Abstract
The serine/threonine kinase Akt is a component of many receptor signal transduction pathways and can prevent cell death following growth factor withdrawal. Here, we show that Akt inhibition of cell death is not dependent on new protein translation. Instead, Akt inhibition of cell death requires glucose hydrolysis through glycolysis. Akt was found to regulate multiple steps in glycolysis via posttranscriptional mechanisms that included localization of the glucose transporter, Glut1, to the cell surface and maintenance of hexokinase function in the absence of extrinsic factors. To test the role of glucose uptake and phosphorylation in growth factor-independent survival, cells were transfected with Glut1 and hexokinase 1 (Glut1/HK1) cells. Glut1/HK1 cells accumulated Glut1 on the cell surface and had high glucose uptake capacity similar to that of cells with constitutively active Akt (mAkt). Unlike mAkt-expressing cells, however, they did not consume more glucose, did not maintain prolonged phosphofructokinase-1 protein levels and activity, and did not maintain pentose phosphate shuttle activity in the absence of growth factor. Nevertheless, expression of Glut1 and HK1 promoted increased cytosolic NADH and NADPH levels relative to those of the control cells upon growth factor withdrawal, prevented activation of Bax, and promoted growth factor-independent survival. These data indicate that Bax conformation is sensitive to glucose metabolism and that maintaining glucose uptake and phosphorylation can promote cell survival in the absence of growth factor. Furthermore, Akt required glucose and the ability to perform glycolysis to prevent Bax activation. The prevention of Bax activation by posttranscriptional regulation of glucose metabolism may, therefore, be a required aspect of the ability of Akt to maintain long-term cell survival in the absence of growth factors.
It is critical that cell numbers are tightly regulated to maintain tissue homeostasis. One mechanism used to accomplish this is the dependence of cells on a limited supply of tissue-specific, cell-extrinsic signals (11). When cells fail to obtain sufficient extrinsic signals, they atrophy and eventually undergo apoptosis (13, 49, 53). Atrophy is characterized by decreases in cell size, protein content, cellular ATP, and the rate of glycolysis. In addition, atrophic cells display delayed activation and proliferation in response to mitogenic stimuli (49). Apoptosis is initiated when proapoptotic Bcl-2 family members, such as Bax, are activated by conformational change and localize to mitochondrial membranes, where they cause the loss of outer mitochondrial membrane integrity (21, 42). Once the outer membrane is breached, cytochrome c, apoptosis-inducing factor, and SMAC/DIABLO are released from the intermembrane space into the cytosol, where they can promote caspase activation and initiate apoptosis (41).
The induction of mitochondrial-mediated apoptosis requires the activation of proapoptotic Bcl-2 family members (38, 60, 68), but the events that lead to the activation of Bax and the loss of mitochondrial integrity are uncertain. One possibility is that Bax activation and mitochondrial dysfunction are the consequences of the decrease in glycolytic metabolism that occurs when cells are deprived of survival-promoting signal transduction pathways. Decreased glucose uptake upon growth factor withdrawal of factor-dependent cell lines may result in a lack of glycolytic substrates for mitochondrial metabolism, such as pyruvate and NADH. Ultimately, the lack of substrates for electron transport and maintenance of inner membrane potential could compromise the ability of mitochondria to properly regulate ion exchange and volume homeostasis (43) and result in the activation of Bax and the loss of mitochondrial integrity. The mechanisms by which extrinsic signals maintain mitochondrial homeostasis and how cancer cells overcome their dependence on extrinsic signals to allow tumor growth are largely unknown.
A key molecule involved in the signal transduction pathways of many cell-extrinsic signals is the proto-oncogene Akt, a serine and threonine kinase. Akt was found as the cellular homologue of v-akt, a viral oncogene isolated from a spontaneous thymoma of an AKR mouse (6, 10, 28). Constitutively active Akt can promote cell survival in the face of a variety of death stimuli, including withdrawal from growth factors (32). In addition, Akt has been shown to regulate aspects of cellular metabolism, such as insulin-responsive glucose transport (23). Upon ligation of the insulin receptor, Akt is recruited to the plasma membrane through interaction of its pleckstrin homology domain and the products of phosphatidylinositol 3-kinase (PI3-K). At the cell membrane, Akt is activated through phosphorylation by kinases, including the PI3-K-dependent kinase PDK1 (2, 52). Akt can be rendered constitutively active by targeting to the plasma membrane by myristoylation (mAkt), where it is phosphorylated by endogenous PI3-K-dependent kinases. When activated, Akt can phosphorylate target proteins that affect glucose uptake and metabolism. For example, insulin activates Akt to promote translocation of the glucose transporter Glut4 to the cell surface to allow glucose uptake in adipocytes (16, 24).
In hematopoietic cells, Akt activity has been shown to maintain cell survival even in the absence of cell-extrinsic survival signals. Unlike survival conferred by antiapoptotic Bcl-2 proteins, maintenance of cell survival by Akt requires the presence of glucose in the culture medium (20, 48). This could be because Akt may mediate its survival effects based on induction of new transcription and translation, such as that caused by Akt-mediated activation of the survival-promoting transcription factor NF-κB (29, 33, 34, 39, 50). In this case, the requirement of Akt for glucose in the medium to support survival may be solely to support macromolecular synthesis, because glucose acts as a source for production of amino acids, ribose sugars, and ATP. Alternatively, Akt may support survival of growth factor-deprived cells as a direct consequence of maintaining nutrient uptake and cellular metabolism. The latter hypothesis is supported by observations with both hematopoietic and neuronal systems that cellular metabolism normally decreases upon withdrawal from growth factor (12, 49, 61) but that Akt can prevent this decline (15, 48) and that Akt can promote short-term survival even in the presence of cycloheximide (CHX) (20).
To analyze the mechanism for long-term Akt-mediated survival of growth factor-withdrawn cells, we analyzed the metabolic and survival characteristics of mAkt-expressing cells and cells expressing early components of the glycolytic pathway. Here, we show that long-term Akt-mediated survival did not require transcription and translation of prosurvival proteins because mAkt expression could promote the survival of interleukin-3 (IL-3)-dependent FL5.12 cells in the absence of IL-3 even when new protein translation was inhibited. mAkt-expressing cells did, however, require a hydrolyzable glucose substrate to mediate IL-3-indepenent survival. Expression of mAkt caused increased glucose uptake and phosphorylation by promoting Glut1 localization to the cell surface, total cellular hexokinase activity, and pentose phosphate shuttle activity. To determine the role of glucose uptake and phosphorylation in the mAkt-mediated survival phenotype, FL5.12 cells were transfected with Glut1 and hexokinase 1 (HK1), individually and together. Unlike mAkt cells, Glut1/HK1 cells did not display elevated glucose consumption relative to that of control cells. The inability of Glut1/HK1 cells to promote a prolonged increase in glucose consumption correlated with a loss of phosphofructokinase 1 (PFK1) protein levels and inability to maintain pentose phosphate shuttle activity following growth factor withdrawal. Nevertheless, Glut1/HK1 cells maintained higher cellular NADH and NADPH [NAD(P)H] levels than did control cells. In addition, expression of Glut1 and HK1 was sufficient to prevent activation of Bax and to promote long-term growth factor-independent survival of some cells, while Akt required glucose and glycolysis to support cell survival in the absence of growth factor. These data demonstrate that Bax conformational change is sensitive to changes in glucose metabolism and indicate that Akt may prevent Bax activation and cell death by promoting glycolysis through multiple posttranscriptional mechanisms, including Glut1 surface localization, stimulation of hexokinase activity, and posttranscriptional regulation of PFK1 expression.
MATERIALS AND METHODS
Cells.
The IL-3-dependent hematopoietic cell line FL5.12 was transfected with rat Glut1 cDNA (a generous gift of M. Birnbaum, University of Pennsylvania, Philadelphia, Pa.) in pSFFV and HK1 in pcDNA3 (a generous gift of J. Wilson, Michigan State University, Lansing, Mich.) either alone or in combination. Control cells were transfected with empty pSFFV. Stable clones were identified after selection with Geneticin (GIBCO-BRL, Grand Island, N.Y.) and immunofluorescence labeling followed by flow cytometric analysis for Glut1 and HK1. Cells expressing mAkt have been previously described (48) or were generated by transfection of FL5.12 cells with mAkt in the pEF6 vector (Invitrogen, Carlsbad, Calif.). Experiments were conducted on three or more clones from each transfection, and results given are from one representative clone of each type. In some experiments, FL5.12 cells overexpressing Bcl-xL were used (clone Bcl-xL 4.1) (49). Cells were grown in RPMI medium with IL-3 as previously described (7, 25, 45). The medium was supplemented with IL-3, either by addition of conditioned medium from the IL-3-producing WEHI 3B cell line or by addition of 400 pg of recombinant murine IL-3/ml (BD-Pharmingen, San Diego, Calif.). Consistent results were obtained with both sources of IL-3. For inhibition of the PI3-K pathway or protein synthesis, cells were cultured in 10 mM LY249002 (LY) or 20 μg of CHX/ml, respectively. Inhibitors were added at the start of each culture and were replenished at day 2 of each experiment. Where glucose was withdrawn, cells were grown in glucose-free RPMI medium (GIBCO-BRL) supplemented with dialyzed fetal calf serum (GIBCO-BRL). Glucose (Sigma, St. Louis, Mo.) was added to some cultures to a final concentration of 10 mM. In other cultures, 2-deoxyglucose (2-DOG; Sigma) was added to a final concentration of 10 mM. Cell concentrations were determined with a Z2 particle counter (Coulter Corp., Miami, Fla.). Glucose and lactate concentrations were measured with diagnostic kits as described by the manufacturer (Sigma). Some cells were cultured in 20 μM iodoacetic acid (IAA; Sigma) in RPMI medium either with or without IL-3 or in the presence of 1 mM methyl-pyruvate (mPyruvate; Sigma).
