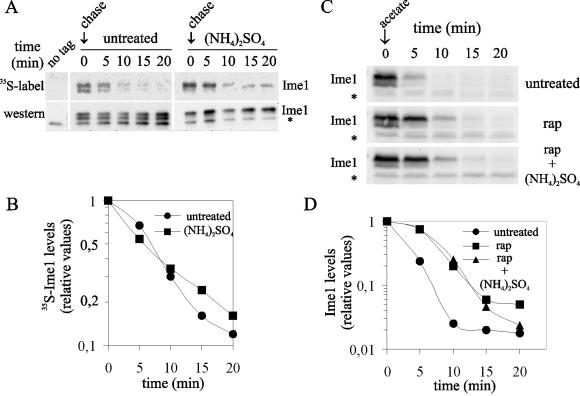
FIG. 5.
The TOR pathway regulates the half-life of Ime1. (A) Pulse-chase analysis of the Ime1-3HA protein (Ime1). 1788(pCM279) cells (adhp-IME1-3xHA) were transferred to sporulation medium with ([NH4]2SO4) or without (untreated) ammonium sulfate. Two hours after transfer to sporulation medium, a pulse-chase analysis was done as described in Materials and Methods. 1788(YCPlac22) cells were labeled and used as a negative control for immunoprecipitation (no tag). A nonspecific band that cross-reacts with the 12CA5 antiboby is indicated by an asterisk. (B) Quantification of 35S-Ime1 radioactivity relative to the total amount of Ime1 for each sample as shown in panel A. (C) Western blot analysis of Ime1-3HA (Ime1) total protein decay. CML268 cells transformed with pCYC129 (GAL1p-IME1-3xHA) growing in YPRafGal were transferred to YPA alone (untreated), YPA with rapamycin (200 ng/ml [rap]), or YPA with rapamycin (200 ng/ml) and ammonium sulfate (rap+[NH4]2SO4). Samples were taken at the indicated time points. A nonspecific band that cross-reacts with the 12CA5 antibody is indicated by an asterisk. (D) Quantification of the Ime1 protein detected in panel C. Values are relative to time zero.
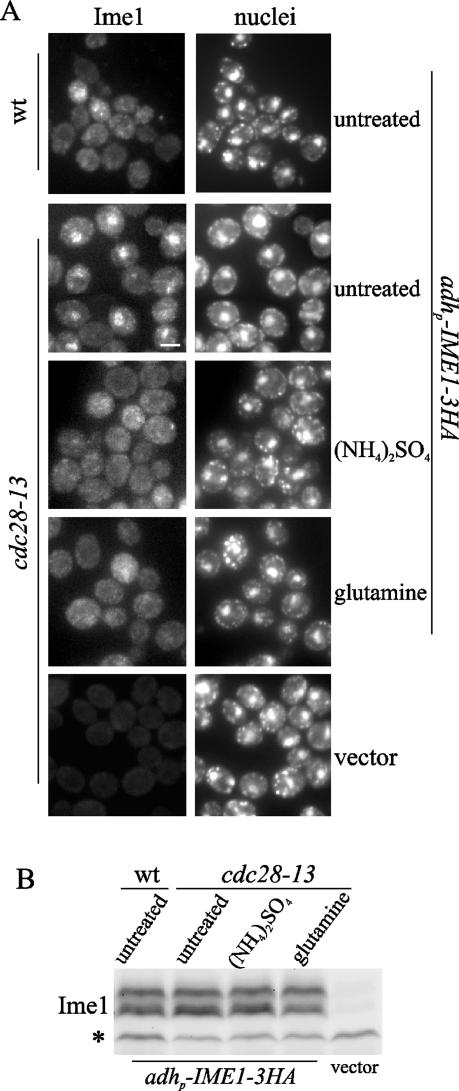
We reasoned that the transient induction of mitotic proliferation could account for release of the Ime1 nuclear signal. To elucidate the importance of G1 cyclins in the ammonium response, we depleted cells of Cln-Cdc28 kinase activity by using a cdc28-13 thermosensitive mutant. We had shown that at restrictive temperature, Ime1 accumulates in the nuclei of cdc28-13 cells and early meiotic genes as SPO13 is expressed (8). cdc28-13 cells transformed with the adhp-IME1-3HA construct were grown in acetate-based rich medium at 25°C. Exponentially growing cells were shifted to 37°C, and ammonium sulfate was added to the cultures 4 h later. Figure 2A shows that ammonia exerted a rapid effect and Ime1 relocalized to the cytoplasm in 15 min. These data strongly suggest the existence of a predominant pathway sensing the presence of ammonia and controlling Ime1 localization independently of G1 cyclins.
FIG. 2.
Relocalization of Ime1 to the cytoplasm after addition of ammonia or glutamine does not require Cdk1 activity. (A) Haploid cdc28-thermosensitive CML200 (cdc28-13) cells transformed with pCM279 (adhp-IME1-3HA) were exponentially grown in acetate-based rich medium (YPA) at 25°C and transferred to 37°C. A sample was taken 4 h after transfer to 37°C (untreated). Then the culture was divided into two halves. The aliquots were maintained at 37°C, and ammonium sulfate ([NH4]2SO4) or glutamine was added to the medium. Samples were removed 15 min after the addition of the nitrogen source. Ime1-3HA was visualized by immunofluorescence (Ime1). Haploid wild-type (wt) cells transformed with pCM279 (adhp-IME1-3HA) growing in YPA for 4 h at 37°C are shown as a control of the localization of Ime1 in the cytoplasm. Untreated CML200 cells harboring the YCplac22 (vector) are shown for background comparison. DNA was stained with DAPI (nuclei). Bar, 5 μm. (B) Ime1 levels were determined by Western blotting in samples taken as described for panel A. A 12CA5 cross-reactive band (*) serves as a control for loading.
