Abstract
The vitamin K-dependent γ-glutamyl carboxylase catalyzes the posttranslational conversion of glutamic acid to γ-carboxyglutamic acid in precursor proteins containing the γ-carboxylation recognition site (γ-CRS). During this reaction, glutamic acid is converted to γ-carboxyglutamic acid while vitamin KH2 is converted to vitamin K 2,3-epoxide. Recombinant bovine carboxylase was purified free of γ-CRS-containing propeptide and endogenous substrate in a single-step immunoaffinity procedure. We show that in the absence of γ-CRS-containing propeptide and/or glutamate-containing substrate, carboxylase has little or no epoxidase activity. Epoxidase activity is induced by Phe-Leu-Glu-Glu-Leu (FLEEL) (9.2 pmol per min per pmol of enzyme), propeptide, residues −18 to −1 of proFactor IX (3.4 pmol per min per pmol of enzyme), FLEEL and propeptide (100 pmol per min per pmol of enzyme), and proPT28 (HVFLAPQQARSLLQRVRRANTFLEEVRK, residues −18 to +10 of human acarboxy-proprothrombin), (5.3 pmol per min per pmol of enzyme). These results indicate that in the absence of propeptide or glutamate-containing substrate, oxygenation of vitamin K by the carboxylase does not occur. Upon addition of propeptide or glutamate-containing substrate, the enzyme is converted to an active epoxidase. This regulatory mechanism prevents the generation of a highly reactive vitamin K intermediate in the absence of a substrate for carboxylation.
Keywords: vitamin K 2,3-epoxide; γ-carboxyglutamic acid
The vitamin K-dependent γ-glutamyl carboxylase catalyzes the posttranslational conversion of glutamic acid to γ-carboxyglutamic acid in precursor proteins that contain the appropriate γ-carboxylation recognition site (γ-CRS) within the propeptide of the precursor (1–3). These proteins include the vitamin K-dependent proteins that support blood coagulation, including factor VII, factor IX, factor X, and prothrombin, and its regulation, including protein C and protein S (4). All of these proteins require this posttranslational modification for biological activity. Two bone proteins, osteocalcin and matrix Gla protein, and Gas6, a recently described vitamin K-dependent protein with homology to protein S (5), also contain the γ-CRS and are γ-carboxylated.
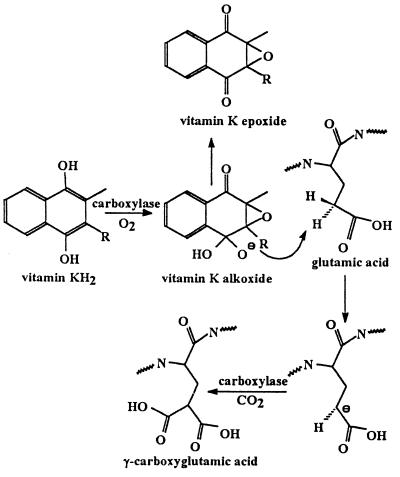
Partially purified preparations of the vitamin K-dependent carboxylase catalyze the conversion of vitamin KH2 to vitamin K 2,3-epoxide. The bulk of evidence suggested that the two activities, γ-glutamyl carboxylation and vitamin KH2 epoxidation, reside in the same enzyme (6–8). The recent availability of purified vitamin K-dependent carboxylase (9, 10) provided the opportunity to demonstrate conclusively that the vitamin K-dependent carboxylase is also a vitamin K epoxidase (11). Strong evidence exists that an active oxygenated species of vitamin K abstracts a hydrogen from the γ-carbon of glutamic acid, with subsequent collapse of the activated vitamin K species to vitamin K epoxide and the addition of CO2 to the γ-carbon of glutamic acid (see Fig. 1 and refs. 6–8). Mechanisms involving either free radical abstraction of a γ-hydrogen (12–14) or formation of a carbanion at the γ-carbon (15, 16) have been proposed. Oxygen and vitamin KH2-dependent exchange of tritium from tritiated water to the γ-carbon of a glutamic acid in a carboxylase substrate at low CO2 concentrations can be taken as evidence of a carbanion mechanism (17). By using a nonenzymatic model, a “base strength amplification mechanism” has been proposed to explain the conversion of vitamin KH2 into an oxygenated intermediate of sufficient basicity to abstract a proton from the γ-carbon of a glutamyl residue in a carboxylase substrate (refs. 18–20 and Fig. 1). This reaction scheme suggests that epoxidation of vitamin KH2 by the vitamin K-dependent carboxylase can occur in the absence of formation of γ-carboxyglutamic acid but that carboxylation cannot occur in the absence of epoxidation. In vitro reaction conditions can be manipulated, for example, by limiting CO2 concentrations, to uncouple epoxidation from carboxylation and achieve rates of epoxidation 5- to 10-fold higher than the rate of carboxylation (13, 21). Carboxylation without concomitant oxidation of vitamin KH2 has never been observed. By using purified recombinant bovine vitamin K-dependent carboxylase free of contaminating glutamate containing substrates or propeptide, we demonstrate, to our knowledge, for the first time that the vitamin K-dependent carboxylase efficiently catalyzes the conversion of vitamin KH2 to vitamin K 2,3-epoxide only in the presence of propeptide and glutamate-containing substrate, indicating a regulatory mechanism that activates the vitamin K epoxidase only in the presence of substrate.
