Abstract
The transforming growth factor β superfamily member, activin, is able to induce mesodermal tissues in animal cap explants from Xenopus laevis blastula stage embryos. Activin can act like a morphogen of the dorsoventral axis in that lower doses induce more ventral, and higher doses more dorsal, tissue types. Activin has also previously been reported to induce neural tissues in animal caps. From cell mixing experiments it was inferred that this might be an indirect effect of induced mesoderm signaling to uninduced ectoderm. Here we demonstrate directly that neural tissues do indeed arise by the action of induced mesoderm on uninduced ectoderm. Dorsal mesoderm is itself subdivided into posterior and anterior domains in vivo, but this had not been demonstrated for induced mesoderm. We therefore tested whether different concentrations of activin recreate these different anteroposterior properties as well. We show that the anteroposterior positional value of induced mesoderm, including its neuroinductive properties, depends on the dose of activin applied to the mesoderm, with lower doses inducing more posterior and higher doses giving more anterior markers. We discuss the implications of these results for patterning signals and the relationship between anteroposterior and dorsoventral axes.
The anteroposterior (A-P) neural axis in vertebrates is thought to be determined by signals from the dorsal mesoderm, often referred to as the Spemann Organizer, which comes to underlie it during gastrulation. Local A-P properties of dorsal mesoderm at the neural plate stage are clearly illustrated by the regionalized neural-inducing ability of portions of dorsal mesoderm taken from different positions along the presumptive A-P axis (1). The mechanisms leading to the regionalization of dorsal mesoderm, however, are still very poorly understood. Presumably A-P axial information is imparted to tissue that is forming dorsal mesoderm as a consequence of the processes that initially generate asymmetries in the embryo: the localization of information following cortical rotation after fertilization and the inductive signals from the adjacent presumptive endoderm (5, 6). Thus, it is tightly linked to dorsoventral (D-V) pattern formation. This problem is a difficult one to study in vivo because it occurs in a relatively small region of the embryo involving both large fragile (endodermal) cells and highly motile gastrulating (mesodermal) cells in a complex three-dimensional arrangement.
The goal of this study has been to gain some insight into the events generating A-P regionalization of the mesoderm by exploiting tools previously applied to the problem of D-V patterning. Specifically, activin and related members of the transforming growth factor β (TGF-β) family have been shown to be crucial in D-V patterning in a variety of ways (reviewed in ref. 7). Several members of this family are mesoderm inducers. Activin can act like a morphogen for part of the D-V axis: relatively low concentrations induce ventrolateral mesoderm and progressively higher levels induce mesoderm of progressively more dorsal character (8). Activin initiates further cell interactions resulting in at least five different cell fates separated by sharp activin dose thresholds (8–10). Expression in vivo of a dominant-negative activin type IIB receptor (11) abolishes formation of dorsal and lateral mesoderm (12), implying action of an activin-like molecule on the dorsal side of the embryo. The same mutant receptor also blocks the ventralizing and epidermalizing activity of TGF-β superfamily member BMP4 (11, 13). BMP4 is expressed zygotically (i.e., after midblastula stages) on the ventral side of the embryo and has been implicated in ventralization of mesoderm and epidermalization of ectoderm (11, 14). However, it is possible to block activin alone: a new activin receptor dominant-negative mutant, comprising only the extracellular domain, has been shown to cause dorsal disruptions without affecting ventral patterning or significantly reducing BMP4 responsiveness (15). In addition, an apparently activin-specific activation sequence has been found in the promoter of the Organizer-specific gene goosecoid (gsc) (16). Taken together, these experiments demonstrate an essential role for activin(s) or very similar molecules in D-V, and especially dorsal, patterning. For historical and practical reasons, most experimental work has been done with activins A and B. However, related molecules, such as Vg1, nodal-related genes Xnr1-3, and activins C and D, might in fact perform or share these functions in vivo (17–20). In as far as these factors are active in inducing assays at all (some are more active than others), the qualitative differences between them are subtle. Until the difficult issues of molecular specificity in vivo are definitively addressed, activin A and B remain the most useful reagents for studying patterning by this class of molecules as a whole.
Activins have also been proposed to have a role in A-P patterning (21). Animal caps treated with three different doses of partially purified activin induced different host neural structures and different levels of a homeodomain gene xhox3. Subsequently, a detailed study of several mesodermal markers showed a ventro-posterior to dorso-anterior trend of inducing effects with increasing activin dose (9). Comparing these data with the D-V data presented above led to the idea of a dorso-anterior to postero-ventral oblique axis patterned by a gradient of activin (see ref. 22). However, this idea has never properly been tested. Specifically, how does activin-induced D-V positional value vary with respect to activin-induced A-P positional value? The high resolution offered by the dissociated cell protocol of Green et al. (9) provided means to approach this issue. We analyzed the positional value of induced mesoderm in two ways. First a direct measure of the A-P character of mesoderm induced by different concentrations of activin could be assayed by examining genes expressed at different A-P positions in dorsal mesoderm. The second approach involved an indirect assay, monitoring the neural-inducing ability of mesoderm induced with different doses of activin. Mesoderm induced with different doses of activin was combined with ectoderm from the animal cap of gastrula stage embryos and later assayed for neural markers induced in the ectoderm that are characteristic of various positions along the A-P axis. Results from both kinds of experiment show that, within the range of concentrations of activin that induce dorsal mesoderm, relatively low levels induces mesoderm with a more posterior character, whereas progressively higher levels induce mesoderm with a progressively more anterior character.
MATERIALS AND METHODS
Embryo and Cell Culture.