Ammonium and glutamine have unrelated effects on Ime1 localization.
To characterize the specificity of the ammonium signal in the regulation of Ime1, we determined the sporulation efficiency after the addition of different nitrogen sources to the sporulation medium (Table 1). Ammonia had the strongest effect and was able to inhibit sporulation at final concentrations as low as 2 mM. Poor nitrogen sources such as proline and urea did not have any effect at 40 mM, but a rich nitrogen source such as glutamine showed complete inhibition of sporulation at this concentration. Figure 3A shows that addition of glutamine also caused relocalization of Ime1 to the cytoplasm, whereas Ime1 remained nuclear after proline addition. Moreover, the effect of glutamine was independent of G1 cyclins, as it was for ammonia addition (Fig. 2A) (described above). The expression of two early meiotic genes, SPO13 and IME2, was repressed after addition of ammonia or glutamine to the sporulation medium (data not shown), which correlates with the relocalization of Ime1 to the cytoplasm.
TABLE 1.
Sporulation efficiency in different nitrogen sources
| Nitrogen source or condition | % Sporulationa |
|---|---|
| Starvation | 78.8 |
| Ammonium sulfate | |
| 40 mM | <0.5 |
| 10 mM | <0.5 |
| 5 mM | <0.5 |
| 2 mM | <0.5 |
| Glutamine | |
| 40 mM | <0.5 |
| 10 mM | 11.7 |
| 5 mM | 41.0 |
| Proline 40 mM | 75.1 |
| Urea 40 mM | 64.2 |
Sporulation was determined after incubation of 1788 cells for 24 h in sporulation medium at 30°C with the different nitrogen sources. Values are the means of triplicate determinations. Standard deviations were less than 15% of the mean.
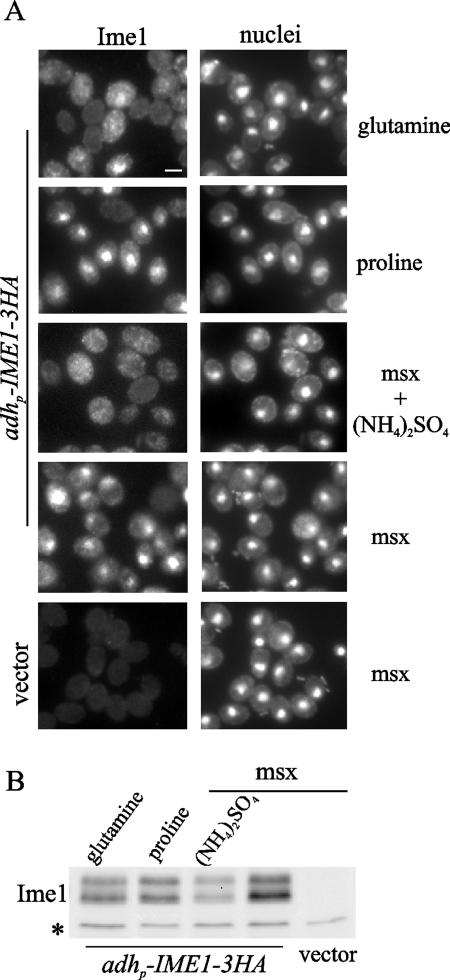
FIG. 3.
Glutamine and ammonia cause relocalization of Ime1 in the cytoplasm by different mechanisms. (A) CML268 (Δime1/Δime1) cells transformed with pCM279 (adhp-IME1-3HA) were transferred to sporulation medium and incubated at 30°C. One hour later, the cell culture was divided into three aliquots. Glutamine or proline was added to two of those aliquots, and samples were collected after 30 min of additional incubation. A third aliquot was pretreated with 2 mM l-methionine-sulfoximine for 20 min to inhibit the glutamine synthetase, and a sample was removed after pretreatment (msx). Then ammonium sulfate was added to the third aliquot, and a sample was taken 30 min later (msx + [NH4]2SO4). CML268 cells transformed with YCplac22 (vector) and treated with 2 mM l-methionine-sulfoximine for 20 min are shown for background comparison. Ime1-3HA was localized by immunofluorescence (Ime1), and nuclei were counterstained with DAPI (nuclei). Bar, 5 μm. (B) Ime1 levels were determined by Western blotting in samples taken as described for panel A. A 12CA5 cross-reactive band (*) serves as a control for loading.
Glutamine is a key intermediate in ammonia utilization (28). Thus, the ammonium signal could be transmitted through the synthesis of glutamine. To test this possibility, we observed the localization of Ime1 in sporulation medium after addition of ammonia in the presence of the glutamine synthetase inhibitor l-methionine-sulfoxamine (10). As shown in Fig. 3A, relocalization of Ime1 to the cytoplasm was not prevented by the presence of the inhibitor. This result suggests that (i) the ammonium signal acts independently of glutamine synthesis and (ii) there is an additional signal originated from glutamine that is transmitted to Ime1 function.