Figure 1.
Reaction scheme for formation of γ-carboxyglutamic acid and vitamin K epoxide. The vitamin K dialkoxide is the hypothetical very strong base proposed by Dowd et al. (20) that abstracts a proton from the γ-carbon of glutamic acid.
EXPERIMENTAL PROCEDURES
Materials.
Vent DNA polymerase and restriction endonucleases were from New England Biolabs. pGEM-7Zf(+) was from Promega. pED and CHO-Dukx-B11 were gifts from Genetics Institute (Cambridge, MA). Lipofectin reagent was from BRL, ECL-Western blotting detection reagents and Hyperfilm-ECL were from Amersham, anti-FLAG1 M2 monoclonal antibody (where FLAG has the sequence DYKDDDDK) and FLAG-bacterial alkaline phosphatase were from Kodak, and peroxidase-conjugated goat anti-rabbit Ig and peroxidase-conjugated goat anti-mouse Ig were from DAKO. Vitamin K1 (10 mg/ml), obtained from Abbott, was chemically reduced with 8 mg of NaBH4. Phe-Leu-Glu-Glu-Leu (FLEEL) and l-α-phosphatidylcholine (type V-E) were from Sigma. NaH[14C]O3 was from Amersham. proPT28, proFIX18 (YVFLDHQDADANLILNRPKR, residues −18 to −1 of human proFactor IX), proPT28γγ (HVFLAPQQARSLLQRVRRANTFLγγVRK), and Phe-Leu-Gla-Gla-Leu (FLγγL) were synthesized by using Fmoc/N-methylpyrrolidone chemistry on an Applied Biosystems model 430A peptide synthesizer. The crude deprotected peptide was purified by HPLC. The peptide sequences were confirmed by direct sequence analysis and the molecular weights confirmed by mass spectral analysis. Partially purified bovine liver carboxylase was prepared as described (2).
Preparation of Soluble FLAG-Bovine Carboxylase.
Construction of the expression vector for FLAG-carboxylase, transfection of CHO cells, and expression of FLAG-carboxylase have been described (22). Cells from a stable colony producing the highest level of enzyme were selected stepwise with increasing concentrations of methotrexate, up to 0.15 μM, to augment carboxylase production. CHO cells expressing FLAG-carboxylase were grown in 500-cm2 tissue culture dishes with MEM [αMEM/10% (vol/vol) fetal bovine serum/2 mM l-glutamine/10 mM Hepes/penicillin (1 unit/ml)/streptomycin (100 μg/ml)] containing 11.1 μM vitamin K and 0.15 μM methotrexate. Confluent cells (10 tissue culture dishes) were harvested with PBS (2.7 mM KCl/1.5 mM KH2PO4/137 mM NaCl/6.5 mM Na2HPO4) containing 5 mM EDTA and washed twice with 200 ml of PBS. To prepare microsomes, approximately 3 × 108 cells resuspended at a density of 1 × 108 cells per ml in PBS/20% (vol/vol) glycerol containing PIC [2 mM dithiothreitol/2 mM EDTA/leupeptin (0.5 μg/ml)/pepstatin A (1 μg/ml)/aprotinin (2 μg/ml)] were homogenized with 30 strokes of a Potter tissue grinder (4 ml, Kontes) with the Teflon pestle attached to a Con-Torque power unit (Eberbach, Ann Arbor, MI). The homogenate was subjected to centrifugation at 900 × g for 5 min and the supernatant was recovered. The homogenization and centrifugation procedures were repeated twice more by using the postcentrifugation cell pellets. The combined supernatants from the three centrifugations were subjected to centrifugation at 150,000 × g for 1 hr at 4°C. The post-centrifugation pellet containing the microsomes was resuspended in 3 ml of PBS/20% glycerol/PIC. The resuspended microsomes were diluted with an equal volume of PBS/20% glycerol/1% CHAPS/0.2% phosphatidylcholine (PC). The solution was placed in an ice bath and subjected to two 5-sec pulses from an Ultrasonic Processor W-220 fitted with a microprobe (Heatsystems-Ultrasonics). After incubation at 4°C for 30 min, the solution was subjected to centrifugation at 150,000 × g for 1 hr at 4°C. The postcentrifugation supernatant was recovered and the pellet containing unsolubilized enzyme was resuspended to 1 ml with PBS/20% glycerol and solubilized as before by addition of an equal volume of PBS/20% glycerol/1% CHAPS/0.2% PC and sonication. The solubilized enzyme was recovered by centrifugation as before and the combined postcentrifugation supernatants stored at −80°C.