Cell fractionation.
Mitochondria were isolated from FL5.12 cells growing in the presence or absence of IL-3 as previously described (19). Plasma membranes were prepared as previously described, with some modifications (31). Briefly, 15 × 106 cells growing in IL-3 or withdrawn from IL-3 for 12 h were washed in plasma membrane-coating buffer (PMCB; 20 mM 2-morpholinoethanesulfonic acid, 150 mM NaCl, and 280 mM sorbitol [pH 6.0]). The cells were resuspended in 1 ml of PMCB and added dropwise to 5 ml of PMCB containing 1% cationic colloidal silica (Ludox-CL; Sigma). The cells were then gently rotated for 20 min at room temperature. The cells were washed three times in 20 ml of PMCB, resuspended in 1 ml of PMCB, and added dropwise to 5 ml of PMCB containing 0.1% polyacrylic acid with a molecular weight of 30K (Sigma). The cells were gently rotated for 20 min at room temperature, washed three times in 20 ml of PMCB, and resuspended in 1 ml of lysis buffer (2.5 mM imidazole σ and 5 mM EDTA). After 30 min on ice, the cells were lysed by using a Dounce homogenizer and tight-fitting pestle. Plasma membranes were isolated by sedimentation through a 70% Nycodenz (Sigma) cushion by centrifugation at 28,000 × g for 30 min. The pellets containing plasma membranes were washed three times in lysis buffer and solubilized from silica by boiling for 5 min in lysis buffer containing 2% sodium dodecyl sulfate. The protein concentrations were then determined and were subjected to Western blotting.
Western blots and immunofluorescence.
Cells were lysed for Western blotting in 0.5% NP-40 NET (100 mM NaCl, 1 mM EDTA, and 10 mM Tris [pH 8] with protease inhibitors; BD-Pharmingen) and precleared by centrifugation. Protein concentrations were determined by bicinchoninic acid protein assay (Pierce, Rockford, Ill.), and 10 μg of protein was run on a sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis gel (Invitrogen). Glut1 was detected by using rabbit anti-Glut1 antisera (Research Diagnostics Inc., Flanders, N.J.), and PFK1 was detected by using goat anti-fructose-6-phosphate kinase (Research Diagnostics), followed by anti-rabbit horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, Calif.) or anti-goat horseradish peroxidase (Santa Cruz Biotechnology), respectively, and detected with ECL-Plus (Amersham Biosciences, Piscataway, N.J.). For immunofluorescence, the cells were fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min. The cells were then permeabilized in 0.03% saponin (Sigma) in PBS and stained in staining solution consisting of 0.3% saponin in PBS containing 10% normal rat serum and 10 μg of primary antibody/ml for 30 min. Glut1 was detected by using rabbit anti-Glut1 (Research Diagnostics, Inc.), and HK1 was detected by using monoclonal mouse immunoglobulin G1 (IgG1) monoclonal anti-HK1 (Chemicon, Temecula, Calif.). Cells unstained by primary antibody served as negative staining controls. After being washed in 0.03% saponin in PBS, the cells were stained with secondary antibody, goat anti-rabbit fluorescein isothiocyanate (FITC; BD-Pharmingen) to detect anti-Glut1 staining and rabbit anti-mouse IgG1-FITC (BD-Pharmingen) to detect anti-HK1 staining, in staining solution for 30 min. The cells were washed and resuspended in 2% fetal calf serum in PBS for flow cytometric analysis or were mounted on slides and analyzed microscopically at ×1,000 with a Nikon E800 microscope (Optical Apparatus, Ardmore, Pa.), Micromax digital camera (Princeton Instruments, Trenton, N.J.), and Metamorph 4.5 imaging software (Universal Imaging, Downington, Pa.).
Northern blots.
Total RNA was isolated by Trizol (GIBCO-BRL), and 10 μg was separated on a 1% agarose-formaldehyde gel. Nitrocellulose filters were probed with mouse PFK1 cDNA (IMAGE clone 533677; Reseach Genetics, Huntsville, Ala.). To demonstrate equivalent loading of total RNA in each lane, nitrocellulose filters were stripped and hybridized with an 18S RNA-specific probe (data not shown).
Flow cytometry.
Cells were analyzed by flow cytometry by using a FACScalibur cytometer (Becton Dickinson, Mountain View, Calif.) and Cell Quest software (Becton Dickinson). To determine cell viability, cells were stained with 10 μg of the vital dye propidium iodide (PI; Molecular Probes, Eugene, Oreg.) per ml. Analysis of changes in Bax conformation in response to IL-3 withdrawal was performed as described previously (40), with small modifications. Briefly, cells were fixed for 5 min with 0.25% paraformaldehyde in PBS and washed twice in PBS. For the primary stain, fixed cells were incubated with 10 μg of a purified mouse IgG1 monoclonal antibody specific for amino acids 12 to 24 of Bax (clone 6A7; BD-Pharmingen) or an isotype control monoclonal antibody (BD-Pharmingen) per ml in staining media containing 100 μg of digitonin (Sigma) per ml and 10% normal rat serum in PBS for 30 min at room temperature. Because FL5.12 cells express Fc receptors that could bind antibody nonspecifically, 5 μg of blocking anti-FcγIII/II (clone 2.4G2; BD-Pharmingen) per ml was added to each primary stain. Cells were washed in PBS with 100 μg of digitonin/ml and incubated with rat anti-mouse IgG1-FITC (clone A85-1; BD-Pharmingen) in digitonin staining medium for 30 min at 4°C. The stained cells were then washed in PBS containing 100 μg of digitonin/ml and stained for DNA content by incubation in PBS containing 100 mg of digitonin/ml, 50 μg of RNase A (Boehringer Mannheim) per ml, and 5 μg of PI/ml for 20 min prior to flow cytometric analysis. To avoid potential staining artifacts that may have occurred in fully apoptotic cells, subdiploid cells were excluded from analysis.
Biochemical assays.
Glucose uptake was determined by oil separation as previously described, with small modifications (61). Briefly, cells were washed one time in PBS and then cultured for 15 min in glucose uptake buffer (8.1 mM Na2HPO4, 1.4 mM KH2PO4, 2.6 mM KCl, 136 mM NaCl, 0.5 mM MgCl2, and 0.9 mM CaCl2 [final pH, 7.4]) at 37°C at a concentration of 5 × 106 cells/ml. Microcentrifuge tubes were prepared to measure glucose uptake by layering 25 μl of 8% sucrose-20% perchloric acid, 100 μl of bromo-dodecane, and 50 μl of glucose uptake buffer containing 1 μCi of [3H]2-DOG. Cells were added to the top layer of glucose uptake buffer with radiolabeled 2-DOG with 5 × 105 cells per tube to initiate the glucose uptake assay. After a 2-min incubation at room temperature, the microcentrifuge tubes were spun at maximum speed for 1 min. Radiolabeled 2-DOG taken up by cells in the 2-min incubation was carried through the bromo-dodecane layer into the lower sucrose-perchloric acid layer, while uninternalized [3H]2-DOG remained in the upper layer. The tubes were then frozen and cut in the center of the bromo-dodecane layer, and radioactivity in the lower layer was determined.
Hexokinase activity was determined as previously described (63). Briefly, a spectraphotometric assay was carried out in which glucose-6-phosphate formation was coupled to NADPH production. Cells (5 × 106) were collected and lysed in a 50 mM sodium phosphate-0.1% (vol/vol) Triton X-100 buffer. Alternatively, 10 μg of protein from purified mitochondria was used for hexokinase determination. Lysates and purified mitochondria were then added to a reaction mixture containing 3 mM glucose, NADP (5 mg/ml), glucose-6-phosphate dehydrogenase (100 U/ml), 220 mM ATP, and 1% monothiolglycerol. NADPH production was measured as the change in absorbance at 340 nm by using a SpectraMax 190 spectrophotometer (Molecular Devices, Sunnyvale, Calif.). Data are expressed as the change in absorbance at 340 nm/second/5 × 105 cells × 10−4.
PFK1 activity was measured as previously described (51). Briefly, cells were resuspended in 50 μl of PFK lysis buffer (50 mM HEPES [pH 7.0], 100 mM KF, and 15 mM EGTA [pH 7.4]), frozen in a dry ice-ethanol bath and thawed at 37°C. Lysates were centrifuged at 30,000 × g for 30 min at 4°C. Supernatants were then added to 950 μl of reaction buffer (50 mM HEPES [pH 7.0], 100 mM KCl, 5 mM MgCl2, 1.5 mM ATP, 0.15 mM NADH, 5 mM NaHPO4, 0.1 mM AMP, 1 mM NH4Cl, 5 U of triose phosphate isomerase/ml, 0.5 U of aldolase/ml, 0.5 U of α-glycerophosphate dehydrogenase/ml, and 5 mM fructose-6-phosphate; all obtained from Sigma). Absorbance at 340 nm was read at room temperature every 15 s for 30 min in a SpectraMax 190 spectrophotometer (Molecular Devices). Data are expressed as the change in absorbance at 340 nm/min/5 × 105 cells × 10−2.
To measure cytosolic NAD(P)H, 3 × 107 FL5.12 cells were cultured in the presence or absence of IL-3 for 12 h. The cells were pelleted and washed in Krebs buffer (115 mM NaCl, 2 mM KCl, 25 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, and 0.25% bovine serum albumin [BSA] [pH 7.4]) supplemented with 10 mM glucose. Cells were resuspended to a density of 107 cells/ml and placed in a cuvette in a Fluoromax spectrofluorimeter (Jovin Yvon, Inc., Edison, N.J.) equipped with a stirring apparatus. NAD(P)H fluorescence was monitored over time by exciting at 340 ± 5 nm and detecting emissions at 461 ± 5 nm. Carbonyl cyanide 4-trifluoromethoxyphenylhydrazone (FCCP; Sigma) and mPyruvate were added to cells by opening the chamber, pipetting the drugs into the cuvette, and mixing several times by pipetting up and down. Data points collected while the chamber was open were removed from the recordings. The final concentration of FCCP in the cuvette was 5 μM, and the final concentration of mPyruvate was 5 mM.