An ammonium signaling pathway has been described controlling pseudohyphal differentiation (27). In this pathway, ammonium availability would be sensed by the ammonium permease Mep2 (32) and transmitted through the α subunit of the guanine nucleotide binding protein Gpa2 (26). We have found that homozygous null mep2 and gpa2 mutant cells were similar to the wild-type cells with regard to the nuclear localization of Ime1 under sporulation conditions and relocalization to the cytoplasm after ammonia addition (data not shown).
TOR regulates the localization of Ime1.
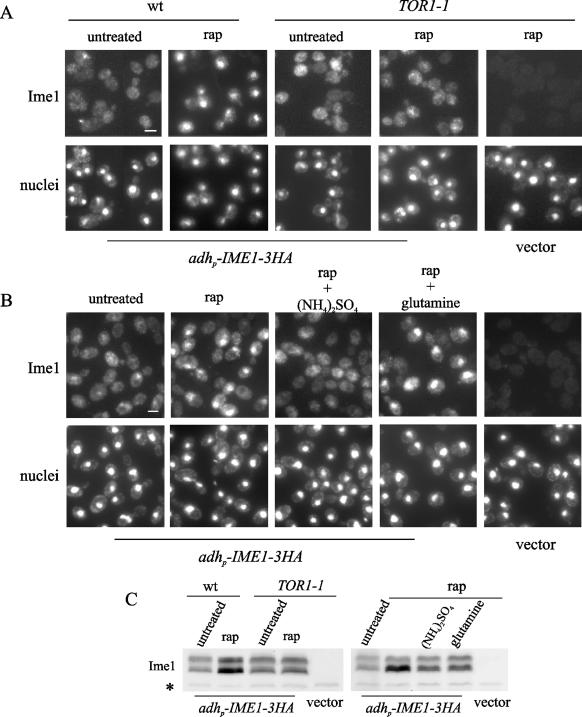
Changes in the intracellular levels of glutamine can be sensed by the TOR pathway (10). Therefore, we considered the possibility that the effect of glutamine on Ime1 localization could be mediated by TOR. First, to check the involvement of the TOR pathway in the regulation of Ime1 localization, we examined the nuclear accumulation of Ime1 after inactivation of TORC1 with rapamycin. Wild-type and rapamycin-resistant TOR1-1 cells transformed with adhp-IME1-3HA were grown in acetate-based rich medium at 30°C and treated with rapamycin. As shown in Fig. 4A, Ime1 was accumulated in the nucleus 15 min after the addition of rapamycin in the wild type, but not in the TOR1-1 rapamycin-resistant cells. These data indicate that the TOR pathway is involved in the regulation of Ime1 function. Rapamycin treatment also produced an increase in Ime1 protein levels only in wild-type cells (Fig. 4C) (described below). Intriguingly, an enrichment of the high-mobility form of Ime1 was usually observed after treatment with rapamycin. TOR modulates the phosphorylation state of different transcriptional activators by controlling the activity of several kinases and type 2 protein phosphatases, such as Sit4 (19). We have observed that in null sit4 mutant cells, the localization and mobility of Ime1 after addition of rapamycin were similar to those in wild-type cells (data not shown), and therefore Sit4 would seem to be unrelated to the control of Ime1 by TOR. Nevertheless, we cannot discard the idea that changes in the phosphorylation state of Ime1 could be associated with the control by TOR.
FIG. 4.
The TOR pathway regulates the nuclear localization of Ime1. (A) Haploid JK9-3da (wild type [wt]) and JH11-1c (TOR1-1) cells harboring pCM279 (adhp-IME1-3xHA) were grown exponentially in YPA at 30°C. One part of the culture was treated with rapamycin (200 ng/ml [rap]), and the rest remained without rapamycin (untreated). Samples were removed 15 min after rapamycin addition. TOR1-1 cells harboring YCplac22 (vector) and treated with rapamycin (200 ng/ml [rap]) for 15 min are shown for background comparison. Ime1-3HA was localized by immunofluorescence (Ime1), and nuclei were counterstained with DAPI (nuclei). Bar, 5 μm. (B) CML268 (Δime1/Δime1) cells with pCM279 (adhp-IME1-3xHA) were grown exponentially in YPA at 30°C. The culture was pretreated for 15 min with rapamycin (200 ng/ml [rap]). One part of the culture remained without rapamycin (untreated). Samples were taken after the pretreatment, and then the cell culture was divided in two aliquots. Ammonium sulfate [(NH4)2SO4] or glutamine was added to the cultures, and samples were taken 30 min later. Ime1-3HA was visualized by immunofluorescence (Ime1). A sample of CML268 cells with YCplac22 (vector) growing exponentially in YPA at 30°C was taken for background comparison. DNA was stained with DAPI (nuclei). Bar, 5 μm. (C) Ime1 levels were determined by Western blotting in samples taken as described for panels A and B. A 12CA5 cross-reactive band (*) serves as a control for loading.