Purification of FLAG-Bovine Carboxylase by Affinity Chromatography.
Chromatography was carried out at 4°C. Anti-FLAG M2 monoclonal antibody affinity resin (1.5-ml packed volume) was washed twice with 20 ml of PBS/20% glycerol/0.5% CHAPS. The washed resin was incubated with 5 ml of solubilized enzyme for 2 hr with gentle shaking. The loaded resin was packed in a chromatography column (1.0 × 10 cm) and unbound material was allowed to flow through the column. The resin was washed five times with 10 ml of PBS/20% glycerol/1 mM EDTA/0.5% CHAPS/0.2% PC and once with 10 ml of PBS/20% glycerol/0.5% CHAPS/0.2% PC. The bound FLAG-carboxylase was eluted by sequential addition of 6 ml of PBS/20% glycerol/0.5% CHAPS/0.2% PC containing FLAG-peptide at 5, 10, 20, 50, 75, 100, 150, 200, 300, and 400 μg/ml. At each step the column bed was incubated with the eluting buffer for 15 min. The isolated enzyme was stored at −80°C.
Western Blotting and Quantitation of FLAG-Carboxylase.
Anti-FLAG M2 monoclonal antibody was used to detect FLAG-carboxylase. Proteins separated in a SDS/10% polyacrylamide gel were transferred to a poly(vinylidene difluoride) membrane, and the membrane was blocked with 5% nonfat dry milk/0.05% Tween 20 in PBS (PBS/T). Membranes were incubated with anti-FLAG M2 monoclonal antibody (1.5 μg/ml) in PBS/T at 4°C overnight. Membranes were then incubated with peroxidase-conjugated goat anti-mouse Ig (0.2 μg/ml) in PBS/T at room temperature for 1 hr. Bound antibodies were detected with the ECL detection kit (Amersham) followed by autoradiography on Hyperfilm ECL (Amersham). Quantitation of FLAG-carboxylase concentration was performed as described (22).
Assay of Carboxylase Activity.
The amount of 14CO2 incorporated into the peptide substrates FLEEL (3.6 mM) or proPT28 (25.0 μM) over 30 min by FLAG-carboxylase was measured in reaction mixtures of 125 μl containing 25 mM Mops (pH 7.0), 0.5 mM NaCl, 0.16% CHAPS, 0.16% PC, 8 mM dithiothreitol, 222 μM chemically reduced vitamin K hydroquinone (vitamin KH2), and 1.4 mM NaH14CO3 (10 μCi; 1 Ci = 37 GBq; Amersham). When FLEEL was used as a substrate, ammonium sulfate (0.8 M), which is known to stimulate carboxylase activity, was included in the assay. ProFIX18 (64 μM) was included as indicated. All the assay components except carboxylase were prepared as a master mixture. The reaction was initiated by adding the master mixture to purified FLAG-carboxylase. Incorporated 14CO2 was assayed as described (2). The concentration of endogenous CO2 in the reaction mixture was determined by the method of Rose (23) as modified by Larson (21).
Assay of Vitamin K Epoxidase Activity.
Epoxidase assays were performed in a 125-μl mixture as described above except that NaH14CO3 was replaced with the same concentration of NaHCO3. Vitamin K epoxide formation was determined as described (24). Briefly, upon completion of the 30-min incubation at 25°C in sealed tubes, the reaction mixture was extracted with 250 μl of ethanol and then with 750 μl of hexane. The organic and aqueous phases were separated by centrifugation at 1000 × g for 10 min. The organic phase was removed and the solvent was evaporated to dryness. The residue was redissolved in 200 μl of methanol. Half of this solution was injected onto a reverse-phase C18 HPLC column (Hypersil ODS, 5 μm, 4.6 × 250 mm; Custom LC, Houston) using a Beckman model 128 HPLC equipped with a Beckman model 168 diode array detector. The column was developed with a mobile phase of 10% dichloromethane/90% methanol, which had been saturated with nitrogen. The flow-rate was 1 ml/min. Vitamin K derivatives were detected at 226 nm and vitamin K epoxide was quantitated by using a purified standard.