Pentose phosphate shuttle activity was determined by culturing 2 × 106 cells that had been grown in IL-3 or withdrawn from IL-3 for 12 h in glucose and sodium bicarbonate-free RPMI medium (Sigma) that had been supplemented with dialyzed fetal calf serum (GIBCO-BRL)-20 mM HEPES-5 mM glucose-0.2 μCi of d-1-14C-glucose or d-6-14C-glucose. The cells were placed in closed vials with raised-center wells containing a filter paper soaked in 100 μl of 5% KOH and incubated at 37°C. After 4 h, the filter papers were removed and their radioactivity was determined. Pentose phosphate shuttle activity was calculated by subtracting the radioactivity levels of samples incubated with d-6-14C-glucose from those incubated with d-1-14C-glucose.
RESULTS
New protein translation is not required for Akt-mediated survival of growth factor-deprived cells.
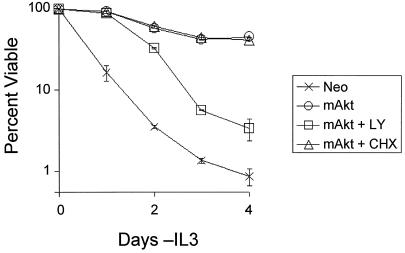
mAkt protects factor-dependent cells from growth factor withdrawal-induced apoptosis (48). To determine if this protection was mediated by activation of a survival-promoting program of transcription and protein translation, mAkt-expressing and control cells were withdrawn from growth factor and cultured in the presence or absence of the protein synthesis inhibitor CHX or the PI3-K inhibitor LY (Fig. 1). CHX and LY were replenished on days 2 and 4. Treatment of cells with CHX did not result in loss of mAkt protein beyond the decline observed for stable endogenous proteins (data not shown). The maintenance of mAkt protein levels was likely due to decreased protein degradation, which has been observed upon inhibition of protein synthesis (17). CHX treatment had no measurable effect on mAkt-mediated cell survival at time points out to 6 days (Fig. 1 and data not shown). Phosphorylation of mAkt was maintained in the absence of IL-3 regardless of CHX treatment (data not shown). Inhibition of mAkt activation by culturing cells in LY, however, caused the dephosphorylation of mAkt (data not shown) and prevented mAkt from mediating its survival effect. While the regulation of mAkt phosphorylation in IL-3 withdrawal and LY treatment is unclear, these data indicate that long-term Akt-mediated cell survival in the absence of growth factors correlates with the ability of Akt to maintain a phosphorylated state rather than induction of new transcription and protein translation.
FIG. 1.
Akt does not require protein synthesis to promote survival of growth factor-deprived cells. Control (Neo) and myristoylated Akt (mAkt)-expressing cells were withdrawn from IL-3 in medium without inhibitors or in medium containing the PI3-K inhibitor LY or the protein translation inhibitor CHX. The inhibitors were replenished on day 2. Treatment of cells with LY resulted in the dephosphorylation and inactivation of mAkt by day 2, while mAkt remained phosphorylated in the absence of IL-3 (data not shown). The cells were analyzed in culture by flow cytometry at various time points to determine viability by PI exclusion. The values shown are the means of the results for triplicate samples; error bars indicate standard deviations.
Constitutively active Akt requires glucose to prevent cell death.
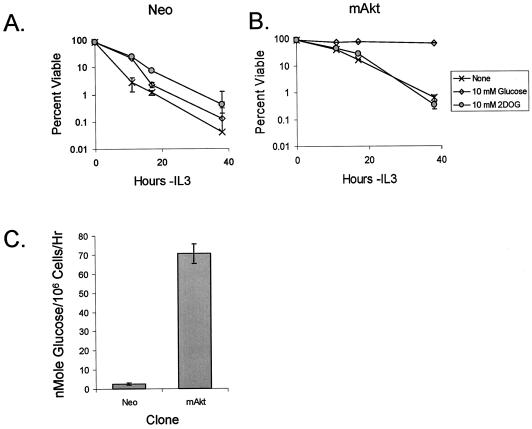
Unlike growth factor-independent survival promoted by antiapoptotic Bcl-2 proteins, mAkt requires the presence of glucose in the culture medium to mediate its protective effect (20, 48). It has been proposed that this dependence of Akt on glucose to promote cell survival is due to Akt-dependent stimulation and maintenance of hexokinase activity and the action of glucose-6-phosphate as a signaling molecule (20). In support of this model, the glucose analog 2-DOG, which can be phosphorylated by hexokinase but cannot be further metabolized through glycolysis, was sufficient to maintain short-term survival of cells following irradiation and serum withdrawal (20). In addition to acting as a potential signaling molecule, 2-DOG can be metabolized through the pentose phosphate pathway to generate NADPH (36, 37). To determine if 2-DOG was sufficient to allow Akt to promote long-term cell survival upon growth factor withdrawal, IL-3-dependent FL5.12 control (Neo) and mAkt-expressing cells were tested for survival upon IL-3 withdrawal in the absence of glucose or in the presence of glucose or 2-DOG. In the presence of glucose or 2-DOG, the control cells died more slowly than cells withdrawn from IL-3 in the absence of glucose (Fig. 2A). Nevertheless, all control cells rapidly underwent apoptosis when withdrawn from IL-3, with less than 1% viable at 38 h regardless of treatment. In contrast, cells expressing mAkt were protected from apoptosis by IL-3 withdrawal in glucose-containing medium (Fig. 2B). Although 2-DOG allowed mAkt to provide a degree of protection from growth factor withdrawal similar to that observed by 2-DOG in control cells, mAkt expression no longer offered long-term protection from death in the absence of glucose or if 2-DOG was substituted for glucose. These data suggested that mAkt cells require glucose hydrolysis through glycolysis for mAkt to mediate a protective effect from growth factor withdrawal. Direct measurement of glucose hydrolysis confirmed that mAkt cells consumed glucose upon IL-3 withdrawal at levels substantially higher than those for the control cells (Fig. 2C). This requirement for glucose hydrolysis was not simply due to a requirement to generate pyruvate as a mitochondrial substrate, because addition of mPyruvate did not affect cell death in cultures either in the absence of glucose or with 2-DOG (data not shown).
FIG. 2.
Akt requires a hydrolyzable glucose substrate to prevent death upon growth factor withdrawal. Control (Neo) (A) and mAkt-transfected IL-3-dependent FL5.12 (B) cells were withdrawn from IL-3 in the absence of glucose or in the presence of 10 mM glucose or 10 mM 2-DOG, a glucose analog that cannot be metabolized through glycolysis. At various time points, cells were analyzed by flow cytometry to determine viability by propidium iodide exclusion. (C) The glycolytic rates of Neo and mAkt cells were measured after 10 h in the absence of IL-3. The values shown are the means of the results for triplicate samples; error bars indicate standard deviations.
Glucose uptake is increased and maintained by mAkt expression.
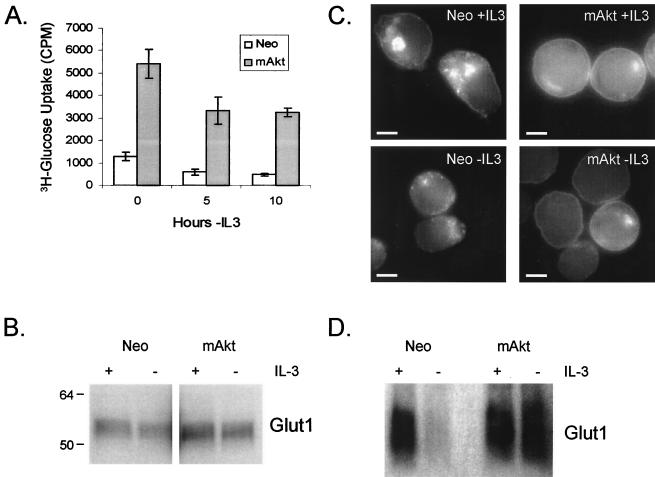
The requirement of mAkt for glucose hydrolysis suggested that mAkt may mediate its protective effects by promoting glucose metabolism. Because Akt has been shown to play an important role in insulin-responsive glucose uptake (55) and can regulate Glut1 mRNA levels (5, 48), we sought to determine if constitutively active Akt affected glucose uptake in hematopoietic cells. Cells expressing mAkt were compared to control cells for their capacity to uptake glucose in the presence or after the removal of IL-3 for periods of 5 or 10 h (Fig. 3A). These time points were chosen because up until 12 h of IL-3 deprivation, control cells can completely recover upon the readdition of IL-3, as demonstrated by an over-90% cloning efficiency (57). The control cells were found to have decreased glucose uptake after both 5 and 10 h of neglect. Constitutive Akt activity increased the capacity of FL5.12 cells to take up glucose at all time points. Because Glut1 is the dominant glucose transporter expressed on hematopoeitic cells, Glut1 expression was analyzed to determine if changes in Glut1 expression caused the increased glucose uptake observed in mAkt cells. Despite the ability of mAkt to attenuate Glut1 mRNA loss in FL5.12 cells following removal from IL-3 (48), the increased glucose uptake capacity of mAkt-expressing cells was not due to increased total Glut1 protein expression. Both in the presence and after 10 h in the absence of IL-3, mAkt-expressing cells were found to have Glut1 expression levels nearly identical to those of the control cells, as determined by Western blot analysis (Fig. 3B) and by flow cytometric analysis of Glut1 total cellular expression by immunofluorescence (Fig. 4A).