In a second experiment, diploid null ime1 cells transformed with adhp-IME1-3HA were exponentially grown in rich medium with acetate as a carbon source. These cells were pretreated with rapamycin for 15 min, and then either ammonium sulfate or glutamine was added to the culture. Rapamycin-inactivated cells were still able to relocalize Ime1 to the cytoplasm in response to the addition of ammonia but not glutamine (Fig. 4B). The same result was observed with cells under sporulation conditions (data not shown). These data confirm that ammonia and glutamine use different mechanisms and suggest that the glutamine signal would be transmitted to Ime1 through the TOR pathway.
The TOR pathway modulates the half-life of Ime1.
Treatment of wild-type cells with rapamycin produced an increase in the overall levels of Ime1 protein (Fig. 4C). This result made likely the participation of the TOR pathway in the control of Ime1 stability. First, we determined whether Ime1 was an unstable protein. Diploid null ime1 cells transformed with adhp-IME1-3HA were incubated for 2 h at 30°C in sporulation medium with or without ammonia. Then the half-life of the Ime1 protein was determined by pulse-chase analysis (see Materials and Methods). Ime1 presented a half-life shorter than 5 min, and the rate of protein decay was not affected by the presence of ammonia (Fig. 5A and B). Second, we analyzed the stability of Ime1 after rapamycin addition. Homozygous Δime1 cells harboring GAL1p-IME1-3HA were induced in galactose-based rich medium and then transferred to acetate medium and treated with rapamycin. Figure 5C and D show that Ime1 was stabilized after rapamycin addition, and the half-life of the protein increased more than twice with respect to that of the untreated culture. Thus, the TOR pathway may positively regulate the turnover of the Ime1 protein. The half-life under untreated conditions was comparable to that obtained by pulse-chase analysis. Remarkably, the stabilization of the Ime1 protein by inactivation of the TOR pathway was observed even in the presence of ammonia (Fig. 5C and D).
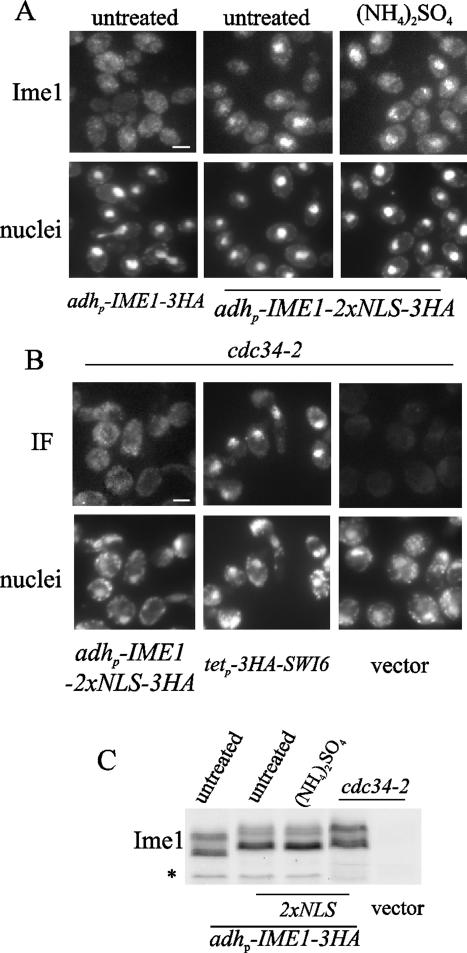
Nuclear accumulation of Ime1 is regulated via different mechanisms.
The SV40 NLS is able to direct the nuclear import of many heterologous proteins (46). Two SV40 NLS were fused to IME1, and the resulting construct (IME1-2xNLS) was expressed under the control of the adh promoter (Fig. 6C). Diploid null ime1 cells harboring adhp-IME1-2xNLS were exponentially grown in acetate-based rich medium at 30°C. As shown in Fig. 6A, the presence of two copies of SV40 NLS caused Ime1 to accumulate in the nucleus under these conditions. Furthermore, Ime1-2xNLS remained nuclear after ammonia addition. However, only a few cells showed nuclear signal after glutamine addition in the same experiment (data not shown). In a second experiment, we subjected Ime1-2xNLS to high levels of G1 cyclins. In haploid cdc34-2 cells at restrictive temperature, high levels of Cln/Cdc28 kinase activity are reached and Ime1 is found in the cytoplasm (8). cdc34-2 cells transformed with adhp-IME1-2xNLS were grown at 25°C in acetate-based rich medium and shifted to 37°C during 4 h. As shown in Fig. 6B, Ime1-2xNLS remained cytoplasmic in the cdc34-2 mutant at restrictive temperature. All of these data support the notion that distinct signals may regulate the accumulation of Ime1 in the nucleus through different mechanisms.
FIG. 6.
Ammonium, TOR, and G1 cyclins regulate nuclear accumulation of Ime1 by different mechanisms. (A) CML268 cells transformed with pCM279 (adhp-IME1-3xHA) or pCM388 (adhp-IME1-2xNLS-3xHA) were grown in YPA at 30°C, and samples were taken from exponential cultures (untreated). Then ammonium sulfate ([NH4]2SO4) was added to the CML268 cells harboring pCM388, and samples were taken 30 min after additional incubation. (B) Haploid cdc34-thermosensitive CML344 (cdc34-2) cells transformed with pCM388 or YCplac22 (vector) were grown in YPA at 25°C and then shifted to 37°C. Samples were taken 4 h after the shift to the restrictive temperature. Under the same growth conditions, cdc34-2 cells expressing the transcriptional factor Swi6 (tetp-3HA-SWI6) were used as an independent control of nuclear localization. Ime1-3HA and 3HA-Swi6 were visualized by immunofluorescence (indicated by Ime1 or IF), and DNA was stained with DAPI (nuclei). Bar, 5 μm. (C) Ime1 levels were determined by Western blotting in samples obtained as described for panels A and B.