Kinetic Studies.
To determine kinetic constants for three substrates, FLEEL, proPT28, and vitamin KH2, the initial rate of 14CO2 incorporation or vitamin K epoxide formation were determined at six or more concentrations of substrate up to 2-fold Km(app). Since FLEEL and vitamin KH2 showed apparent substrate inhibition as reported (11), concentrations of FLEEL and vitamin KH2 were used below the inhibitory concentrations. Fixed concentrations of FLEEL or proPT28 of 3.6 mM and 25 μM, respectively, were used when kinetic parameters were determined for vitamin KH2. Vitamin KH2 was 222 μM when determining kinetic parameters for FLEEL and proPT28. Kinetic constants were determined by nonlinear regression analysis using the Michaelis–Menten equation (deltagraph pro 3, DeltaPoint)
RESULTS
Construction of the FLAG-carboxylase cDNA and expression of the recombinant enzyme in Chinese hamster ovary (CHO) cells has been described (22). A clone of CHO cells stably expressing the FLAG-carboxylase was subjected to stepwise selection with methotrexate to amplify expression of the enzyme. The clone chosen for production of FLAG-carboxylase expressed about 350 ng of enzyme per 106 cells. The specific activity of this carboxylase in CHO cell microsomes for carboxylation of FLEEL is 7 × 107 cpm per mg per 30 min, about 500-fold higher than that of the enzyme in bovine liver microsomes. The carboxylase was purified from CHO cell microsomes by immunoaffinity chromatography using anti-FLAG antibodies covalently bound to Sepharose. Bound carboxylase was eluted with FLAG peptide. The enzyme isolated from the CHO cell microsomes in this single-step procedure has a specific activity for carboxylation of FLEEL of 1.8 × 109 cpm per mg per 30 min, equivalent to the specific activity of isolated bovine carboxylase, 2.0 × 109 cpm per mg per 30 min (9, 10). The enzyme, purified 30-fold from the CHO cell microsomal preparation, is essentially homogeneous by SDS/PAGE analysis and has a molecular weight of 94,000 (Fig. 2). In contrast to highly purified carboxylase prepared by affinity chromatography on resin bearing a peptide containing the γ-CRS and eluted with a similar peptide, the FLAG-carboxylase preparation is not exposed to propeptide.
Figure 2.
SDS/polyacrylamide gel of purified FLAG-carboxylase. Proteins were electrophoresed in a 10% polyacrylamide gel in the presence of SDS and 2-mercaptoethanol and visualized with a silver stain. The positions of migration of molecular weight markers are indicated on the left. Lanes: A, Crude solubilized FLAG-carboxylase; B, FLAG-carboxylase eluted from the anti-FLAG M2 monoclonal antibody column.
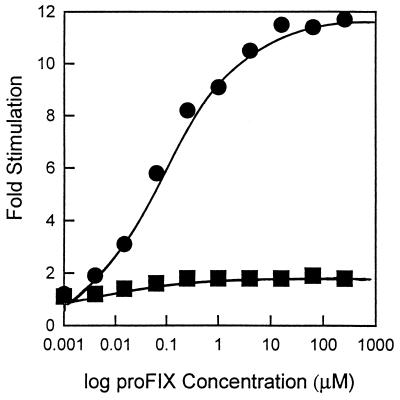
The rate of carboxylation of substrates, such as FLEEL, that do not include a propeptide-like sequence is stimulated 1.5- to 3-fold by the addition of propeptide when crude or partially purified bovine microsomal carboxylase is used (2, 25). The purified FLAG-carboxylase, which is free of contaminating endogenous substrate or propeptide, showed a maximal stimulation of 12- to 28-fold. Half-maximal stimulation occurs at about 0.1 μM proFIX18, 10-fold higher than the 0.01 μM proFIX18 required for maximal stimulation of the partially purified enzyme (Fig. 3). This is consistent with the observation that human microsomal carboxylase from 293 cells, a transformed human embryonic kidney cell line, is stimulated 12.5-fold by the factor X propeptide, but the microsomal enzyme from 293 cells transfected with the cDNA for factor IX was stimulated only 1.9-fold (26).
Figure 3.