FIG. 3.
Constitutively active Akt promotes glucose uptake not by regulating Glut1 protein levels but by promoting Glut1 cell surface localization. (A) Control (Neo) and mAkt-expressing IL-3-dependent cells were cultured in the presence of IL-3 or in the absence of IL-3 for 5 or 10 h. The cells were then harvested, and their ability to uptake the nonhydrolyzable glucose analog 2-DOG was measured. The values shown are the means of the results for triplicate samples; error bars indicate standard deviations. (B) Control and mAkt cells were analyzed by Western blot for expression of the glucose transporter Glut1, either in the presence of IL-3 or after 10 h of withdrawal from IL-3. Each lane was loaded with 10 μg of protein. (C) Control (Neo) and mAkt cells were stained by immunofluorescence for Glut1 localization in the presence of IL-3 and after 10 h in the absence of IL-3. Each micrograph is individually contrasted to allow the best visualization of Glut1 localization. The bars represent 5 μm. (D) Plasma membranes were isolated from control and mAkt-expressing cells in the presence of IL-3 or after 12 h in the absence of IL-3, and 10 μg of protein was analyzed by Western blot for cell surface expression of Glut1.
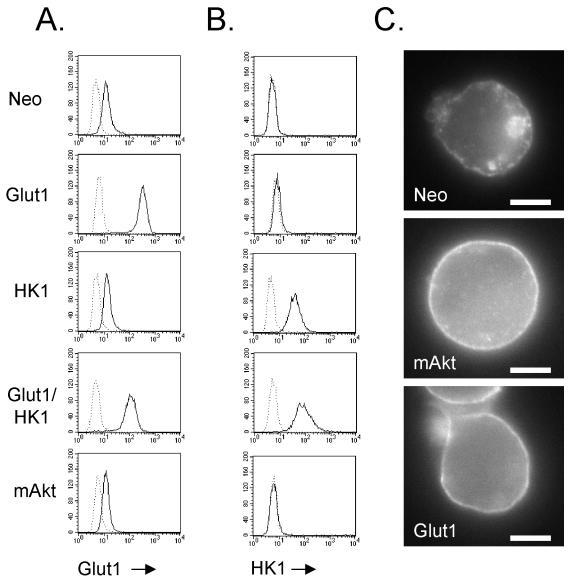
FIG. 4.
Glut1 expression promotes accumulation of Glut1 surface localization. (A and B) Control and mAkt-expressing FL5.12 cells were compared to cells transfected with Glut1, HK1, or Glut1 and HK1 together (Glut1/HK1) for Glut1 expression (A) and HK1 expression (B). (C) Glut1 localization was determined in control, mAkt, and Glut1 cells by immunofluorescence staining of permeabilized cells for Glut1 protein. Each micrograph is individually contrasted to allow the best visualization of Glut1 localization. Surface Glut1 immunofluorescence of Glut1-overexpressing cells was sufficiently bright that intracellular Glut1 became obscured upon contrasting the images (data not shown). The bars represent 5 μm.
Constitutively active Akt promotes and maintains Glut1 surface localization.
Glucose transporters can be regulated at many levels, including transcription, mRNA stability, and protein localization (3-5, 27). Because Akt has been demonstrated to play an important role in the translocation of Glut4 to the cell surface following insulin signaling in adipose and muscle cells (55), we tested whether mAkt affected Glut1 localization in hematopoietic cells. The control and mAkt-expressing cells were permeabilized, stained, and analyzed by immunofluorescence for Glut1 (Fig. 3C and 4C). In the control cells growing in IL-3, Glut1 was found mainly intracellularly (Fig. 3C, upper left panel, and 4C) with a ring of Glut1 at the cell surface. mAkt expression resulted in Glut1 localization predominantly on the cell surface (Fig. 3C, upper right panel, and 4C). Glut1-containing vesicles were detectable in the cytosol of mAkt-expressing cells but to a much lesser degree than in the control cells. When the control cells were deprived of IL-3 for 10 h, cell surface expression of Glut1 was significantly reduced and the ring of cell surface Glut1 staining was no longer discernible (Fig. 3C, lower left panel). Instead, Glut1 appeared to be localized almost exclusively in cytoplasmic vesicles. In contrast, 10 h after removal from IL-3, constitutively active Akt maintained cell surface Glut1 localization. To confirm these results, plasma membranes were isolated from the control and mAkt-expressing cells grown in the presence or after 12 h in the absence of IL-3 (Fig. 3D). As suggested by immunofluorescence, plasma membrane-associated Glut1 was found to decrease in control cells when removed from IL-3. Plasma membrane-associated Glut1 on mAkt cells, however, remained high even in the absence of IL-3. These data indicate that Akt activity is sufficient to affect Glut1 localization and suggest that the increased glucose uptake capacity of mAkt cells was the result of surface localization of Glut1 rather than increased Glut1 protein levels.
Akt increases hexokinase activity.
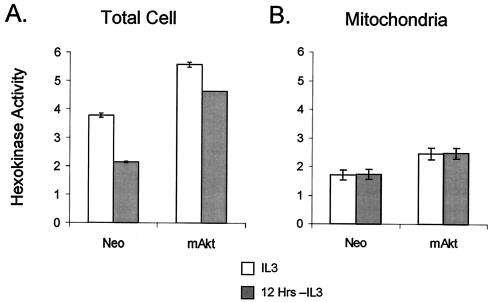
Glucose transporters are facilitative and transport glucose down concentration gradients. Cells cannot retain glucose in the cytoplasm until it is phosphorylated by hexokinase. Cells maintained in IL-3 or withdrawn from IL-3 for 12 h were prepared, and total cellular hexokinase activity was measured to determine the effect of IL-3 withdrawal on hexokinase activity and whether constitutive activation of Akt affected hexokinase function (Fig. 5A). Like glucose uptake, hexokinase activity decreased in control cells upon IL-3 withdrawal. Cells with constitutively active Akt had increased hexokinase activity in the presence of IL-3 relative to that in the control cells. When IL-3 was withdrawn, mAkt-expressing cells had only a small decrease in total cellular hexokinase activity, maintaining twofold-greater activity than the control cells.
FIG. 5.
Constitutively active mAkt cells have enhanced hexokinase activity that they maintain upon withdrawal from IL-3. (A) Control (Neo) and mAkt cells were grown in IL-3 or withdrawn from IL-3 for 12 h. Whole-cell lysates were analyzed for total hexokinase activity. (B) Mitochondria were isolated from control and mAkt cells in the presence of IL-3 or after 12 h in the absence of IL-3, and 10 μg of protein was analyzed for hexokinase activity. The values shown are the means of the results for triplicate samples; error bars indicate standard deviations.
Hexokinase activity can be cytosolic or mitochondrially associated, and mitochondrial-associated hexokinase activity has been suggested to play an important role in apoptosis (20, 47). Mitochondria from control and mAkt-expressing cells were, therefore, prepared from cells grown in the presence of IL-3 or from cells withdrawn from IL-3 for 12 h. While cells expressing mAkt were found to have higher mitochondrial hexokinase activity than the control cells, mitochondrial hexokinase activity was not affected by IL-3 withdrawal in either the control or mAkt-expressing cells (Fig. 5B). Loss of mitochondrial hexokinase activity, therefore, is not the cause of cell death upon growth factor withdrawal.
Overexpression of Glut1 promotes Glut1 surface localization.
Because mAkt maintained the uptake and phosphorylation of glucose in growth factor-withdrawn cells, we next sought to determine if these activities were sufficient to mimic the effects of mAkt on cell metabolism and survival. Four primary isoforms of hexokinase exist in mammals, with types 1 and 2 being mitochondrially targeted while types 3 and 4 are cytosolic (62). Because Akt has been reported to maintain mitochondrial hexokinase activity and HK1 overexpression can support short-term viability upon apoptotic stimuli (8, 20), FL5.12 cells were transfected with Glut1 and HK1 individually or together. Stable Glut1, HK1, and Glut/HK1 double-expressing clones were selected based on total Glut1 or HK1 protein levels as determined by flow cytometric analysis of permeabilized cells. All clones expressed endogenous Glut1. Cells transfected with Glut1, however, had significantly increased levels of Glut1 protein relative to that for cells not transfected with Glut1, with similarly high Glut1 expression levels in Glut1-only cells and Glut1/HK1 double-expressing clones (Fig. 4A). Likewise, cells transfected with HK1 had high levels of detectable HK1 protein compared to cells not transfected with HK1 (Fig. 4B). Cells not transfected with HK1 stained negative for HK1 because FL5.12 cells do not express endogenous HK1 but rather express HK2. Because the mechanism of Glut1 localization to the cell surface is unknown, Glut1-transfected cells were analyzed to determine if Glut1 overexpression resulted in an increase in cell surface Glut1 protein. Control, mAkt, and Glut1 cells were permeabilized, and Glut1 localization was observed by immunofluorescence (Fig. 4C). Glut1-overexpressing cells were found to have high levels of cell surface Glut1 protein. This localization pattern correlated with high glucose uptake capacity of Glut1 and Glut1/HK1 cells (data not shown). Glut1 and Glut1/HK1 cells also had significant intracellular pools of Glut1 (data not shown), but the high concentration of Glut1 on the cell surfaces obscured the view of these pools by immunofluorescence. Large amounts of cell surface Glut1 were maintained in Glut1- and Glut1/HK1-overexpressing cells upon withdrawal from IL-3, as determined by immunofluorescence and glucose uptake experiments (data not shown).
mAkt increases total cellular glucose consumption while expression of Glut1 and HK1 does not.