Adult Xenopus laevis were obtained from Xenopus I (Ann Arbor, MI), and embryos prepared as described (23). Embryos were cultured in 0.1× normal amphibian medium (NAM) (24). When required, Fldx lineage label (Molecular Probes) was injected into one-cell embryos (25).
Cells to be treated with activin were isolated from animal caps of stage 8 embryos (26) in Ca2+/Mg2+-free medium as described (22). Dishes of dispersed cells (each containing cells from ≈15 embryos) were exposed to activin at concentrations ranging from 1 to 64 units/ml. Activin A was partially purified from conditioned medium from CHO cells transfected with the human activin β-A gene (9). A bioassay was used to standardize activin, with 1 unit/ml (typically ≈5 pM) being the minimum concentration required to induce elongation of animal caps (27, 28). After 1 hr of incubation each dish of cells was reaggregated by centrifugation at 165 × g, washed and allowed to incubate until control embryo stage 10–10.5. Aggregates monitored for mesodermal gene expression were cultured further as described below. Those used in experiments assaying the neural-inducing ability of aggregates, were cut into pieces, sandwiched between two lineage-labeled animal caps from stage 10.5–11 embryos so that the mass of mesoderm and ectoderm was approximately equal (approximately two cap equivalents), and cultured as described below.
Assays for A-P Regionalization in Dorsal Mesoderm.
Reaggregates cultured alone for mesodermal tissue analysis were harvested at control stage 40. Tissues were fixed overnight in 4% paraformaldehyde, dehydrated, embedded in paraffin, and sectioned at 10 μm. Slides were processed for immunofluorescent detection of the primary antibodies 12/101 for muscle (ref. 29; obtained from the Developmental Biology Hybridoma Bank, Iowa City) and MZ15 (30) (a gift from F. Watt). Expression of the Xenopus homologue of the mouse brachyury gene, Xbra (31), gsc (32), and Xsox2 (R.M.G., K. Gulding, and C. Zygar, this work and unpublished data) in whole embryos was monitored by in situ hybridization (33). For double-staining embryos, Digoxigenin-labeled gsc antisense probes were made from SmaI-cut pGSC2 transcribing with T3 RNA polymerase to yield a 525-base RNA; BM Purple (Boehringer Mannheim) was used as the alkaline phosphatase substrate for this probe. Fluorescein-labeled Xbra probes were made from EcoRV-cut pXT1 transcribing with T7 polymerase, producing a 2,210-base RNA probe; 5-bromo-4-chloro-3-indoyl phosphate p-toluidine salt was used as the alkaline phosphatase substrate for this probe (33). Digoxigenin-labeled Xsox2 probes were made from XbaI-cut pXSox2 transcribing with T7 RNA polymerase yielding a probe of 1,115 bases. RNA levels in reaggregates, harvested at stage 20, were determined by RNase protection assays with probes for Xbra, gsc, and EF1-α as described by Green et al. (9).
Neural induction in recombinants of reaggregates of activin-induced cells and animal caps were monitored in several ways. The 2G9 antibody (34) (kindly provided by E. A. Jones) is a pan-neural marker, though it reacts far less intensely with forebrain than with more posterior neural tissues. Binding was monitored with a rhodamine detection system in sections of reaggregates that had been harvested at stage 40 and processed as described above. Animal cap ectoderm was Fldx-labeled, as mentioned above, to delineate mesoderm and ectoderm. An affinity purified polyclonal antibody against Hox-B9 protein (ref. 35; kindly provided by C. Wright and T. Doniach) and a monclonal antibody against the engrailed gene product, En (ref. 36; Developmental Biology Hybridoma Bank) were also used. For detection of Hox-B9 and En, recombinants were harvested at stage 30 for whole mount immunohistochemistry as described by Doniach et al. (37). These recombinants were sectioned after staining.
RNase protection was used to assay neural markers in recombinants, all harvested at st. 30. The HoxB-9 probe is described by Green et al. (9). Probe for engrailed was made from a 301-bp PstI-SacI (371–672) fragment of Xenopus engrailed 2 (GenBank accession no. X62973) cloned into pBluescript SK, linearized, and transcribed to give a probe length 436 bases (construct a gift of A. Hemmati-Brivanlou and R. Harland). The probe for the Xotx-2 gene was made from an ≈200-bp NotI–EcoRV fragment of pOTX30.1 cDNA cloned into pBluescript SK linearized by NotI and transcribed to give a probe length of about 250 bases (T. Lamb, S. Stachel, and R. Harland, personal communication). A 220-base probe for Xsox2 was prepared from plasmid Xsox2.s2 (R.M.G., K. Gulding, and C. Zygar, unpublished work), yielding a protected fragment of 145 bases.
RESULTS
Experimental Design.
The procedures used here for assessing the proposal that a concentration range of activin can induce dorsal mesoderm with posterior to anterior character were based on those of Green et al. (9). As illustrated in Fig. 1, animal caps were isolated from blastula embryos [stage 8 of Nieuwkoop and Faber (26)] when this tissue is highly competent to form mesoderm in response to activin treatment. Untreated caps form only ciliated epidermis. Cells were dissociated from caps by treatment in Ca2+/Mg2+-free medium and incubated with different concentrations of activin. Single cells are used in this assay because they show sharper thresholds for particular responses to activin than do intact animal caps (9, 22). For cells to form differentiated mesoderm, however, they must be reaggregated after treatment. The A-P character of aggregates was assessed either directly, by examining expression of mesodermal markers of different regions of the A-P axis, or indirectly, by monitoring their ability to induce neural markers indicative of different A-P position in gastrula animal caps. Gastrula animal caps were used in the latter experiments because they have a high degree of neural competence but are no longer able to form mesoderm. If caps used in these induction experiments had mesodermal competence there might be a concern that residual levels of activin remaining in aggregates after treatment could induce mesoderm which could in turn induce neural tissue, but not via the route intended in these experiments. When recombinants were to be stained with antibodies to regional markers, embryos used as donors for gastrula animal caps were injected at the one-cell stage with the lineage label Fldx to be able to discern whether markers were expressed in mesodermal aggregrates or neuralized animal caps.