Comparison of propeptide stimulation of carboxylation of FLEEL by the bovine microsomal carboxylase and FLAG-carboxylase. The carboxylation of FLEEL was measured under standard assay conditions except that the concentration of proFIX18 was varied as indicated in the figure. Squares, bovine microsomal carboxylase; circles, purified FLAG-carboxylase.
The kinetic parameters for purified FLAG-bovine carboxylase were determined. Substrate inhibition of the carboxylase by FLEEL or vitamin KH2 have been noted (11, 27, 28). Thus, the concentration of FLEEL that inhibits the purified FLAG-bovine carboxylase was determined and initial rates were determined for concentrations of FLEEL that are below the inhibitory concentration. We have demonstrated (22) that the effect of free propeptide concentration and vitamin KH2 concentration on the rate of carboxylation of FLEEL are interrelated. The kinetic parameters for vitamin KH2 epoxidation were determined in the presence of 64 μM proFIX18. Under these conditions substrate inhibition by vitamin KH2 is not observed up to concentrations of vitamin KH2 of 90 μM. For purified FLAG-bovine carboxylase, the Km(app) values for FLEEL, proPT28, and vitamin KH2 are 0.7 mM, 7.8 μM, and 24 μM, respectively. The kcat for carboxylation of FLEEL by the purified FLAG-carboxylase is 1.4 sec−1. The Km(app) value and kcat value for carboxylation of proPT28 by the purified FLAG-carboxylase are 7.8 μM and 0.04 sec−1.
These kinetic parameters for FLAG-carboxylase are equivalent to those previously measured for partially purified bovine microsomal carboxylase, purified bovine and human carboxylase, and microsomal FLAG-carboxylase (2, 11, 22, 29–31). The addition of the FLAG epitope to the amino terminus of the enzyme does not alter its activity as a γ-glutamyl carboxylase or as a vitamin KH2 epoxidase, and the FLAG-carboxylase is not altered by the purification procedure employed. This method of isolation of the vitamin K-dependent carboxylase has significant advantage over those beginning with liver microsomes. Unlike isolation from bovine liver, the isolation of recombinant FLAG-carboxylase from CHO cells provides enzyme of high purity and free of propeptide. In addition, the isolation method is rapid requiring a single day to obtain purified enzyme from a microsomal preparation.
Studies with crude enzyme suggested that FLEEL could stimulate vitamin K epoxide formation (21, 32). In addition, we have recently studied a series of mutant carboxylase enzymes in which two or three charged residues in close proximity of one another in the protein sequence were mutated to alanines (22). Three of these mutant carboxylase species were defective in their ability to bind propeptide. The epoxidase activities of the mutant enzymes are decreased in parallel with carboxylase activity. Thus these data strongly suggest that propeptide- or the glutamate-containing substrate can stimulate epoxidation as well as carboxylation.
The availability of vitamin K-dependent carboxylase free of contaminating glutamate-containing substrate or propeptide afforded a unique opportunity to explore this hypothesis and to determine to what extent vitamin K epoxidation by the carboxylase is regulated by the presence of a carboxylatable substrate or propeptide. Initially we monitored epoxide formation at varying vitamin KH2 concentration in the presence of saturating concentrations of either FLEEL or proPT28. The kcat value is 1.68 sec−1 when FLEEL is the glutamate-containing substrate and 0.08 sec−1 when proPT28 is the glutamate-containing substrate, suggesting that the nature of the glutamate-containing substrate influences the rate of vitamin KH2 epoxidation by the carboxylase.
When FLEEL and bicarbonate are present at saturating levels, the rates of epoxidation and carboxylation by purified FLAG-carboxylase are, as expected, coupled (Table 1 and Fig. 4). Vitamin K epoxide is formed at 100 pmol per min per pmol of enzyme. When both FLEEL and propeptide are omitted from the reaction mixture but the concentrations of vitamin KH2 and oxygen are the same as in the complete reaction mixture, little or no vitamin K epoxide is formed above the background rate in the absence of enzyme. The rate of epoxide formation can be augmented by the addition of FLEEL alone, increasing the rate greater than 5-fold to 9.2 pmol per min per pmol of enzyme. Propeptide also increases the rate of formation of epoxide about 2-fold above the background rate in the absence of enzyme, to 3.4 pmol per min per pmol of enzyme. Similar results were obtained in eight independent experiments. The γ-carboxylated form of FLEEL, Phe-Leu-Gla-Gla-Leu, does not stimulate epoxide production, and stimulation of epoxide production in the presence of proFIX18 and Phe-Leu-Gla-Gla-Leu is essentially that observed with proFIX18 alone. Half-maximal stimulation of epoxidation occurs at 0.5 μM proFIX18, about the same concentration as for half-maximal stimulation of FLEEL carboxylation by proFIX18 (Fig. 5). When proPT28 and bicarbonate are present at saturating levels the rates of epoxidation and carboxylation are, as expected, coupled; vitamin K epoxide is formed at 5.3 pmol per min per pmol of enzyme (Table 2). An analog of proPT28 in which the two carboxylatable glutamic acid residues are replaced by γ-carboxyglutamate can also increase the rate of formation epoxide although the rate, 3.1 pmol per min per pmol of enzyme, is lower than that in the presence of proPT28.