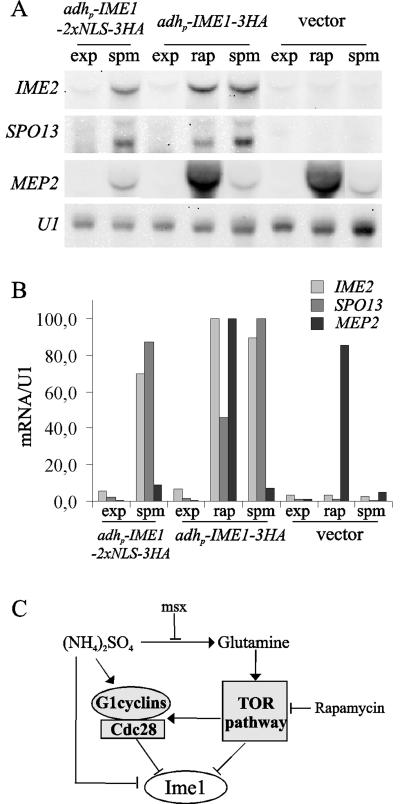
Loss of TOR function promotes activation of Ime1-dependent transcription.
The preceding results show that Ime1 needs to interact with the DNA binding protein Ume6 to induce meiotic expression, and this interaction is regulated by the nutritional status of the cell (40, 49). Then the nuclear barrier could be a first control step, and additional mechanisms would modulate the function of Ime1 once in the nucleus. In order to confirm this possibility, we analyzed the induction of early meiotic genes under those conditions in which Ime1 accumulates in the nucleus. First, samples of the diploid null ime1 mutant transformed with adhp-IME1-2XNLS-3HA were obtained from logarithmic-phase cultures in acetate-based rich medium at 30°C. Even though Ime1-2xNLS was detected in the nucleus of cells growing in acetate-based rich medium (Fig. 6A), this was not sufficient to induce SPO13 and IME2 expression (Fig. 7A). As expected, the same cells incubated in sporulation medium were able to induce the SPO13 and IME2 transcripts (Fig. 7A). These data corroborate the existence of additional nutritional controls once Ime1 is localized in the nucleus. Second, diploid Δime1 cells harboring adhp-IME1-3HA or an empty vector were exponentially grown in acetate-based rich medium at 30°C and treated with rapamycin for 30 min. As shown in Fig. 7A, the addition of rapamycin caused an induction of SPO13 and IME2 expression similar to that of MEP2, a TOR-controlled gene (2, 6). The transcripts of SPO13 and IME2 were already detected 15 min after the addition of rapamycin and showed a maximum level of expression at 30 min (data not shown). However, the expression of IME2 showed a transitory induction similar to MEP2 (6), since the level of transcript was greatly reduced 2 h after the addition of rapamycin (data not shown). It is important to note that the rapid induction of SPO13 and IME2 was totally dependent on Ime1 (Fig. 7A). This finding is in agreement with the localization of Ime1 in the nuclei of TOR-inactivated cells in rich medium and strongly suggests that TOR is also involved in the nutritional control of the activation of Ime1-dependent transcription once Ime1 is localized in the nucleus.
FIG. 7.
(A) CML268 cells transformed with pCM279 (adhp-IME1-3xHA), pCM388 (adhp-IME1-2xNLS-3xHA), or YCPlac22 (vector) were grown in YPA at 30°C. Samples were taken from exponential-phase cultures (exp). Then one part of the cultures was treated with 200 ng of rapamycin per ml, and samples were taken 30 min later (rap). Another part was transferred to sporulation medium and incubated for 3 h before the samples were collected (spm). RNA levels for the meiotic early genes IME2 and SPO13 were determined by Northern blotting. The TOR-regulated gene MEP2 was used as a control of rapamycin treatment. snU1 mRNA serves as a loading control. (B) Relative gene expression of samples in panel A. The loading was normalized to the U1 signal, and relative gene expression was determined by setting the maximal signal for each transcript to 100. (C) Schematic diagram of different elements involved in the regulation of the localization of Ime1. See Discussion for details.
DISCUSSION
In a previous work, we described that G1 cyclins negatively regulate the initiation of meiosis by preventing the accumulation of Ime1 in the nucleus (8). Here we report the existence of additional pathways, unrelated to the role of G1 cyclins, that would control the localization of Ime1 in response to nitrogen signals. On the one hand, the sudden addition of rich nitrogen sources such as ammonia or glutamine induces a relocalization of Ime1 to the cytoplasm in Cdk1 activity-depleted cells. Also, the decrease of Ime1 nuclear signal in wild-type starved cells is very fast and takes place only 15 min after the nitrogen source addition, whereas G1 cyclin levels need longer times to recover after refeeding (our unpublished results). Then we conclude that ammonia and glutamine are able to originate a signal transmitted to Ime1 in parallel to the activation of the mitotic cycle. Considering that ammonia is metabolically incorporated mainly as glutamine (28), we reasoned that a single pathway could transmit both signals. By using a glutamine synthetase inhibitor, we show here that the effects due to ammonium addition are independent of glutamine formation. Therefore, ammonium and glutamine seem to trigger two separate signals.