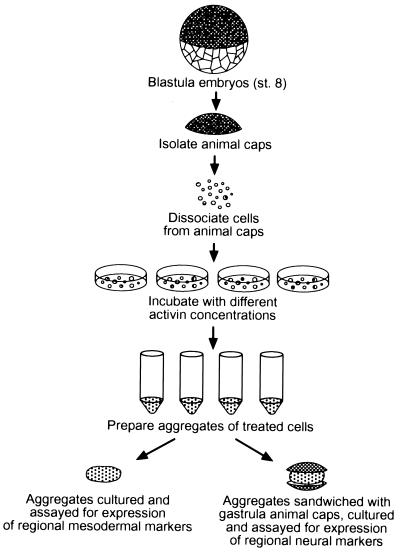
Figure 1.
Assays for A-P character of induced mesoderm. Animal caps were isolated from blastula (stage 8) embryos, and cells were dissociated and incubated with different concentrations of activin. Aggregrates were made by gentle centrifugation and either cultured alone, and assayed subsequently for mesodermal markers, or cultured in combination with gastrula animal cap ectoderm to assay for expression of regional neural markers in this ectoderm (see Materials and Methods).
A-P Character of Activin-Induced Dorsal Mesoderm.
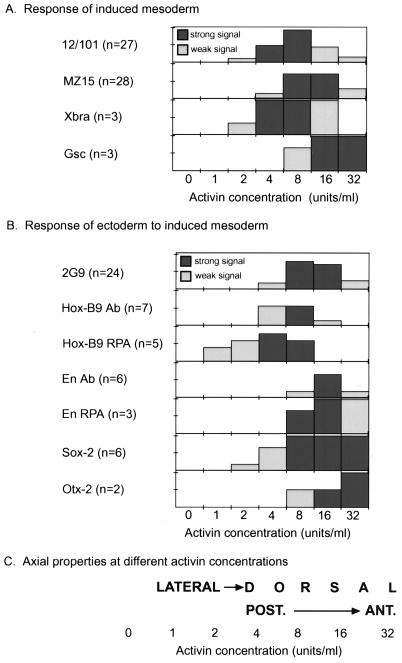
Preliminary experiments were performed to establish the overall dose-response of animal cap cells for forming differentiated mesoderm, as assessed by muscle and notochord formation. As can be seen in Fig. 2, muscle [detected using 12/101 antibodies (29)] is induced primarily at 4–8 units/ml of activin and notochord [detected with MZ15 antibody (30)] primarily at 8–16 units/ml. This confirms the results found by Green and Smith (22) and suggests a concentration range in which to examine A-P character of induced mesoderm, since notochord is not found along the entire A-P axis: it underlies spinal cord and hindbrain and is absent from regions anterior to this.
Figure 2.
Dose-response profiles for activin-induced mesoderm and recombinants of activin-induced mesoderm and gastrula ectoderm. (A) Responses in induced mesoderm. The number of experiments using each marker is indicated in parentheses. Bar heights indicate the percentage of positive cases for each activin concentration (y axis tick marks denote 50% and 100%). Bars filled with dark gray are those in which the response was strong whereas those filled with light gray indicate a weak response. Antibody staining was scored as strong if the positive tissue comprised >10% of the relevant area of tissue section and weak if only a few cells were positive (no intermediate cases). For RNase protection assays, weak and strong are relative terms within a given assay such that that a weak response is about 10% or less of a strong one. (B) Responses of gastrula ectoderm to induced mesoderm (labeling and scoring criteria as in A). (C) Summary of the relationship of activin concentration to the type of mesoderm induced (see text).
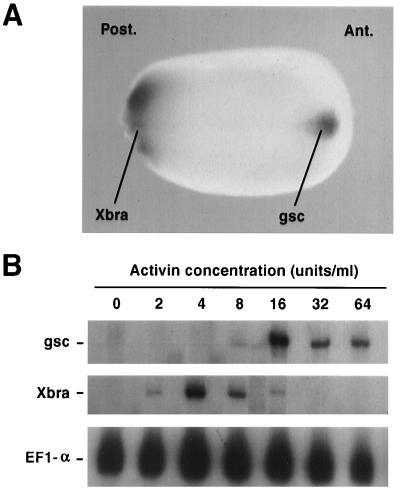
While there are few mesodermal markers with highly discrete expression domains along the A-P axis at the earliest stages at which A-P position is unambiguous, goosecoid (gsc) is one such gene (32). It is expressed during neurula stages in head mesoderm (Fig. 3A), and Xbra (31), a homologue of the mouse Brachyury gene (38), is found in posterior mesoderm at the same stage (Fig. 3A). The expression of gsc observed in Fig. 3A is due to the combined expression of both anterior neural and anterior mesodermal staining in register with one another. Expression in the mesoderm is unambigously seen by examining sections of embryos after in situ hybridization (data not shown). As seen in Fig. 3B and Fig. 2, Xbra is activated in cells treated with 4–8 units/ml of activin, corresponding to levels that also activate notochord formation. However gsc is only activated at much higher concentrations of activin, most strongly at 32–64 units/ml. This is a little higher in absolute units/ml than in previous reports (8), but is consistent it the plus-or-minus 2-fold error inherent in the unit assay is taken into account (27) and was entirely consistent within the batch of activin used for these experiments. Most importantly, the relative induction of responses was very consistent. Thus, within the range of activin concentrations that induce dorsal mesoderm, relatively higher concentrations of activin induce mesoderm with a more anterior character.