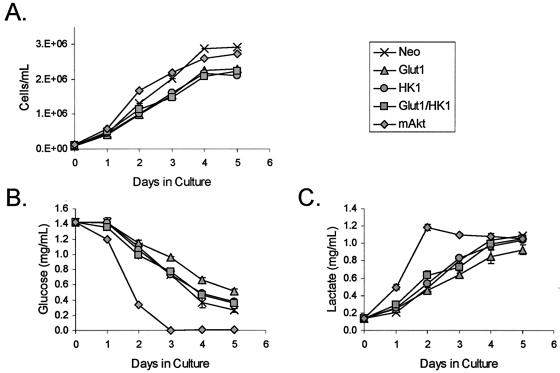
Since mAkt cells displayed increased glycolytic rates compared to those of the control cells, we next determined if the constitutive expression of Glut1 and HK1 was sufficient to increase glucose consumption. To determine if Glut/HK1 cells differed from the control cells in their glucose consumption, control cells, mAkt-expressing cells, and cells expressing Glut1 and HK1 were grown in the presence of IL-3. Cell cultures were established, and cell concentrations and the contents of glucose and lactate in the culture supernatant were determined daily for 5 days. All clones grew at similar rates (Fig. 6A). Only mAkt cells, however, depleted their cultures of glucose (Fig. 6B). This glucose appeared to be metabolized through glycolysis and not stored as glucose-6-phosphate or glycogen or metabolized through other pathways because mAkt expression caused an increase in lactate in the medium that was comparable to the depletion of glucose (Fig. 6C).
FIG. 6.
Constitutively active Akt increases total cellular glucose consumption. Control (Neo), Glut1, HK1, Glut1/HK1, and mAkt cells were cultured in the presence of IL-3 and observed each day for 5 days. (A) Cell proliferation was determined by measuring cell concentration. Glucose consumption (B) and lactate production (C) were determined by measuring their concentrations in the media. The values shown are the means of the results for triplicate cultures; error bars indicate standard deviations.
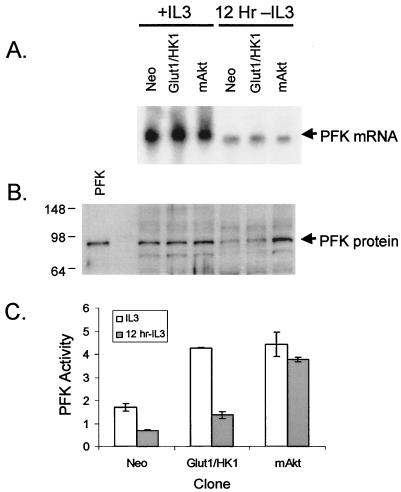
PFK1 protein levels and activity are maintained by mAkt upon IL-3 withdrawal.
The critical commitment step for glycolysis is the phosphorylation of fructose-6-phosphate by PFK1. Prior to this step, glucose or fructose-6-phosphate can be shunted through the pentose phosphate pathway. ATP is hydrolyzed by PFK1 in the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate. As a result of the hydrolysis of ATP, this step represents an irreversible commitment of glucose to glycolysis. PFK1 expression and activity were, therefore, measured in control, Glut1/HK1, and mAkt cells in the presence of IL-3 or after 12-h withdrawal from IL-3. mRNA for PFK1 decreased upon IL-3 withdrawal in all samples, indicating that Akt activity did not significantly affect transcription of PFK1 (Fig. 7A). PFK1 protein levels were also observed to decrease significantly in control and Glut1/HK1 cells following IL-3 withdrawal (Fig. 7B). PFK1 protein levels remained high, however, in mAkt cells after culture for 12 h in the absence of IL-3. The maintenance of PFK1 protein levels by mAkt may have been due to a prevention of its degradation because growth factor-withdrawn cells initiate a cellular atrophy program characterized by significant proteolysis that Akt can prevent (15). To confirm that PFK1 protein levels correlated with PFK1 function, we tested PFK1 activity in cell lysates from cells cultured in the presence or absence of IL-3 for 12 h (Fig. 7C). Neither control nor Glut1/HK1 cells maintained PFK1 activity upon removal from IL-3. Constitutively active Akt, however, maintained PFK1 activity despite IL-3 deprivation.
FIG. 7.
Akt prevents loss of PFK1 protein levels and activity upon growth factor withdrawal. Control (Neo), Glut1/HK1, and mAkt cells were grown in the presence of IL-3 or for 12 h in the absence of IL-3. (A) RNA was isolated and PFK1 expression was probed by northern hybridization. (B) Whole-cell lysates were analyzed by Western blot for expression of PFK1 protein. Purified rabbit muscle PFK1 is shown as a size standard. (C) Cell lysates were tested for PFK1 activity. The values shown are the means of the results for triplicate samples; error bars indicate standard deviations.
High cytosolic NAD(P)H is maintained by mAkt during IL-3 withdrawal.
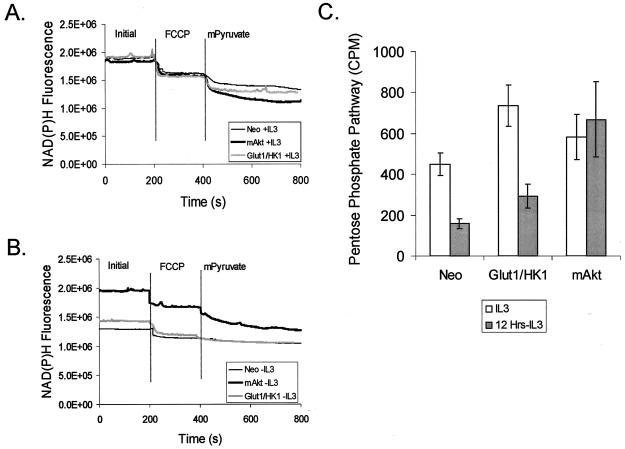
To determine if constitutively active Akt could affect the activity of glycolytic enzymes and generation of mitchondrial substrates downstream of hexokinase upon IL-3 withdrawal, NAD(P)H levels were measured in control, Glut1/HK1, and mAkt cells. To measure NAD(P)H, intact whole cells were analyzed by spectrofluorimetic detection of NAD(P)H (Fig. 8). Total cellular NAD(P)H levels, as well as mitochondrial-accessible and cytosol-accessible NAD(P)H levels, were determined. Mitochondrial NAD(P)H was measured by the addition of the protonophore FCCP to cause the uncoupling of electron transport from F1F0 ATPase, thus shifting the electrochemical equilibrium to favor oxidation of NADH to NAD and consumption of mitochondrial NADH. The decrease in NAD(P)H fluorescence from initial total cellular NAD(P)H fluorescence following FCCP addition, therefore, indicates the relative amount of mitochondrial-accessible NADH. To determine what fraction of remaining fluorescence could be attributed to cytosolic NAD(P)H, mPyruvate was added. mPyruvate is cell permeable and causes the oxidation of cytosolic NADH to NAD by lactate dehydrogenase. The further decrease in NAD(P)H fluorescence as a consequence of the reduction of mPyruvate to lactate, therefore, serves as an indicator of cytoplasmic NADH.
FIG. 8.
Glut1/HK1 cells partially and mAkt cells fully maintain NAD(P)H levels upon removal from IL-3. Control (Neo), Glut1/HK1, and mAkt cells were grown in the presence of IL-3 (A) or withdrawn from IL-3 (B) for 12 h. The cells were then subjected to spectrofluorimetric analysis for NAD(P)H fluorescence to determine the total, mitochondrial-accessible, and cytosolic-accessible NAD(P)H content of each cell type. After establishing a baseline for fluorescence, the cells were treated with the protonophore FCCP to stimulate oxidation of mitochondrial NADH (added at the vertical line). Cytosolic NADH content was then estimated for each sample by observing the decrease in NADH fluorescence over time after the addition of mPyruvate (added at the vertical line). Through the action of the cytosolic enzyme lactate dehydrogenase, mPyruvate causes the depletion of cytosolic NADH. (C) Pentose phosphate shuttle activity was determined in control, Glut1/HK1, and mAkt cells in the presence of IL-3 or after 12 h in the absence of IL-3. The values shown are the means of the results for triplicate samples; error bars indicate standard deviations.
In this assay, control, Glut1/HK1, and mAkt cells had similar initial and FCCP-treated fluorescence profiles in the presence of IL-3 (Fig. 8A). Glut1/HK1 and mAkt cells, however, had increased NAD(P)H available to cytoplasmic lactate dehydrogenase because their absorbances were more significantly affected than those of the control cells by addition of mPyruvate. In the absence of IL-3, the control cells had significantly decreased NAD(P)H fluorescence and their residual fluorescence was less affected by FCCP and mPyruvate than in the presence of IL-3 (Fig. 8B). IL-3-withdrawn Glut1/HK1 cells also had significantly decreased cellular NAD(P)H but nevertheless maintained a higher level of FCCP and mPyruvate-sensitive pool NAD(P)H than that of the control cells. Cells with constitutively active Akt, however, retained their FCCP and mPyruvate-sensitive fluorescence in the absence of IL-3 at levels similar to those in the presence of IL-3, suggesting that mAkt caused cells to continue glycolysis and maintain cytosolic NAD(P)H production. These data indicate that, although unable to maintain production of NAD(P)H to the extent of that of mAkt cells upon removal from IL-3, Glut1/HK1 cells can maintain higher levels of production of glucose-derived metabolic substrates than can control cells. Consistent with this finding, Glut1-expressing cells were found to maintain increased rates of glycolysis relative to that of control cells upon IL-3 withdrawal (data not shown).