Table 1.
Formation of vitamin K epoxide in the presence of proFIX18 and FLEEL
| Omission(s) | γ-Carboxyglutamic acid, pmol per min per pmol of enzyme | Vitamin K 2,3-epoxide, pmol per min per pmol of enzyme |
|---|---|---|
| None | 116.0 | 100.0 |
| Minus vitamin K | 0.05 | ND |
| Minus FLAG-carboxylase | 0.10 | 1.5 |
| Minus propeptide, minus FLEEL | 0.07 | 1.7 |
| Minus propeptide | 9.0 | 9.2 |
| Minus FLEEL | 0.28 | 3.4 |
Complete reaction mixture contains 64 μM proFIX18, 3.6 mM FLEEL, 222 μM vitamin K, and 1.24 pmol of FLAG-carboxylase in a reaction volume of 125 μl. NaH14CO2 is added to 1.54 mM and is diluted 2.4-fold by endogenous CO2 as determined by the method of Larson et al. (21). The pmol of γ-carboxyglutamic acid formed has been corrected to reflect this dilution of the C14 labeled CO2. ND, not detectable.
Figure 4.
Regulation of epoxide formation. The formation of γ-carboxyglutamic acid (solid bars) and vitamin K epoxide (shaded bars) in pmol per min per pmol of carboxylase is shown in the presence and absence of either proFIX18 or FLEEL.
Figure 5.
Propeptide stimulation of vitamin K epoxidation. The epoxidation of vitamin KH2 was studied under standard reaction condition except that FLEEL was omitted from the reaction mixture and the concentration of proFIX18 was varied as shown in the figure.
Table 2.
Formation of vitamin K epoxide in the presence of proPT28
| Omission | γ-Carboxyglutamic acid, pmol per min per pmol of enzyme | Vitamin K 2,3-epoxide, pmol per min per pmol of enzyme |
|---|---|---|
| 5.1 | 5.3 | |
| Minus vitamin K | 0.015 | ND |
| Minus FLAG-carboxylase | 0.03 | 0.56 |
| Minus proPT28 | 0.03 | 0.70 |
Complete reaction mixture contains 25 μM proPT28, 222 μM vitamin K, and 1.24 pmol of FLAG-carboxylase in a reaction volume of 125 μl. NaH14CO2 is added to 1.54 mM and is diluted 2.4-fold by endogenous CO2 as determined by the method of Larson et al. (21). The pmol of γ-carboxyglutamic acid formed has been corrected to reflect this dilution of the C14-labeled CO2. ND, not detectable.
DISCUSSION
We have recently created and expressed a form of recombinant bovine vitamin K-dependent γ-glutamyl carboxylase that bears the FLAG epitope on the amino terminus (22). In the current work, the FLAG-carboxylase has been isolated by affinity chromatography using an monoclonal antibody directed against the FLAG epitope. The modified recombinant enzyme behaves equivalently to the wild-type enzyme with regard to kinetic parameters of carboxylation of several glutamate-containing peptide substrates and to epoxidation of vitamin KH2. This carboxylase preparation is essentially homogeneous and is free of contaminating endogenous substrate and free of contamination by any propeptide of a vitamin K-dependent protein substrate. This purified FLAG-carboxylase provides, to our knowledge, the first opportunity to explore the relationship of carboxylation to epoxidation while controlling the concentrations of all of the substrates and known modulators of carboxylase function independently.