Figure 3.
Expression of the mesodermal markers gsc and Xbra in whole embryos and in cell aggregates treated with activin. (A) At late neural plate stages (stage 16 is shown here) up to tailbud stages (data not shown), gsc is expressed in a small area in the anterior of Xenopus embryos. Expression is in anterior regions (Ant.) in both neuroectoderm fated to make forebrain tissue and underlying mesoderm, as confirmed by sectioning of embryos (not shown). Xbra is most strongly expressed at this stage in the posterior (Post.) mesoderm. At slightly earlier neural plate stages it is also expressed in notochord as well, but this becomes undetectable in whole mounts as neurulation proceeds. (B) RNase protection assay of Xbra and gsc expression in aggregates of cells induced with different concentrations of activin (control stage 20). The gsc gene is activated strongly only at the highest concentrations of activin (16–64 units/ml), though transcripts are occasionally detectable, in very low amounts, in aggregates treated with 8 units/ml of activin. Xbra is expressed strongly in aggregates treated with lower doses of activin (4–8 units/ml) and weakly at concentrations just below (2 units/ml) and above (8 units/ml) this level. EF1-α expression is used as a loading control, because it is expressed ubiquitously in Xenopus embryos at the stages used in these experiments (39).
A-P Character of Neural Tissue Formed in Response to Activin-Induced Mesoderm.
Although the activation of posterior and anterior mesodermal markers at relatively low and high doses of activin, respectively, provides some evidence that activin can act as a morphogen determining the A-P axis of dorsal mesoderm, the expression of these markers has not been rigorously shown to be indicator of A-P character in this assay. The markers’ dynamic induction dose-response profiles cannot simply be correlated with expression in vivo (8, 10). To definitively examine induction of Organizer by activin, studies of the neural-inducing properties of this mesoderm were undertaken. Not only are there many more markers available for assaying the A-P axial position in neural tissue than along the A-P mesodermal axis, but these experiments provide a functional test of the A-P character of activin-induced mesoderm.
In our initial experiments, activin-induced aggregrates were combined with competent animal cap ectoderm and the ectoderm then scored for neural tube-like morphology after culture to tailbud stages when neural tissue is well differentiated morphologically. Morphologically differentiated neural tissue was consistently seen in recombinants at the concentrations of activin in which mesoderm might be expected to induce neural tissue (4–32 units/ml; data not shown). To confirm these observations with a definitive neural marker, the 2G9 antibody was used to stain recombinants. This antigen stains most neural tissue, though much less intensely in anterior brain than in spinal cord (34). As can be seen in Fig. 2, staining occurred primarily in recombinants at 8–16 units of activin. This is approximately the concentration range that would be expected to show staining based on morphological responses. Reduced staining in recombinants made with mesoderm induced at 32 units/ml of activin is again consistent with the proposal that high doses of activin are inducing anterior mesoderm, which is then in turn inducing anterior neural tissue in which 2G9 staining is weak. In all cases, all neural markers and morphology were restricted to the indirectly induced cap wraps, and were absent in the cell aggregates, as identified by the lineage labeling (Fig. 4 and data not shown).
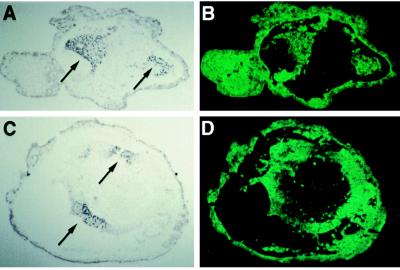
Figure 4.
Restriction to ectoderm of expression of Hox-B9 and En in recombinants of activin-induced mesoderm and gastrula ectoderm. (A) Section through a recombinant reacted with Hox-B9 antibody; mesoderm was induced with 4 units/ml of activin. Horseradish peroxidase reaction product indicative of Hox-B9 expression is labeled with arrows. Nuclear stain is consistent with the role of this gene product as a transcription factor. (B) Lineage-labeling in section from A. Hox-B9-staining cells are labeled with Fldx, indicating that expression of this gene is seen exclusively in the ectodermal portion of the recombinant. (C) Section through a recombinant reacted with En antibody; mesoderm in this recombinant had been induced with 32 units/ml of activin. (One of few cases where strong En staining was seen at this high concentration of activin, illustrating experimental variation even when activin is applied to dissociated cells.) En expression is labeled with arrows. (D) Lineage labeling in section from C. En-staining cells are labeled with Fldx, indicating that expression of this gene is also seen exclusively in the ectodermal portion of the recombinant.
Neural markers with a more definitive localization along the A-P axis were then examined in recombinants. Hox-B9 is expressed in the posterior part of the spinal cord and thus serves as a good posterior neural marker (35). As can been seen in Fig. 2, Hox-B9 antibody staining is most intense in recombinants at 8 units/ml, consistent with our proposal that this dose is inducing mesoderm of a more posterior character. An example showing that staining with Hox-B9 is occurring in the ectodermal part of the recombinant (Fldx-labeled) is shown in Fig. 4 A and B. This is particularly important since Hox-B9 is also expressed in posterior/lateral mesoderm (35). In Fig. 2, RNase protection assays with this probe show a response at lower values than for the antibody staining. This is expected due to some residual expression in the mesodermal part of the recombinant because HoxB9 is expressed in ventral/lateral mesoderm and is known to be turned on in response to relatively low doses of activin (0.9–2.6 units/ml) and persists at least to tailbud stage (9).