As we show here, the inactivation of the TOR pathway with rapamycin blocks the response of cells to the sudden addition of glutamine but not ammonia. Thus, the glutamine signal would be transmitted to Ime1 through the TOR pathway, while there would be an uncharacterized pathway that would specifically transmit the ammonium signal. In agreement with this, it has been proposed that TOR is able to sense changes in the intracellular levels of glutamine, because transcriptional activators such as Gln3 or Rgt1/3 are translocated to the nucleus in a TOR-dependent manner after glutamine starvation (10). The nuclear accumulation of both Gln3 and Rgt1/3 is also induced by inactivation of the TOR pathway with rapamycin (2, 21). In accordance with these data, we also show that the addition of rapamycin induces the accumulation of Ime1 within the nucleus in wild-type cells growing in rich medium, but not in rapamycin-resistant TOR1-1 cells. Thus, Ime1 seems to behave like the TOR-controlled transcription activators. Furthermore, we report here that Ime1 fused to two SV40 NLS is able to counteract the effects caused by a sudden addition of ammonia but not glutamine (data not shown) or high levels of G1 cyclins. This finding emphasizes that ammonium, TOR, and G1 cyclins would control the localization of Ime1 through distinct mechanisms. A general mechanism to ensure the cytoplasmic localization of several TOR-controlled transcriptional activators is mediated by the interaction with specific cytoplasmic retention factors (2). One of these factors, Ure2, is involved in the cell response to changes in the quality of nitrogen sources (2, 6, 15). A null ure2 mutant accumulated Ime1 in the nucleus after rapamycin treatment like the wild-type strain (our unpublished results), suggesting that TOR may not involve Ure2 in the prevention of the nuclear accumulation of Ime1.
Previously, the TOR pathway has been described as controlling the turnover of several proteins involved in the initiation of translation and in the cell response to starvation (3, 42). We show here that TOR partially modulates the turnover of Ime1, because the inactivation of TOR with rapamycin causes an increase in the overall levels of the Ime1 protein in wild-type but not in TOR1-1 cells, and this increase is due to the increase in the half-life of Ime1. Thus, TOR would regulate Ime1 function by controlling both localization and turnover of the protein. Our results also indicate that the control of Ime1 turnover is specific for TOR and independent of the presence of ammonia. This finding agrees with the fact that (i) inactivation of TOR and (ii) nitrogen starvation do not cause equivalent responses at the transcriptome level (15).
The nuclear localization of Ime1 (Ime1-2xNLS) is not sufficient to turn on Ime1-dependent transcription. However, we report here that loss of TOR function promotes the induction of early meiotic genes in acetate-rich medium. This finding suggests that TOR could transmit nutritional signals to control additional steps of Ime1-dependent transcription, besides the localization of Ime1. Although our results would seem contradictory to the published data describing the transcriptome profile induced by rapamycin (15), there are two important methodological differences to be noted. First, we have grown the cells in acetate instead of glucose as the carbon source, and glucose may impose additional mechanisms on the regulation of Ime1-dependent transcription (18). Thus, our data would be consistent with the fact that rapamycin promotes sporulation in glucose-rich medium only in saturated cultures, when glucose becomes exhausted (50). Second, we have used the S. pombe adh promoter to release the expression of IME1 from nutritional controls. In wild-type cells expressing the endogenous IME1 gene under its own promoter, rapamycin did not induce SPO13 and IME2 expression (data not shown). Then an ectopic source of Ime1 would be required to observe the activation of Ime1-dependent transcription by rapamycin. Finally, in our model, only a few diploid cells transformed with adhp-IME1-3HA produced tetrads 2 days after incubation in acetate-based rich medium in the presence of rapamycin (data not shown). Additional work will be required to analyze the physiological relevance of TOR inactivation during the whole sporulation process.
We propose here that subcellular localization of Ime1 is specifically controlled by nutritional as well as cell cycle signals (Fig. 7C). Three independent mechanisms, TOR, ammonia, and G1 cyclins, act in parallel to prevent the accumulation of Ime1 in the nucleus. We cannot discard partial overlap among these three signaling processes, because ammonia and TOR are able to upregulate G1 cyclin levels (1). The TOR pathway has been proposed to involve glutamine to integrate both nitrogen and carbon metabolism (10), suggesting that TOR could play an important role in sensing the optimal combination of nitrogen- and carbon-limiting conditions for entry into meiosis or pseudohyphal differentiation. It is important to note that TOR would play opposite roles in regulating the distinct developmental pathways, because filamentous growth requires an active TOR pathway (11). Finally, signaling of Ime1 by independent mechanisms would facilitate cells to decide the suitable response to different nutritional situations. The meiotic program is an energetically expensive process, and diploid yeast cells only initiate meiosis under nutrient-limiting situations that fulfill two main requirements: (i) starvation or low quality of carbon and nitrogen sources and (ii) arrest of mitotic proliferation. When cells are faced with intermediate proliferating and/or nutrition-limiting situations, the regulation of Ime1 through independent nutritional and cell cycle mechanisms would help cells to prevent inappropriate entry into the meiotic program.