NADH and NADPH display fluorescence profiles indistinguishable by the means used here. To determine the rate of NADPH generation as an indicator of the contribution of NADPH to NAD(P)H fluorescence, pentose phosphate pathway activity was measured (Fig. 8C). Control, Glut1/HK1, and mAkt-expressing cells were grown in the presence or in the absence of IL-3 for 12 h, and glucose flux through the pentose phosphate pathway was measured. Growth factor withdrawal caused a twofold reduction in pentose phosphate pathway activity for both control and Glut1/HK1 cells. Cells expressing mAkt, however, were found to maintain their level of glucose flux through the pentose phosphate pathway even in the absence of IL-3. This maintenance of pentose phosphate activity was unlikely, due simply to the maintenance of glucose uptake and phosphorylation that mAkt promotes, because Glut1/HK1 cells also maintained high glucose uptake and phosphorylation upon IL-3 withdrawal yet had reduced pentose phosphate activity. Rather, these data suggest that Akt can promote the maintenance of this pathway directly.
Glut1 and HK1 increase cell survival upon IL-3 withdrawal by preventing change in Bax conformation.
These data suggest that one mechanism by which Akt may promote growth factor-independent survival is through the support of glucose metabolism. To determine if the maintenance intracellular stores of hydrolyzable glucose was sufficient to prevent cell death upon growth factor withdrawal, control, Glut1, HK1, and Glut1/HK1 cells were observed upon removal from IL-3. The control cells died rapidly upon IL-3 withdrawal (Fig. 9A). Cells expressing both Glut1 and HK1, however, had substantially enhanced survival without IL-3. Surviving Glut1/HK1 cells were fully viable and could be recovered by the addition of IL-3 even after up to 5 days in the absence of IL-3, whereas control cells could not be rescued by IL-3 at this time (data not shown).
FIG. 9.
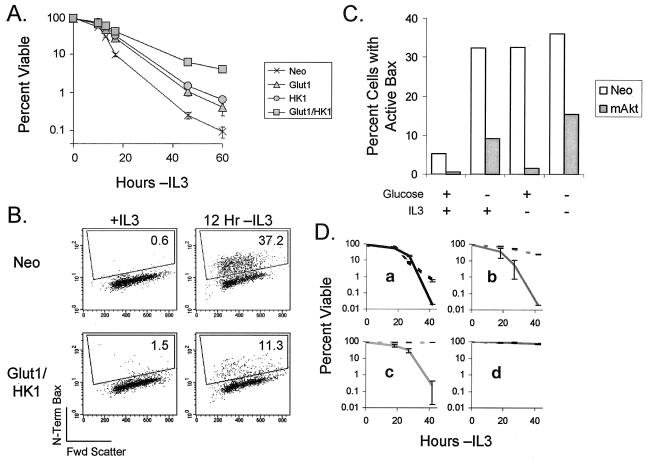
Coexpression of Glut1 and HK1 increases survival in the absence of IL-3 and prevents change in Bax conformation. (A) Cells were withdrawn from IL-3, and viabilities at various time points were measured by flow cytometry by propidium iodide exclusion. The values shown are the means of the results for cell survival from triplicate samples; error bars indicate standard deviations. (B) Bax activation was measured by flow cytometry in cells that were maintained in IL-3 or withdrawn from IL-3 for 12 h. Percentages for cells staining positive for change in Bax conformation are given. (C) Control (Neo) and mAkt-expressing cells were cultured in the presence or absence of glucose or IL-3, as indicated, for 12 h, and the percentage of cells with activated Bax was determined. (D) Control (a), Glut1/HK1 (b), mAkt (c), and Bcl-xL (d)-overexpressing FL5.12 cells were cultured in the absence of IL-3 either with (solid lines) or without (dashed lines) the glycolytic inhibitor IAA. Cell viabilities at various time points after the start of culture were measured by flow cytometry by propidium iodide exclusion. The values shown are the means for the results of cell survival from triplicate samples; error bars indicate standard deviations.
Because the proapoptotic Bcl-2 family member Bax has been shown to play an important role in initiating the mitochondrial cell death pathway (38, 60, 68), we next sought to determine if the maintenance of intracellular stores of hydrolyzable glucose in Glut1/HK1 cells might promote cell survival by affecting the regulation of Bax. Bax is normally present in the cytosol in an inactive conformation. Upon apoptotic stimulation, an unknown event causes Bax to translocate to mitochondria and undergo a conformational change that reveals a hidden N-terminal epitope (21, 42). After conformational change, Bax is activated and can promote mitochondrial release of cytochrome c (30). Control and Glut1/HK1 cells, therefore, were analyzed by flow cytometry for the presence of activated Bax by using an N-terminal Bax-specific antibody (Fig. 8B). Control cells withdrawn from IL-3 for 12 h were found to have a substantial population (37.2%) of cells with Bax in an activated conformation. Glut1/HK1 cells, in contrast, had only a small number of cells (11.3%) in which Bax had undergone conformational change. These data indicate that Bax conformational change may be sensitive to changes in glucose metabolism.
Since Glut1/HK1 expression can promote survival, and since Akt requires glucose to promote survival, it is possible that Akt-dependent increases in glucose metabolism are required for survival. Bax activation in control and mAkt-expressing cells was therefore measured in cells deprived of IL-3 or glucose. Previously, Bax mitochondiral redistribution and conformational change had been observed upon glucose withdrawal in cell populations by immunoprecipitation with activated Bax conformation-specific antibodies (58). Here, using single-cell analysis by flow cytometry, we observed Bax to become activated in control cells upon withdrawal of either IL-3 or glucose (Fig. 9C). In agreement with previous work (56, 65), we found that cells expressing constitutively active Akt resist Bax conformational change after withdrawal of IL-3 in the presence of glucose. Removal of glucose from the culture medium in either the presence or absence of IL-3, however, caused a >10-fold increase in mAkt-expressing cells with active-conformation Bax.
To further test the role of glycolysis in mAkt-mediated survival, growth factor-withdrawn cells were treated with IAA, an inhibitor of glyceraldehyde-3-phosphate dehydrogenase (22, 64). While control cells underwent cell death upon IL-3 withdrawal and IAA had little effect, IAA treatment prevented the protection of cells from growth factor withdrawal for both Glut1/HK1 and mAkt-expressing cells (Fig. 9D). This was not due to general cell toxicity of IAA because FL5.12 cells expressing Bcl-xL were unaffected by IAA, possibly because Bcl-xL maintains cell viability in the absence of growth factor even when glycolysis becomes depressed (48, 49). These data suggest that Glut1/HK1 and mAkt-expressing cells require glycolysis to promote cell survival. Further, these data indicate that Bax conformational change may be sensitive to changes in glucose metabolism and suggest that promotion of glucose metabolism in both Glut1/HK1 and mAkt cells may maintain survival by preventing metabolism-sensitive Bax conformational change and activation.
DISCUSSION
The serine/threonine kinase, Akt, participates in many signal transduction pathways that can prevent cell death upon withdrawal from extrinsic signals. The mechanism by which mAkt maintains survival has been unclear but has been shown to depend on glucose (20, 48). Here, we show that Akt does not require new protein translation but rather utilizes a posttranslational mechanism that depends on glucose hydrolysis to promote survival of growth factor-withdrawn cells. Furthermore, control of glucose metabolism by Akt occurs at multiple glycolytic control points and may be a critical component of Akt-dependent survival in the absence of growth factors because maintenance of glucose metabolism on its own is sufficient to attenuate Bax conformational change and growth factor withdrawal-induced cell death.
How precisely Akt-mediated regulation of glucose metabolism acts to prevent Bax activation and contribute to cell survival is unclear. One possible mechanism is that Akt-driven glucose uptake and phosphorylation by glucose transporters and hexokinase, respectively, cause an accumulation of glucose-6-phosphate that can then act to provide cell survival signals. This possibility is supported by the observation that the glucose analog 2-DOG is sufficient to replace glucose in short-term survival assays (20). Phospho-2-DOG may provide only a short-term survival advantage because accumulation of phospho-2-DOG has been shown to cause dissociation of hexokinase proteins from outer mitochondrial membranes (54), which may lead to apoptosis (20, 47). Alternatively, the ability of 2-DOG to be metabolized through the pentose phosphate shuttle but not through glycolysis (36, 37) may suggest that generation and regulation of other metabolites, such as NADPH, may be important for short-term cell survival. The ability of mAkt to maintain pentose phosphate shuttle activity even in the absence of growth factor may support continued NADPH generation and cellular redox regulation to promote short-term cell survival. The failure of 2-DOG to support long-term mAkt-mediated survival, however, indicates that pentose phosphate shuttle activity is not sufficient to allow long-term survival. Instead, Akt-driven glucose metabolism may promote survival by stimulating glucose hydrolysis and generation of substrates for mitochondrial metabolism through glycolysis. In support of the latter hypothesis are observations that Akt activity can increase rates of glycolysis and promote glucose consumption and lactate production (18, 20, 48).
Akt regulates cellular metabolism through multiple mechanisms. Intracellular localization of nutrient transporters is an important primary mechanism to regulate metabolism. Akt has also been shown to regulate the localization of Glut4 in myoblasts and adipocytes, as insulin-stimulated translocation of Glut4 has been shown to depend on both the Rho family GTPase TC10 and the phosphatidylinositol 3-kinase/Akt pathway (9, 55). Myoblasts and adipocytes also express Glut1, but in these cases, Glut1 is apparently constitutively localized to the cell surface. In contrast, hematopoietic cells do not express Glut4; instead, Glut1 is the dominant glucose transporter. Here, we show that Glut1 localization in hematopoietic cells is regulated by the cytokine IL-3 and by the kinase Akt. In addition, Akt has been shown to promote surface localization of the amino acid transporter-associated protein 4F2hc, transferrin receptor, and low-density lipoprotein receptor in an mTOR-dependent manner (15). The mechanism by which localization of Glut1 may be regulated in hematopoietic cells, however, is unclear. Because Glut1 translocates poorly in response to insulin in myoblasts and adipocytes (14), Glut1 localization in hematopoietic cells may be regulated through pathways different from those for Glut4. Alternatively, the regulation of Glut1 localization may be different in myoblasts and adipocytes than in hematopoietic cells. In this view, regulation of Glut1 localization in hematopoietic cells may resemble that of Glut4 in myoblasts and adipocytes.