A somewhat more anterior neural marker is the engrailed gene product, En, expressed at the mid-brain/hindbrain boundary (36). Staining with an En antibody (Fig. 4 C and D), as well as RNase protection assays, show the most intense response at 16 units/ml (Fig. 2), again consistent with a progressively more anterior character in the mesoderm induced with higher doses of activin.
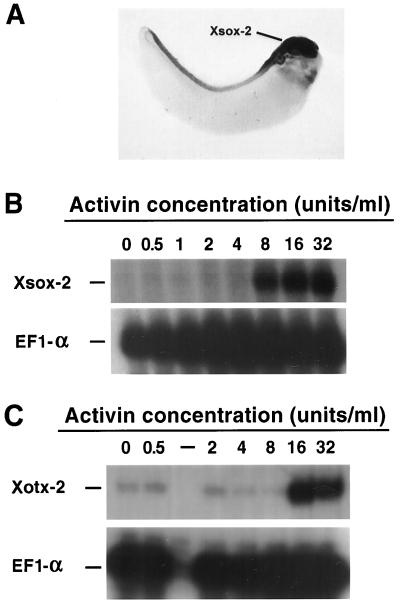
Two markers were used in recombinants to assay mesoderm hypothesized to have a very anterior character. Xsox-2 is a member of the high mobility group-box class of transcriptional regulators that is expressed primarily in forebrain, and to a lesser extent in hindbrain and at low levels in spinal cord (R.M.G., K. Gulding, and C. Zygar, unpublished work). It is highly related in sequence to the mouse Sox-2 gene which has similar expression properties (40). Xotx-2, a homeobox gene thought to be a homologue of the Drosophila orthodenticle gene, is expressed in anterior neural tissue and in ectoderm anterior to the neural plate at neurula stages. It is additionally expressed in underlying mesoderm in register with the ectoderm (41).
As is seen in Figs. 5 and 2, expression of both genes in recombinants indicates that they are activated only at the highest concentrations of activin, consistent with the proposal that high levels of activin induce dorsal mesoderm with anterior character. The highest levels of Xsox-2 were seen in recombinants with mesoderm induced with 16–32 units of activin. In addition, however, there was some signal at lower doses. Because Xsox-2 is expressed in posterior regions of the brain and in the spinal cord at reduced level, the signal at low activin doses may represent low level expression of this gene seen in posterior neural tissue. Xotx-2 expression is only seen in recombinants at high activin doses. We do not know whether all of the expression seen in these recombinants is due to neural expression of Xotx-2 or due to some expression in the mesoderm as well. However, in either case, the expression is extremely anterior and is consistent with the argument that high levels of activin induce mesoderm of dorsal and anterior character.
Figure 5.
Expression and induction of forebrain markers. (A) Whole mount in situ hybridization of stage 30 embryo with Xsox2 probe showing strong forebrain expression. This sample was overstained to show up the weaker neural tube expression (see text). (B and C) RNase protection assays examining expression of Xsox-2 and Xotx-2 in recombinants of activin-induced mesoderm and gastrula ectoderm. (B) Xsox-2 expression is seen in recombinants with mesoderm induced at relatively high levels of activin (primarily at 8–32 units/ml). (C) Xotx-2 expression is seen in recombinants with mesoderm induced at high levels of activin (primarily at 16–32 units/ml). A low level of maternal Xotx-2 is seen in all samples, but is not related to the activin response. EF1-α was used as a loading control.
DISCUSSION
The experiments described here show that there is a dose range of activin that can induce dorsal mesoderm with a wide range of A-P properties, suggesting that activin, or a signaling system related to it, may define A-P character of mesoderm during its formation in the embryo in addition to its proposed role in defining D-V character in mesoderm. While these observations provide some of the first hints about signaling molecules that might play a role in determining the A-P and D-V axes in mesoderm, they raise some questions as well.
One Molecule on Two Axes?
First among the issues that remain unanswered is whether a single molecule of any kind could perform the suggested role initiating patterning processes in both A-P and D-V axes during normal development. It is hard, for example, to see how position in two dimensions can be defined by a single variable in the egg (see ref. 42 for a clear reductio ad absurdam of naive single signal models). However, a simple resolution of this paradox is to separate patterning of the axes by time: one axis is patterned first and the signal source then moves before superimposing further pattern in a second direction on the now-modified responding cells. An example of this is the torpedo/gurken system in Drosophila (43) but there is ample experimental evidence for fixing of pattern in one axis (D-V) before the other (A-P) in amphibians. Maternal cytoplasmic components are crucial for formation of at least some part of ventral and dorsal mesoderm (44, 45). Induction of the remaining dorsal mesoderm by the Nieuwkoop Center in dorsal vegetal cells is initiated during cleavage stages and so presumably involves maternal components as well (46) though the final D-V patterning of mesoderm is not thought to be fixed until gastrula stages (47). There is little information about the regionalization of mesoderm along the A-P axis at pre-gastrula stages, though the gene gsc is activated by early gastrula stage (stage 10.5) in part as a result of maternal cytoplasmic determinants (49), and this gene does have an exclusively anterior domain of expression in neurulae, as we show here. Vodicka and Gerhart (49) report that certain genes expressed in dorsal mesoderm of early gastrulae already show presumptive A-P regionalization (including a more anterior pattern for gsc and a more posterior one for Xbra), suggesting that some A-P patterning has occurred at these stages. However, their work, and a number of other studies (2, 4, 50, 51) argue that A-P patterning in dorsal mesodermal cells is not fixed until neurula stages. Conclusions that emerge are that, while D-V and A-P axes are intimately related (see ref. 22), D-V properties are likely to be fixed prior to A-P properties.