Acknowledgments
We thank C. Gallego, E. Vergés, and H. Wang for help in some experiments. We gratefully acknowledge J. Torres for helpful advice. We also thank Sònia Rius for technical assistance.
This work was funded by the Ministry of Science and Technology of Spain and FEDER to M.A. E.G. is a researcher of the Ramon y Cajal Program.
REFERENCES
- 1.Barbet, N. C., U. Schneider, S. B. Helliwell, I. Stansfield, M. F. Tuite, and M. N Hall. 1996. TOR controls translation initiation and early G1 progression in yeast. Mol. Biol. Cell 7:25-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck, T., and M. N. Hall. 1999. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402:689-692. [DOI] [PubMed] [Google Scholar]
- 3.Berset, C., H. Trachsel, and M. Altmann. 1998. The TOR (target of rapamycin) signal transduction pathway regulates the stability of translation initiation factor eIF4G in the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 95:4264-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blondel, M., and C. Mann. 1996. G2 cyclins are required for the degradation of G1 cyclins in yeast. Nature 384:279-282. [DOI] [PubMed] [Google Scholar]
- 5.Bowdish, K. S., H. E. Yuan, and A. P. Mitchell. 1994. Analysis of RIM11, a yeast protein kinase that phosphorylates the meiotic activator IME1. Mol. Cell. Biol. 14:7909-7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardenas, M. E., N. S. Cutler, M. C. Lorenz, C. J. Di Como, and J. Heitman. 1999. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 13:3271-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu, S., J. DeRisi, M. Eisen, J. Mulholland, D. Botstein, P. O. Brown, and I. Herskowitz. 1998. The transcriptional program of sporulation in budding yeast. Science 282:699-705. [DOI] [PubMed] [Google Scholar]
- 8.Colomina, N., E. Garí, C. Gallego, E. Herrero, and M. Aldea. 1999. G1 cyclins block the Ime1 pathway to make mitosis and meiosis incompatible in budding yeast. EMBO J. 18:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crespo, J. L., and M. N. Hall. 2002. Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 66:579-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crespo, J. L., T. Powers, B. Fowler, and M. N. Hall. 2002. The TOR-controlled transcription activators GLN3, RTG1, and RTG3 are regulated in response to intracellular levels of glutamine. Proc. Natl. Acad. Sci. USA 99:6784-6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutler, N. S., X. Pan, J. Heitman, and M. E. Cardenas. 2001. The TOR signal transduction cascade controls cellular differentiation in response to nutrients. Mol. Biol. Cell 12:4103-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freese, E. B., M. I. Chu, and E. Freese. 1982. Initiation of yeast sporulation of partial carbon, nitrogen, or phosphate deprivation. J. Bacteriol. 149:840-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallego, C., E. Garí, N. Colomina, E. Herrero, and M. Aldea. 1997. The Cln3 cyclin is down-regulated by translational repression and degradation during the G1 arrest caused by nitrogen deprivation in budding yeast. EMBO J. 16:7196-7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttmann-Raviv, N., S. Martin, and Y. Kassir. 2002. Ime2, a meiosis-specific kinase in yeast, is required for destabilization of its transcriptional activator, Ime1. Mol. Cell. Biol. 22:2047-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardwick, J. S., F. G. Kuruvilla, J. K. Tong, A. F. Shamji, and S. L. Schreiber. 1999. Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins. Proc. Natl. Acad. Sci. USA 96:14866-14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heitman, J., N. R. Movva, and M. N. Hall. 1991. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253:905-909. [DOI] [PubMed] [Google Scholar]
- 17.Honigberg, S. M., and R. H. Lee. 1998. Snf1 kinase connects nutritional pathways controlling meiosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:4548-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honigberg, S. M., and K. Purnapatre. 2003. Signal pathway integration in the switch from the mitotic cell cycle to meiosis in yeast. J. Cell Sci. 116:2137-2147. [DOI] [PubMed] [Google Scholar]
- 19.Jacinto, E., and M. N. Hall. 2003. Tor signalling in bugs, brain and brawn. Nat. Rev. Mol. Cell. Biol. 4:117-126. [DOI] [PubMed] [Google Scholar]
- 20.Kassir, Y., D. Granot, and G. Simchen. 1988. IME1, a positive regulator gene of meiosis in S. cerevisiae. Cell 52:853-862. [DOI] [PubMed] [Google Scholar]
- 21.Komeili, A., K. P. Wedaman, E. K. O'Shea, and T. Powers. 2000. Mechanism of metabolic control. Target of rapamycin signaling links nitrogen quality to the activity of the Rtg1 and Rtg3 transcription factors. J. Cell Biol. 151:863-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupiec, M., B. Byers, R. E. Esposito, and A. P. Mitchell. 1997. Meiosis and sporulation in Saccaromyces cerevisiae, p. 889-1036. In J. R. Pringle, J. R. Broach, and E. W. Jones (ed.), The molecular and cellular biology of the yeast Saccharomyces, vol. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Kuruvilla, F. G., A. F. Shamji, and S. L. Schreiber. 2001. Carbon- and nitrogen-quality signaling to translation are mediated by distinct GATA-type transcription factors. Proc. Natl. Acad. Sci. USA 98:7283-7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, R. H., and S. M. Honigberg. 1996. Nutritional regulation of late meiotic events in Saccharomyces cerevisiae through a pathway distinct from initiation. Mol. Cell. Biol. 16:3222-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loewith, R., E. Jacinto, S. Wullschleger, A. Lorberg, J. L. Crespo, D. Bonenfant, W. Oppliger, P. Jenoe, and M. N. Hall. 2002. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10:457-468. [DOI] [PubMed] [Google Scholar]