The enhanced survival of Glut1/HK1 cells upon IL-3 withdrawal demonstrates that glucose uptake and capture can promote cell survival when cells are withdrawn from growth factor. The ability of Glut1/HK1-expressing cells to survive IL-3 withdrawal indicates that glucose and its metabolic derivatives become limiting in growth factor deprivation and thus contribute to apoptosis, possibly through activation of Bax. Nevertheless, Glut1/HK1 cells did not survive as well as mAkt-expressing cells. Glucose uptake and phosphorylation must be accompanied by other events to allow survival at a level similar to that in mAkt cells. As indicated by the failure of 2-DOG to replace glucose in mAkt-mediated long-term survival in the absence of growth factor, accumulation of phosphoglucose or its analogs was not on its own sufficient to promote survival. Instead, phosphoglucose must be further metabolized to support mAkt-dependent survival. Therefore, by allowing IL-3-deprived cells continued access to phosphoglucose, Glut1 and HK1 expression may support these metabolic pathways. The failure of Glut1/HK1 cells to fully maintain PFK1 activity, the inability of Glut1 and HK1 expression to fully maintain NAD(P)H levels equivalent to those of mAkt cells in the absence of IL-3, and the failure to maintain pentose phosphate shunt activity, however, may explain the lower rate of long-term survival of Glut1/HK1 cells compared to that of mAkt cells when deprived of IL-3.
The death of cells withdrawn from extrinsic signals requires the actions of the proapoptotic molecules Bax or Bak (38, 60, 68). Both Bax and Bak require some form of activation to initiate mitochondrial dysfunction and release of the contents of the mitochondrial intermembrane space. Bax must change conformation and translocate to the mitochondria (21, 42), and Bak must homo-oligomerize (60). The source of each of these activation events is unknown. The ability of glucose starvation to cause Bax conformational change and translocation (58) and the ability of Glut1 and HK1 expression to prevent Bax activation, however, indicate that Bax is sensitive to regulation by glucose metabolism. The effects of the loss of glucose metabolism and the mitochondrial hypopolarization that occurs during cellular atrophy of growth factor-withdrawn cells may therefore result in Bax and Bak activation leading to the loss of mitochondrial homeostasis. While Akt has been shown to prevent the activation of Bax upon withdrawal of cells from cytokine (56, 65), the mechanism has been unclear. Akt does, however, require glucose and cellular ability to perform glycolysis to prevent Bax activation and cell death. One possible mechanism used by Akt to prevent Bax activation and the loss of mitochondrial integrity, therefore, may be the promotion of glucose capture and commitment by its actions at multiple points of glycolysis, including regulation of glucose transporters, maintenance of hexokinase function, and stabilization of PFK1 protein levels and activity. It is unclear, however, what metabolite or metabolic pathway to which Bax may respond and if this response is direct or indirect, through actions of BH3-only proteins (46).
The regulation of glucose metabolism may also play an important role in tumorigenesis. It has long been appreciated that cancer cells have increased glycolytic metabolism, termed the Warburg effect (59). Recently, the regulation of glucose transport on its own has been implicated in the development and progression of cancer, as increased Glut1 expression has been associated with lung and colorectal carcinoma (26, 66, 67) and correlated with poor prognosis in breast cancer (35). In addition, suppression of glucose transport and glucose metabolism have been shown to inhibit tumor growth (1, 44). The ability of Akt to promote glucose uptake and initiate glycolysis through posttranscriptional regulation of glycolytic enzymes, therefore, may represent key aspects of Akt-mediated prevention of cell death, promotion of cell growth, and contribution to tumorigenesis.
Acknowledgments
We thank M. J. Birnbaum (University of Pennsylvania, Philadelphia, Pa.) and J. E. Wilson (Michigan State University, Lansing, Mich.) for their advice and generous gifts of Glut1 and HK1 cDNAs, respectively. We also thank R. Elstrom, A. Edinger, and K. Frauwirth for technical assistance and critical discussions.
This work was supported by grants from the National Cancer Institute. J.C.R. and D.R.P. were supported by the Irvington Institute for Immunological Research. C.J.F. was supported by a chapter grant from the Arthritis Society. J.C.R. was also supported by a Howard Temin K01 Career Development Award from the National Cancer Institute (grant K01 CA91905).
REFERENCES
- 1.Aft, R. L., F. W. Zhang, and D. Gius. 2002. Evaluation of 2-deoxy-d-glucose as a chemotherapeutic agent: mechanism of cell death. Br. J. Cancer 87:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7:261-269. [DOI] [PubMed] [Google Scholar]
- 3.Atasoy, U., J. Watson, D. Patel, and J. D. Keene. 1998. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J. Cell Sci. 111:3145-3156. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin, S. A., L. F. Barros, and M. Griffiths. 1995. Trafficking of glucose transporters—signals and mechanisms. Biosci. Rep. 15:419-426. [DOI] [PubMed] [Google Scholar]
- 5.Barthel, A., S. T. Okino, J. Liao, K. Nakatani, J. Li, J. P. J. Whitlock, and R. A. Roth. 1999. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J. Biol. Chem. 274:20281-20286. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa, A., J. R. Testa, S. P. Staal, and P. N. Tsichlis. 1991. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254:274-277. [DOI] [PubMed] [Google Scholar]
- 7.Boise, L. H., M. Gonzalez-Garcia, C. E. Postema, L. Ding, T. Lindsten, L. A. Turka, X. Mao, G. Nunez, and C. B. Thompson. 1993. bcl-x, a bcl-2 related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597-608. [DOI] [PubMed] [Google Scholar]
- 8.Bryson, J. M., P. E. Coy, K. Gottlob, N. Hay, and R. B. Robey. 2002. Increased hexokinase activity, of either ectopic or endogenous origin, protects renal epithelial cells against acute oxidant-induced cell death. J. Biol. Chem. 277:11392-11400. [DOI] [PubMed] [Google Scholar]
- 9.Chiang, S. H., C. A. Baumann, M. Kanzaki, D. C. Thurmond, R. T. Watson, C. L. Neudauer, I. G. Macara, J. E. Pessin, and A. R. Saltiel. 2001. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410:944-948. [DOI] [PubMed] [Google Scholar]
- 10.Coffer, P. J., and J. R. Woodgett. 1991. Molecular cloning and characterisation of a novel protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur. J. Biochem. 201:475-481. [DOI] [PubMed] [Google Scholar]
- 11.Conlon, I., and M. Raff. 1999. Size control in animal development. Cell 96:235-244. [DOI] [PubMed] [Google Scholar]
- 12.Deckwerth, T. L., and E. M. J. Johnson. 1993. Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J. Cell Biol. 123:1207-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshmukh, M., J. Vasilakos, T. L. Deckwerth, P. A. Lampe, B. D. Shivers, and E. M. J. Johnson. 1996. Genetic and metabolic status of NGF-deprived sympathetic neurons saved by an inhibitor or ICE family proteases. J. Cell Biol. 135:1341-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducluzeau, P. H., L. M. Fletcher, H. Vidal, M. Laville, and J. M. Tavare. 2002. Molecular mechanisms of insulin-stimulated glucose uptake in adipocytes. Diabetes Metab. 28:85-92. [PubMed] [Google Scholar]
- 15.Edinger, A. L., and C. B. Thompson. 2002. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell 13:2276-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foran, P. G. P., L. M. Fletcher, P. B. Oatey, N. Mohammed, J. O. Dolly, and J. M. Tavare. 1999. Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3-L1 adipocytes by a pathway involving SNAP-23, synaptobrevin-2, and/or cellubrevin. J. Biol. Chem. 274:28087-28095. [DOI] [PubMed] [Google Scholar]
- 17.Franklin, J. L., and E. M. Johnson. 1998. Control of neuronal size homeostasis by trophic factor-mediated coupling of protein degradation to protein synthesis. J. Cell Biol. 142:1313-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frauwirth, K. A., J. L. Riley, M. H. Harris, R. V. Parry, J. C. Rathmell, D. R. Plas, R. L. Elstrom, C. H. June, and C. B. Thompson. 2002. The CD28 signaling pathway regulates glucose metabolism. Immunity 16:769-777. [DOI] [PubMed] [Google Scholar]
- 19.Gottlieb, E., S. M. Armour, and C. B. Thompson. 2002. Mitochondrial respiratory control is lost during growth factor deprivation. Proc. Natl. Acad. Sci. USA 99:12801-12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlob, K., N. Majewski, S. Kennedy, E. Kandel, R. B. Robey, and N. Hay. 2001. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 15:1406-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross, A., J. Jockel, M. C. Wei, and S. J. Korsmeyer. 1998. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 17:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo, Z., J. Lee, M. Lane, and M. Mattson. 2001. Iodoacetate protects hippocampal neurons against excitotoxic and oxidative injury: involvement of heat-shock proteins and Bcl-2. J. Neurochem. 79:361-370. [DOI] [PubMed] [Google Scholar]
- 23.Hajduch, E., G. J. Litherland, and H. S. Hundal. 2001. Protein kinase B (PKB/Akt)—a key regulator of glucose transport? FEBS Lett. 492:199-203. [DOI] [PubMed] [Google Scholar]