Perhaps a diffusible molecule like activin is produced in the Nieuwkoop Center. Thus, on the dorsal vegetal side of the embryo, higher concentrations of inducer would give mesoderm with dorsal character, whereas at more distant locations ventrally, lower concentrations would induce more ventral mesoderm. From the data presented here, more anterior dorsal mesoderm could be induced at sites close to the Nieuwkoop Center and posterior mesoderm at more distant locations toward the animal pole. The problem, then, is to explain how a single gradient emanating from Nieuwkoop Center at blastula stages both ventrally and toward the animal pole could generate the A-P axis in mesoderm overlying it and not do so in mesoderm more ventral to it, where it must generate D-V pattern. This problem is resolvable because basic D-V pattern is established first while A-P patterning continues into gastrulation stages. Cells from the region of the Nieuwkoop Center do involute along with dorsal anterior mesoderm during gastrulation (49) and could be a continuing source of an activin-like signal resulting in generation of anterior properties in adjacent mesoderm during this period. Such cells are separated from more ventral mesoderm by the developing archenteron, which would then become the barrier preventing this tissue from being signaled to adopt more dorsal or anterior fate. Alternatively, a second source of a molecule like activin could become active during gastrula stages providing the higher levels of activin necessary to generate A-P properties on the dorsal side of the embryo.
Why Is Activin a Relatively Poor Axis Inducer?
A number of experiments have shown that activin, unlike wnt-family molecules and Vg1, is a relatively poor axis inducing molecule (52, 53). Specifically, activin-induced secondary axes or axes rescued in UV-treated embryos lack anterior structures. From this, one would have predicted that activin could not induce functional anterior Organizer capable of inducing anterior neural ectoderm. It is surprising, therefore, that activin is able to induce very anterior neural markers in our assay. Presumably, the difference has something to do with the presentation of the activin molecule to responding cells. Perhaps activin diffusibility plays a role: activin has been shown to be more highly diffusible than other related molecules (54) and this may prevent buildup of sufficiently high local activin concentration by RNA injection. In practice, if one raises the amount of injected RNA, the entire embryo appears to become Organizer-like, gastrulation is totally disrupted and an organized secondary axis never forms (J.B.A.G., unpublished observations). It may also be that injected RNA cannot reproduce the appropriate timing and spatial restriction of synthesis (see above).
Activin or Not Activin?
Our experiments only crudely mimic the situation in vivo where a number of molecules undoubtedly work in concert to generate both D-V and A-P pattern in mesoderm. Even if activin has a very important role, it has to be a member of a cascade of developmental events (see Introduction and refs. 8 and 10). The conundrum of reconciling the D-V and A-P fates with a single source like activin might be resolved if a second molecule localized on the ventral side of the embryo, like a member of the BMP family (e.g., BMP4; see ref. 55), were to modify the effect of the activin-like signal along the D-V axis to differentiate it from the A-P patterning signal propagated toward the animal cap. This, of course, presupposes a pre-existing BMP gradient and relegates activin to an actuator or elicitor of a D-V prepattern. There is, in fact, evidence for such a prepattern in the animal cap (56). Alternatively multiple members of the TGF-β family (whose receptors might all be activated in our experiments with activin) might generate the complex patterning events in both axes. For example, a source in the Nieuwkoop Center could act together with a molecule like TGF-β family member Xnr-3 [which is expressed in surface epithelial cells that come to underlie dorsal mesoderm during gastrulation (57)] to impart the high levels of a common intracellular second messenger required to generate A-P pattern in dorsal mesoderm. It should further be added that a multiple threshold dose response is not unique to activin, but is also found with soluble Vg1 protein (J.B.A.G., unpublished data). Thus, defining the role and identity of any one activin-related molecule in vivo is a nontrivial problem, even though we can be sure that several have crucial roles in the patterning processes under discussion.
Clearly further study of expression and genetic manipulations of TGF-β family members is required to determine whether members of this gene family are involved in the process we describe here. Recently identified activin D (20) is a possible candidate for such a molecule in vivo. Targeted inactivation of the activins A and B gene has no effect on axis formation in mouse embryos (58), although a dominant-negative activin construct does interfere with fish embryo development in a way consistent with our results: low doses inhibit anterior dorsal development, and higher doses result in more posterior defects (59). Perhaps the most dramatic result is that a dominant-negative activin receptor expressed in Xenopus embryos can abolish all mesoderm (12). This mutant receptor blocks more than activin (11, 60), but it is a clear demonstration of the requirement for activin-related molecules in vivo. In a recent paper by Dyson and Gurdon (15) the extracellular domain of an activin receptor acts as a dominant negative mutant in an apparently activin-specific way and generates a dorsally disrupted embryo. This provides to most compelling evidence to date for a role for activin in vivo.
Despite the above-mentioned result, an alternative explanation for the data presented here is that neither activin, nor a related molecule is responsible for generating mesodermal pattern, but that we are fortuitously mimicking another signaling pathway which is responsible for patterning in the embryo. If activin receptors can activate downstream pathways used by other signaling molecules then our experiments may amalgamate the effects of one or more signaling molecules that act in vivo [e.g., adding fibroblast growth factor and Xwnt8 to animal caps gives dorsal mesoderm (61)]. In that case, the properties of activin we see in our experiments might not reflect signaling systems used in vivo for patterning as such (though they may still be required for mesoderm induction), but nonetheless still illustrate the principle that a morphogen-like molecule can act to generate A-P as well as D-V properties in mesoderm and neurectoderm.