The propeptide of the vitamin K-dependent proteins has been previously demonstrated to play a dominant role in recognition of the glutamate-containing substrate by the vitamin K-dependent carboxylase. Previously proposed mechanisms for enzymatic oxidation of vitamin KH2 to vitamin K epoxide and the formation of γ-carboxyglutamic acid from glutamic acid (for example, see Fig. 1) do not include a role for the glutamate-containing substrate in the process of oxygen reduction and epoxidation of vitamin KH2. However, the data presented herein indicate that little or no carboxylase-catalyzed formation of vitamin K epoxide occurs in the absence of a vitamin K-dependent protein propeptide or a glutamate-containing substrate. Several studies, performed with detergent solubilized microsomal carboxylase preparations, suggest that the glutamate-containing substrate can stimulate epoxidation (21, 32). The extent to which the propeptide and glutamate containing substrate regulate the epoxidase activity of the enzyme could not be observed in these earlier experiments as endogenous substrate was always a contaminant of the enzyme preparation. It has further been suggested that epoxidation will proceed in the absence of carboxylation as evidenced by studies in which carboxylation of endogenous substrate was measured and epoxide production continued at a constant rate after carboxylation of the endogenous substrate was complete (8). However, this observation is likely due to the presence of the carboxylated form of the precursor proteins, still bearing a propeptide. In our experiments proPT28γγ stimulates epoxidation of purified FLAG-carboxylase albeit at a reduced rate from that achieved in the presence of proPT28. The stimulation of epoxidation by proPT28γγ presumably occurs because the γ-CRS-containing region of this peptide can still bind to the carboxylase. Similarly, carboxylated precursor vitamin K-dependent proteins that remain in the reaction mixture are probably capable of supporting continued vitamin KH2 epoxidation by binding to the carboxylase through their propeptides. Thus, interpretation of kinetic data obtained in the presence of endogenous vitamin K-dependent protein precursors may be confounded by the presence of these precursors and their propeptide moieties.
We propose that although the oxidation of vitamin KH2 precedes reaction at the glutamate γ-C-H, vitamin KH2 oxygenation chemistry is not initiated by the enzyme until a carboxylatable substrate is bound. No uncoupling is seen even though γ-C-H cleavage and CO2 fixation occurs after formation of an oxygenated vitamin K intermediate. This would be a mechanism for preventing oxidation of vitamin KH2 to the highly reactive vitamin K intermediate required for proton abstraction from the γ-carbon of glutamic acid in the absence of a suitable carboxylatable substrate. There is precedent for such a microenvironmental switch for electron flow or O2 reduction affected by substrate. Flavoprotein monooxygenase requires the presence of a sulfhydryl containing substrate as well as NADPH and O2 to produce the intermediate hydroperoxide. Uncoupling of the reactions to produce water and H2O2 does not occur, thus, preventing the production of a reactive intermediate in the absence of the substrate on which it acts. The present data do not permit molecular definition of how the vitamin K-dependent substrate affects this switch in enzyme-activated vitamin KH2 epoxidation. It is possible that binding of the propeptide and glutamate-containing substrate effects the binding of vitamin KH2 or that binding of the propeptide and glutamate-containing substrate results in a conformational change in the enzyme, rendering it catalytically competent or of much greater efficiency for vitamin KH2 oxidation. The availability of pure vitamin K-dependent γ-glutamyl carboxylase uncontaminated by substrate or propeptide provides the opportunity to explore these possibilities.
Acknowledgments
We thank Dr. Beth Bouchard for helpful discussion and assistance in preparation of this manuscript. Support for this work was provided by Grant HL-42443 from the National Institutes of Health.
ABBREVIATIONS
- PC
phosphatidylcholine
- FLEEL