- 26.Lorenz, M. C., and J. Heitman. 1997. Yeast pseudohyphal growth is regulated by GPA2, a G protein alpha homolog. EMBO J. 16:7008-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenz, M. C., and J. Heitman. 1998. The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J. 17:1236-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magasanik, B. 1992. Regulation of nitrogen utilization, p. 283-317. In E. W. Jones J. R. Pringle, and J. R. Broach (ed.), The molecular and cellular biology of the yeast Saccharomyces, vol. 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Mai, B., and L. Breeden. 2000. CLN1 and its repression by Xbp1 are important for efficient sporulation in budding yeast. Mol. Cell. Biol. 20:478-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malathi, K., Y. Xiao, and A. P. Mitchell. 1997. Interaction of yeast repressor-activator protein Ume6p with glycogen synthase kinase 3 homolog Rim11p. Mol. Cell. Biol. 17:7230-7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malathi, K., Y. Xiao, and A. P. Mitchell. 1999. Catalytic roles of yeast GSK3beta/shaggy homolog Rim11p in meiotic activation. Genetics 153:1145-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marini, A.-M., S. Soussi-Boudekou, S. Vissers, and B. Andre. 1997. A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:4282-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell, A. P. 1994. Control of meiotic gene expression in Saccharomyces cerevisiae. Microbiol. Rev. 58:56-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nash, R., G. Tokiwa, S. Anand, K. Erickson, and A. B. Futcher. 1988. The WHI1+ gene of Saccharomyces cerevisiae tethers cell division to cell size and is a cyclin homolog. EMBO J. 7:4335-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Primig, M., R. M. Williams, E. A. Winzeler, G. G. Tevzadze, A. R. Conway, S. Y. Hwang, R. W. Davis, and R. E. Esposito. 2000. The core meiotic transcriptome in budding yeasts. Nat. Genet. 26:415-423. [DOI] [PubMed] [Google Scholar]
- 36.Purnapatre, K., S. Piccirillo, B. L. Schneider, and S. M. Honigberg. 2002. The CLN3/SWI6/CLN2 pathway and SNF1 act sequentially to regulate meiotic initiation in Saccharomyces cerevisiae. Genes Cells 7:675-691. [DOI] [PubMed] [Google Scholar]
- 37.Raught, B., A. C. Gingras, and N. Sonenberg. 2001. The target of rapamycin (TOR) proteins. Proc. Natl. Acad. Sci. USA 98:7037-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohde, J., J. Heitman, and M. E. Cardenas. 2001. The TOR kinases link nutrient sensing to cell growth. J. Biol. Chem. 276:9583-9586. [DOI] [PubMed] [Google Scholar]
- 39.Rose, M. D., F. Winston, and P. Hieter. 1990. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 40.Rubin-Bejerano, I., S. Mandel, K. Robzyk, and Y. Kassir. 1996. Induction of meiosis in Saccharomyces cerevisiae depends on conversion of the transcriptional represssor Ume6 to a positive regulator by its regulated association with the transcriptional activator Ime1. Mol. Cell. Biol. 16:2518-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sagee, S., A. Sherman, G. Shenhar, K. Robzyk, N. Ben-Doy, G. Simchen, and Y. Kassir. 1998. Multiple and distinct activation and repression sequences mediate the regulated transcription of IME1, a transcriptional activator of meiosis-specific genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:1985-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt, A., T. Beck, A. Koller, J. Kunz, and M. N. Hall. 1998. The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 17:6924-6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shenhar, G., and Y. Kassir. 2001. A positive regulator of mitosis, Sok2, functions as a negative regulator of meiosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:1603-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, H. E., S. S. Y. Su, L. Neigeborn, S. E. Driscoll, and A. P. Mitchell. 1990. Role of IME1 expression in regulation of meiosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:6103-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, H. E., S. E. Driscoll, R. A. Sia, H. E. Yuan, and A. P. Mitchell. 1993. Genetic evidence for transcriptional activation by the yeast IME1 gene product. Genetics 133:775-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stade, K., C. S. Ford, C. Guthrie, and K. Weis. 1997. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90:1041-1050. [DOI] [PubMed] [Google Scholar]
- 47.Vershon, A. K., and M. Pierce. 2000. Transcriptional regulation of meiosis in yeast. Curr. Opin. Cell Biol. 12:334-339. [DOI] [PubMed] [Google Scholar]
- 48.Vidan, S., and A. P. Mitchell. 1997. Stimulation of yeast meiotic gene expression by the glucose-repressible protein kinase Rim15p. Mol. Cell. Biol. 17:2688-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao, Y., and A. P. Mitchell. 2000. Shared roles of yeast glycogen synthase kinase 3 family members in nitrogen-responsive phosphorylation of meiotic regulator Ume6p. Mol. Cell. Biol. 20:5447-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng, X. F., and S. L. Schreiber. 1997. Target of rapamycin proteins and their kinase activities are required for meiosis. Proc. Natl. Acad. Sci. USA 94:3070-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]