Acknowledgments
We thank Kay Gulding and Carol Zygar for technical assistance. This work was supported a Miller Fellowship and Claudia Adams Barr Investigator Award (to J.B.A.G.), by the Howard Hughes Institute (to J.C.S.), and a Wellcome Travel Grant and National Science Foundation grant (to R.M.G.).
ABBREVIATIONS
- A-P
anteroposterior
- D-V
dorsoventral
- TGF-β
transforming growth factor β
References
- 1.Mangold O. Naturwissenschaften. 1933;21:761–766. [Google Scholar]
- 2.Durston A J, Timmermans J P, Hage W J, Hendriks H F, de Vries N J, Heideveld M, Nieuwkoop P D. Nature (London) 1989;340:140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- 3.Sive H L, Hattori K, Weintraub H. Cell. 1989;58:171–180. doi: 10.1016/0092-8674(89)90413-3. [DOI] [PubMed] [Google Scholar]
- 4.Saha M S, Grainger R M. Neuron. 1992;8:1003–1014. doi: 10.1016/0896-6273(92)90123-u. [DOI] [PubMed] [Google Scholar]
- 5.Gerhart, J., Danilchik, M., Doniach, T., Roberts, S., Rowning, B. & Stewart, R. (1989) Development (Cambridge, U.K.) 107 (Suppl.), 37–51. [DOI] [PubMed]
- 6.Nieuwkoop P D. Curr Top Dev Biol. 1977;11:115–132. doi: 10.1016/s0070-2153(08)60744-9. [DOI] [PubMed] [Google Scholar]
- 7.Smith J C. Curr Opin Cell Biol. 1995;7:856–861. doi: 10.1016/0955-0674(95)80070-0. [DOI] [PubMed] [Google Scholar]
- 8.Green J B, Smith J C, Gerhart J C. Development (Cambridge, UK) 1994;120:2271–2278. doi: 10.1242/dev.120.8.2271. [DOI] [PubMed] [Google Scholar]
- 9.Green J B A, New H V, Smith J C. Cell. 1992;71:731–739. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- 10.Wilson P A, Melton D A. Curr Biol. 1994;4:676–686. doi: 10.1016/s0960-9822(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 11.Wilson P A, Hemmati-Brivanlou A. Nature (London) 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- 12.Hemmati-Brivanlou A, Melton D A. Nature (London) 1992;359:609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- 13.New H V, Kavka A I, Smith J C, Green J B A. Mech Dev. 1997;61:175–186. doi: 10.1016/s0925-4773(96)00639-9. [DOI] [PubMed] [Google Scholar]
- 14.Graff J M, Thies R S, Song J J, Celeste A J, Melton D A. Cell. 1994;79:169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- 15.Dyson S, Gurdon J B. Curr Biol. 1997;7:81–84. doi: 10.1016/s0960-9822(06)00030-3. [DOI] [PubMed] [Google Scholar]
- 16.Watabe T, Kim S, Candia A, Rothbacher U, Hashimoto C, Inoue K, Cho K W. Genes Dev. 1995;9:3038–3050. doi: 10.1101/gad.9.24.3038. [DOI] [PubMed] [Google Scholar]
- 17.Thomsen G H, Melton D A. Cell. 1993;74:433–441. doi: 10.1016/0092-8674(93)80045-g. [DOI] [PubMed] [Google Scholar]
- 18.Dale L, Matthews G, Colman A. EMBO J. 1993;12:4471–4480. doi: 10.1002/j.1460-2075.1993.tb06136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotten G, Neidhardt H, Schneider C, Pohl J. Biochem Biophys Res Commun. 1995;206:608–613. doi: 10.1006/bbrc.1995.1086. [DOI] [PubMed] [Google Scholar]
- 20.Oda S, Nishimatsu S, Murakami K, Ueno N. Biochem Biophys Res Commun. 1995;210:581–588. doi: 10.1006/bbrc.1995.1699. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz i Altaba A, Melton D A. Nature (London) 1989;341:33–38. doi: 10.1038/341033a0. [DOI] [PubMed] [Google Scholar]
- 22.Green J B A, Smith J C. Nature (London) 1990;347:391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- 23.Henry J J, Grainger R M. Dev Biol. 1987;124:200–214. doi: 10.1016/0012-1606(87)90472-6. [DOI] [PubMed] [Google Scholar]
- 24.Peng H B. In: Xenopus laevis: Practical Uses in Cell and Molecular Biology. Kay B K, Peng H B, editors. Vol. 36. New York: Academic; 1991. pp. 657–662. [PubMed] [Google Scholar]
- 25.Gimlich R L, Braun J. Dev Biol. 1985;109:509–514. doi: 10.1016/0012-1606(85)90476-2. [DOI] [PubMed] [Google Scholar]
- 26.Nieuwkoop P D, Faber J. Normal Table of Xenopus laevis. Amsterdam: North–Holland; 1967. [Google Scholar]
- 27.Cooke J, Smith J C, Smith E J, Yaqoob M. Development (Cambridge, UK) 1987;101:893–908. doi: 10.1242/dev.101.4.893. [DOI] [PubMed] [Google Scholar]
- 28.Green J B, Howes G, Symes K, Cooke J, Smith J C. Development (Cambridge, UK) 1990;108:173–183. doi: 10.1242/dev.108.1.173. [DOI] [PubMed] [Google Scholar]
- 29.Brockes J P, Kintner C R. Cell. 1986;45:301–306. doi: 10.1016/0092-8674(86)90394-6. [DOI] [PubMed] [Google Scholar]