- 24.Hill, M. M., S. F. Clark, D. F. Tucker, M. J. Birnbaum, D. E. James, and S. L. Macaulay. 1999. A role for protein kinase Bβ/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 19:7771-7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hockenbery, D., G. Nunez, C. Milliman, R. D. Schreiber, and S. J. Korsmeyer. 1990. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348:334-336. [DOI] [PubMed] [Google Scholar]
- 26.Ito, T., Y. Noguchi, N. Udaka, H. Kitamura, and S. Satoh. 1999. Glucose transporter expression in developing fetal lungs and lung neoplasms. Histol. Histopathol. 14:895-904. [DOI] [PubMed] [Google Scholar]
- 27.Jain, R. G., L. G. Andrews, K. M. McGowan, P. H. Pekala, and J. D. Keene. 1997. Ectopic expression of Hel-N1, an RNA-binding protein, increases glucose transporter (GLUT1) expression in 3T3-L1 adipocytes. Mol. Cell. Biol. 17:954-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones, P. F., T. Jakubowicz, F. J. Pitossi, F. Maurer, and B. A. Hemmings. 1991. Molecular cloning and identification of a serine/theonine protein kinase of the second-messenger subfamily. Proc. Natl. Acad. Sci. USA 88:4171-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones, R. G., M. Parsons, M. Bonnard, V. S. F. Chan, W.-C. Yeh, J. R. Woodgett, and P. S. Ohashi. 2000. Protein kinase B regulates T lymphocyte survival, nuclear factor κB activation, and Bcl-XL levels in vivo. J. Exp. Med. 191:1721-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurgensmeier, J. M., Q. Deveraux, L. Ellerby, D. Bredensen, and J. C. Reed. 1998. Bax directly induces cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95:4997-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamsteeg, E.-J., and P. M. T. Deen. 2001. Detection of aquaporin-2 in the plasma membranes of oocytes: a novel isolation method with improved yield and purity. Biochem. Biophys. Res. Commun. 282:683-690. [DOI] [PubMed] [Google Scholar]
- 32.Kandel, E. S., and N. Hay. 1999. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 253:210-229. [DOI] [PubMed] [Google Scholar]
- 33.Kane, L. P., M. N. Mollenauer, Z. Xu, C. W. Turck, and A. Weiss. 2002. Akt-dependent phosphorylation specifically regulates Cot induction of NF-κB-dependent transcription. Mol. Cell. Biol. 22:5962-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kane, L. P., V. S. Shapiro, D. Stokoe, and A. Weiss. 1999. Induction of NF-κB by the Akt/PKB kinase. Curr. Biol. 9:601-604. [DOI] [PubMed] [Google Scholar]
- 35.Kang, S. S., Y. K. Chun, M. H. Hur, H. K. Lee, Y. J. Kim, S. R. Hong, J. H. Lee, S. G. Lee, and Y. K. Park. 2002. Clinical significance of glucose transporter 1 (GLUT1) expression in human breast carcinoma. Jpn. J. Cancer Res. 93:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Goffe, C., G. Vallette, L. Charrier, T. Candelon, C. Bou-Hanna, J.-F. Bouhours, and C. L. Loboisse. 2002. Metabolic control of resistance of human epithelial cells to H2O2 and NO stress. Biochem. J. 364:349-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Goffe, C., G. Vallette, A. Jarry, C. Bou-Hanna, and C. L. Laboisse. 1999. The in vivo manipulation of carbohydrate metabolism: a new strategy for deciphering the cellular defence mechanisms against nitric oxide attack. Biochem. J. 344:643-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindsten, T., A. J. Ross, A. King, W. X. Zong, J. C. Rathmell, H. A. Shiels, E. Ulrich, K. G. Waymire, P. Mahar, K. Frauwirth, Y. Chen, M. Wei, V. M. Eng, D. M. Adelman, M. C. Simon, A. Ma, J. A. Golden, G. Evan, S. J. Korsmeyer, G. R. MacGregor, and C. B. Thompson. 2000. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6:1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madrid, L. V., C. Y. Wang, D. C. Guttridge, A. J. Schottelius, A. S. Baldwin, Jr., and M. W. Mayo. 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol. Cell. Biol. 20:1626-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandic, A., K. Viktorsson, M. Molin, G. Akusjarvi, H. Eguchi, S. I. Hayashi, M. Toi, J. Hansson, S. Linder, and M. C. Shoshan. 2001. Cisplatin induces the proapoptotic conformation of Bak in a ΔMEKK1-dependent manner. Mol. Cell. Biol. 21:3684-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuyama, S., and J. C. Reed. 2000. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 7:1155-1165. [DOI] [PubMed] [Google Scholar]
- 42.Nechushtan, A., C. L. Smith, Y. T. Hsu, and R. J. Youle. 1999. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 18:2330-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholls, D. G., and S. J. Ferguson. 1992. Bioenergetics 2, 2nd. ed. Academic Press, London, England.
- 44.Noguchi, Y., A. Saito, Y. Miyagi, S. Yamanaka, D. Marat, C. Doi, T. Yoshikawa, A. Tsuburaya, T. Ito, and S. Satoh. 2000. Suppression of facilitative glucose transporter 1 mRNA can suppress tumor growth. Cancer Lett. 154:175-182. [DOI] [PubMed] [Google Scholar]
- 45.Nunez, G., L. London, D. Hockenbery, M. Alexander, J. P. Mckearn, and S. J. Korsmeyer. 1990. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J. Immunol. 144:3602-3610. [PubMed] [Google Scholar]
- 46.Opferman, J. T., and S. J. Korsmeyer. 2003. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 4:410-415. [DOI] [PubMed] [Google Scholar]
- 47.Pastorino, J. G., N. Shulga, and J. B. Hoek. 2002. Mitochondria binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 277:7610-7618. [DOI] [PubMed] [Google Scholar]
- 48.Plas, D. R., S. Talapatra, A. L. Edinger, J. C. Rathmell, and C. B. Thompson. 2001. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 276:12041-12048. [DOI] [PubMed] [Google Scholar]
- 49.Rathmell, J. C., M. G. Vander Heiden, M. H. Harris, K. A. Frauwirth, and C. B. Thompson. 2000. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell 6:683-692. [DOI] [PubMed] [Google Scholar]
- 50.Romashkova, J. A., and S. S. Makarov. 1999. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86-90. [DOI] [PubMed] [Google Scholar]
- 51.Sapico, V., and R. L. Anderson. 1970. Regulation of d-fructose 1-phosphate kinase by potassium ion. J. Biol. Chem. 245:3252-3256. [PubMed] [Google Scholar]
- 52.Scheid, M. P., P. A. Marignani, and J. R. Woodgett. 2002. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol. Cell. Biol. 22:6247-6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sofroniew, M. V., J. D. Cooper, C. N. Svendsen, P. Crossman, N. Y. Ip, R. M. Lindsay, and F. Zafra. 1993. Atrophy but not death of adult septal cholinergic neurons after ablation of target capacity to produce mRNAs for NGF, BDNF, and NT3. J. Neurosci. 13:5263-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sui, D., and J. E. Wilson. 1997. Structural determinants for the intracellular localization of the isozymes of mammalian hexokinase: intracellular localization of fusion constructs incorporating structural elements from the hexokinase isozymes and the green fluorescent protein. Arch. Biochem. Biophys. 345:111-125. [DOI] [PubMed] [Google Scholar]
- 55.Summers, S. A., V. P. Yin, E. L. Whiteman, L. A. Garza, H. Cho, R. L. Tuttle, and M. J. Birnbaum. 1999. Signaling pathways mediating insulin-stimulated glucose transport. Ann. N. Y. Acad. Sci. 892:169-186. [DOI] [PubMed] [Google Scholar]
- 56.Tsuruta, F., N. Masuyama, and Y. Gotoh. 2002. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J. Biol. Chem. 277:14040-14047. [DOI] [PubMed] [Google Scholar]
- 57.Vander Heiden, M. G., N. S. Chandel, E. K. Williamson, P. T. Schumacker, and C. B. Thompson. 1997. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91:627-637. [DOI] [PubMed] [Google Scholar]
- 58.Vander Heiden, M. G., D. R. Plas, J. C. Rathmell, C. J. Fox, M. H. Harris, and C. B. Thompson. 2001. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol. Cell. Biol. 21:5899-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warburg, O. 1956. On the origin of cancer cells. Science 123:309-314. [DOI] [PubMed] [Google Scholar]
- 60.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whetton, A. D., G. W. Brazill, and T. M. Dexter. 1984. Haemopoietic cell growth factor mediates cell survival via its action of glucose transport. EMBO J. 3:409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson, J. E. 1995. Hexokinases. Rev. Physiol. Biochem. Pharmacol. 126:65-198. [DOI] [PubMed] [Google Scholar]
- 63.Wilson, J. E. 1989. Rapid purification of mitochondrial hexokinase from rat brain by a single affinity chromatography step on Affi-Gel blue. Prep. Biochem. 19:13-21. [DOI] [PubMed] [Google Scholar]
- 64.Winkler, B. S., M. W. Sauer, and C. A. Starnes. 2003. Modulation of the Pasteur effect in retinal cells: implications for understanding compensatory metabolic mechanisms. Exp. Eye Res. 76:715-723. [DOI] [PubMed] [Google Scholar]
- 65.Yamaguchi, H., and H. G. Wang. 2001. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene 20:7779-7786. [DOI] [PubMed] [Google Scholar]
- 66.Younes, M., R. W. Brown, M. Stephenson, M. Gondo, and P. T. Cagle. 1997. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer 80:1046-1051. [DOI] [PubMed] [Google Scholar]
- 67.Younes, M., L. V. Lechago, and J. Lechago. 1996. Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases. Clin. Cancer Res. 2:1151-1154. [PubMed] [Google Scholar]
- 68.Zong, W. X., T. Lindsten, A. J. Ross, G. R. MacGregor, and C. B. Thompson. 2001. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 15:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]