Phe-Leu-Glu-Glu-Leu
- γ-CRS
γ-carboxylation recognition site
References
- 1.Jorgensen M J, Cantor A B, Furie B C, Brown C L, Shoemaker C B, Furie B. Cell. 1987;48:185–191. doi: 10.1016/0092-8674(87)90422-3. [DOI] [PubMed] [Google Scholar]
- 2.Ulrich M M W, Furie B, Jacobs M R, Vermeer C, Furie B C. J Biol Chem. 1988;263:9697–9702. [PubMed] [Google Scholar]
- 3.Foster D C, Rudinski M S, Schach B G, Berkner K L, Kumar A A, Hagen F S, Sprecher C A, Insley M Y, Davie E W. Biochemistry. 1987;26:7003–7011. doi: 10.1021/bi00396a022. [DOI] [PubMed] [Google Scholar]
- 4.Furie B, Furie B C. Cell. 1988;53:505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- 5.Manfioletti G, Brancolini C, Avanzi G, Schneider C. Mol Cell Biol. 1993;13:4976–4985. doi: 10.1128/mcb.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suttie J W. Annu Rev Biochem. 1985;54:459–477. doi: 10.1146/annurev.bi.54.070185.002331. [DOI] [PubMed] [Google Scholar]
- 7.Vermeer C. Biochem J. 1990;266:625–636. doi: 10.1042/bj2660625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood G M, Suttie J W. J Biol Chem. 1988;263:3234–3239. [PubMed] [Google Scholar]
- 9.Wu S-M, Morris D P, Stafford D W. Proc Natl Acad Sci USA. 1991;88:4611–4615. [Google Scholar]
- 10.Kuliopulos A, Cieurzo C E, Furie B, Furie B C, Walsh C T. Biochemistry. 1992;31:9436–9444. doi: 10.1021/bi00154a016. [DOI] [PubMed] [Google Scholar]
- 11.Morris D P, Soute B A M, Vermeer C, Stafford D W. J Biol Chem. 1993;268:8735–8742. [PubMed] [Google Scholar]
- 12.Gallop P M, Friedman P A, Henson E M. In: Vitamin K Metabolism and Vitamin K-Dependent Proteins. Suttie J W, editor. Baltimore, MD: University Park Press; 1980. pp. 408–412. [Google Scholar]
- 13.DeMetz M, Soute B A, Hemker H C, Fokkens R, Lugtenberg J, Vermeer C. J Biol Chem. 1982;257:5326–5329. [PubMed] [Google Scholar]
- 14.Slama J T, Satsangi R K, Simmons A, Lynch V, Bolger R E, Suttie J W. J Med Chem. 1990;33:824–832. doi: 10.1021/jm00164a056. [DOI] [PubMed] [Google Scholar]
- 15.Suttie J W, Larson A E, Canfield L M, Carlisle T L. Fed Proc Fed Am Soc Exp Biol. 1978;37:2605–2609. [PubMed] [Google Scholar]
- 16.Dubois J, Gaudry M, Bory S, Azerad R, Marquet A. J Biol Chem. 1983;258:7897–7899. [PubMed] [Google Scholar]
- 17.McTigue J J, Suttie J W. J Biol Chem. 1983;258:12129–12131. [PubMed] [Google Scholar]
- 18.Ham S W, Dowd P. J Am Chem Soc. 1990;112:1660–1661. [Google Scholar]
- 19.Dowd P, Ham S W, Geib S J. J Am Chem Soc. 1991;113:7734–7743. [Google Scholar]
- 20.Dowd P, Hershline R, Ham S W, Naganathan S. Science. 1995;269:1684–1691. doi: 10.1126/science.7569894. [DOI] [PubMed] [Google Scholar]
- 21.Larson A E, Friedman P A, Suttie J W. J Biol Chem. 1981;256:11032–11035. [PubMed] [Google Scholar]
- 22.Sugiura I, Furie B, Walsh C T, Furie B C. J Biol Chem. 1996;271:17837–17844. doi: 10.1074/jbc.271.30.17837. [DOI] [PubMed] [Google Scholar]
- 23.Rose J G. Anal Biochem. 1976;75:358–360. doi: 10.1016/0003-2697(76)90294-3. [DOI] [PubMed] [Google Scholar]
- 24.Roth D A, Whirl M L, Velazquez-Estades L J, Walsh C T, Furie B, Furie B C. J Biol Chem. 1995;270:5305–5311. doi: 10.1074/jbc.270.10.5305. [DOI] [PubMed] [Google Scholar]
- 25.Knobloch J E, Suttie J W. J Biol Chem. 1987;262:1534–15337. [PubMed] [Google Scholar]
- 26.Lingenfelter S E, Berkner K L. Biochemistry. 1996;35:8234–8243. doi: 10.1021/bi9523318. [DOI] [PubMed] [Google Scholar]
- 27.Vermeer C, Soute B A M, de Metz M, Hemker H C. Biochim Biophys Acta. 1982;714:361–365. doi: 10.1016/0304-4165(82)90346-4. [DOI] [PubMed] [Google Scholar]
- 28.Uotila L. Arch Biochem Biophys. 1988;264:135–143. doi: 10.1016/0003-9861(88)90578-4. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard B R, Jacobs M, Ulrich M M W, Walsh C T, Furie B, Furie B C. J Biol Chem. 1989;264:14145–14150. [PubMed] [Google Scholar]
- 30.Wu S-M, Soute B A M, Vermeer C, Stafford D W. J Biol Chem. 1990;265:13124–13129. [PubMed] [Google Scholar]
- 31.Soute B A M, Ulrich M M W, Watson D J, Maddison J E, Ebberink R H M, Vermeer C. Thromb Haemostasis. 1992;68:521–525. [PubMed] [Google Scholar]
- 32.Suttie J W, Geweke L O, Martin G S L, Willingham A K. FEBS Lett. 1980;109:267–270. doi: 10.1016/0014-5793(80)81102-1. [DOI] [PubMed] [Google Scholar]