- 30.Smith J C, Watt F M. Differentiation. 1985;29:109–115. doi: 10.1111/j.1432-0436.1985.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith J C, Price B M J, Green J B A, Weigel D, Herrmann B G. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- 32.Cho K W, Blumberg B, Steinbeisser H, De Robertis E M. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harland R M. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- 34.Jones E A, Woodland H R. Development (Cambridge, UK) 1989;107:785–791. doi: 10.1242/dev.107.4.785. [DOI] [PubMed] [Google Scholar]
- 35.Wright C V, Morita E A, Wilkin D J, De Robertis E M. Development (Cambridge, UK) 1990;109:225–234. doi: 10.1242/dev.109.1.225. [DOI] [PubMed] [Google Scholar]
- 36.Patel N H, Martin-Blanco E, Coleman K G, Poole S J, Ellis M C, Kornberg T B, Goodman C S. Cell. 1989;58:955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- 37.Doniach T, Phillips C R, Gerhart J C. Science. 1992;257:542–545. doi: 10.1126/science.1636091. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann B G, Labeit S, Poustka A, King T R, Lehrach H. Nature (London) 1990;343:617–622. doi: 10.1038/343617a0. [DOI] [PubMed] [Google Scholar]
- 39.Krieg P A, Varnum S M, Wormington W M, Melton D A. Dev Biol. 1989;133:93–100. doi: 10.1016/0012-1606(89)90300-x. [DOI] [PubMed] [Google Scholar]
- 40.Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow P N, Lovell-Badge R. Development (Cambridge, UK) 1996;122:509–520. doi: 10.1242/dev.122.2.509. [DOI] [PubMed] [Google Scholar]
- 41.Blitz I L, Cho K W. Development (Cambridge, UK) 1995;121:993–1004. doi: 10.1242/dev.121.4.993. [DOI] [PubMed] [Google Scholar]
- 42.Slack J. Nature (London) 1991;349:17–18. doi: 10.1038/349017a0. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Reyes A, Elliott H, St. Johnston D. Nature (London) 1995;375:654–658. doi: 10.1038/375654a0. [DOI] [PubMed] [Google Scholar]
- 44.Gurdon J B, Mohun T J, Fairman S, Brennan S. Proc Natl Acad Sci USA. 1985;82:139–143. doi: 10.1073/pnas.82.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakai M. Development (Cambridge, UK) 1996;122:2207–2214. doi: 10.1242/dev.122.7.2207. [DOI] [PubMed] [Google Scholar]
- 46.Gimlich R L, Gerhart J C. Dev Biol. 1984;104:117–130. doi: 10.1016/0012-1606(84)90042-3. [DOI] [PubMed] [Google Scholar]
- 47.Dale L, Slack J M. Development (Cambridge, UK) 1987;100:279–295. doi: 10.1242/dev.100.2.279. [DOI] [PubMed] [Google Scholar]
- 48.Lemaire P, Gurdon J B. Development (Cambridge, UK) 1994;120:1191–1199. doi: 10.1242/dev.120.5.1191. [DOI] [PubMed] [Google Scholar]
- 49.Vodicka M A, Gerhart J C. Development (Cambridge, UK) 1995;121:3505–3518. doi: 10.1242/dev.121.11.3505. [DOI] [PubMed] [Google Scholar]
- 50.Holtfreter-Ban H. Differentiation Capacities of Spemann’s Organizer Investigated in Explants of Diminishing Size. Rochester, NY: Univ. of Rochester; 1965. [Google Scholar]
- 51.Sive H L, Draper B W, Harland R M, Weintraub H. Genes Dev. 1990;4:932–942. doi: 10.1101/gad.4.6.932. [DOI] [PubMed] [Google Scholar]
- 52.Cooke J. Development (Cambridge, UK) 1989;107:229–241. doi: 10.1242/dev.107.2.229. [DOI] [PubMed] [Google Scholar]
- 53.Steinbeisser H, De Robertis E M, Ku M, Kessler D S, Melton D A. Development (Cambridge, UK) 1993;118:499–507. doi: 10.1242/dev.118.2.499. [DOI] [PubMed] [Google Scholar]
- 54.Jones C M, Smith J C. Curr Biol. 1996;6:1468–1475. doi: 10.1016/s0960-9822(96)00751-8. [DOI] [PubMed] [Google Scholar]
- 55.Fainsod A, Steinbeisser H, De Robertis E M. EMBO J. 1994;13:5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sokol S, Melton D A. Nature (London) 1991;351:409–411. doi: 10.1038/351409a0. [DOI] [PubMed] [Google Scholar]
- 57.Smith W C, McKendry R, Ribisi S, Jr, Harland R M. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 58.Matzuk M M, Kumar T R, Vassalli A, Bickenbach J R, Roop D R, Jaenisch R, Bradley A. Nature (London) 1995;374:354–356. doi: 10.1038/374354a0. [DOI] [PubMed] [Google Scholar]
- 59.Wittbrodt J, Rosa F M. Genes Dev. 1994;8:1448–1462. doi: 10.1101/gad.8.12.1448. [DOI] [PubMed] [Google Scholar]
- 60.Schulte-Merker S, Smith J C, Dale L. EMBO J. 1994;13:3533–3541. doi: 10.1002/j.1460-2075.1994.tb06660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christian J L, Olson D J, Moon R T. EMBO J. 1992;11:33–41. doi: 10.1002/j.1460-2075.1992.tb05024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]