Abstract
We previously isolated a novel rat cDNA encoding a basic helix–loop–helix transcription factor named Relax, whose expression in the developing central nervous system is strictly limited to discrete domains containing precursor cells. The timing of Relax expression coincides with neuronal differentiation. To investigate the involvement of Relax in neurogenesis we tested whether Relax activated neural genes in the ectoderm by injecting Relax RNA into Xenopus embryos. We demonstrate that ectopic Relax expression induces a persistent enlargement of the neural plate and converts presumptive epidermal cells into neurons. This indicates that Relax, when overexpressed in Xenopus embryos, has a neuronal fate-determination function. Analyses both of Relax overexpression in the frog and of the distribution of Relax in the rat neural tube strongly suggest that Relax is a neuronal fate-determination gene.
Keywords: basic helix–loop–helix, ectopic expression, neurogenesis, cell fate
Neural fate determination in Drosophila is promoted by a family of proteins encoded by the genes of the Achaete-scute complex (achaete, scute, lethal of scute, and asense) (1) and atonal (2). These genes are known as proneural genes because they determine the competence of ectodermal cells to become neural precursors. Mutations that inactivate proneural genes deplete particular subsets of neural precursors, whereas ectopic expression of proneural genes causes ectopic formation of sensory organs.
Several vertebrate homologs of Drosophila proneural genes have been identified, including the Achaete-scute homologs Mash-1 and Xash-3 and the atonal homologs NeuroD and X-ngnr-1 (3–6). Their involvement in neurogenesis has been tested using inactivating mutations for Mash-1 (7) and ectopic expression in early Xenopus embryos for Xash-3, NeuroD, and X-ngnr-1 (4–6, 8, 9). Microinjection of RNA encoding Xenopus Achaete-scute homolog-3 (Xash-3) into fertilized frog embryos causes a lateral expansion of the neural plate at the expense of the epidermis (5). In addition, NeuroD and X-ngnr-1 are capable of converting most of the embryonic ectoderm into neurons (4, 8). Comparative analyses of the effects of overexpression of these two genes and their expression patterns suggest that the gene products are part of a regulatory cascade in which X-ngnr-1 controls the expression of NeuroD.
Recently, we isolated a novel rat cDNA named Relax, for Rat embryonic longitudinal axis, which encodes a member of the basic helix–loop–helix (bHLH) family of transcription factors and is related to the Drosophila proneural gene atonal (10). The gene has been isolated in the mouse and named ngn3 (11). Relax transcripts are transiently detected in the developing central nervous system (CNS). Their expression is localized in cells of the ventricular zone of the neural tube, where neural progenitors originate and the time of the gene’s expression coincides with neuronal differentiation. This unique expression pattern suggests that Relax is involved in neural fate determination. To investigate further the contribution of Relax to neurogenesis, we tested whether it can activate neural genes in the ectoderm by injecting Relax RNA into Xenopus embryos. The effect of this ectopic expression was analyzed by in situ hybridization and immunocytochemistry with pan-neural and pan-neuronal markers. We demonstrate that Relax induces an enlargement of the neural plate and converts presumptive epidermal cells into neurons. This suggests that Relax behaves, in Xenopus, as a neuronal fate determination gene.
METHODS
In Vitro RNA Synthesis.
Capped Relax RNAs were synthesized from 1 μg of XbaI-linearized pcDNA3, containing the entire coding sequence of Relax, with the T7 RNA polymerase using the mMessage mMachine in Vitro Transcription Kit (Ambion, Austin, TX). Capped XE12 and XASH3 RNAs were transcribed by SP6 and T7 polymerases using template linearized with XbaI and HindIII, respectively. After DNase treatment, RNAs were extracted with phenol/chloroform and precipitated with an equal volume of isopropyl alcohol. RNAs were resuspended in water for injection.
Embryo Injections.
Embryos were dejellied with 2% cysteine 1hr after fertilization and kept at 16°C in NAM 0.1× containing 5% Ficoll, penicillin, and streptomycin until they reached the four-cell stage. Each of the two left blastomeres was injected with 5 nl of mRNA solution using an Eppendorf pressure-driven microinjector (150 pg of mRNA per blastomere). After injection, the embryos were allowed to develop at 18°C to the desired stage.
Whole-Mount Immunocytochemistry.
Embryos were fixed in dimethyl sulfoxide:methanol (20:80), bleached by addition of 10% H2O2 in the fixative medium, and stained with antibodies essentially as described by Dent et al.(12). Samples were incubated with anti-N-CAM and anti-β-tubulin primary antibodies diluted 1:100 and then with biotinylated anti-rabbit IgG for the former and anti-mouse IgG for the latter (second antibody). Vectastain kits (Vector Laboratories) were used for immunohistochemical staining. The embryos were cleared in benzyl alcohol:benzyl benzoate (1:2, vol:vol) prior to photography.
Whole-Mount in Situ Hybridization.
Embryos were fixed in MEMFA medium (0.1 M MOPS, pH 7.4/2 mM EGTA/1 mM MgSO4/3.7% formaldehyde) for 2 hr, stored in ethanol at −20°C, then stained with an N-CAM digoxygenin-labeled riboprobe as described by Harland (13).
RESULTS
We used microinjection into Xenopus embryos to investigate the role of Relax in early neurogenesis. We injected synthetic RNA encoding different bHLH proteins including Relax into the two left blastomeres of four cell-stage embryos. The first cleavage in Xenopus embryos usually defines the bilateral symmetry, and, consequently, such injections restrict the introduced RNA to one side of the developing embryo. The uninjected side can therefore be used as a control for the injected side. The embryos were allowed to develop until various stages. For each RNA species injected, we analyzed at least 40 embryos for every developmental stage investigated. We first followed neural gene expression at the early neurula stage by in situ hybridization using a marker of undifferentiated neural tissue, N-CAM, then by immunocytochemistry with two antibodies, one directed against the N-CAM protein and the other against a neuronal marker, the β-tubulin protein.
Relax, like other bHLH transcription factors, is likely to act as a heterodimer (14) with a ubiquitious bHLH partner such as the protein E12, of which there may be limiting amounts in the embryo (15). For example, Ferreiro et al. (9) have shown that only coinjection and not individual injection of Xash-3 and XE12 significantly induced neural gene expression. We therefore injected either Relax alone or Relax combined with XE12 and compared the effects on the production of N-CAM mRNA and N-CAM and β-tubulin proteins. XE12 was used as a negative control and Xash-3/XE12, as a positive control. Some embryos were coinjected with 1 ng of RNA coding for β-galactosidase, such that the progeny of the injected blastomeres could be followed and toxic effects of the RNA preparations could be assessed. The precise developmental stage of the injected embryos was determined by comparison with uninjected embryos.
Relax Ectopic Expression Induces Early Neural Plate Defects.
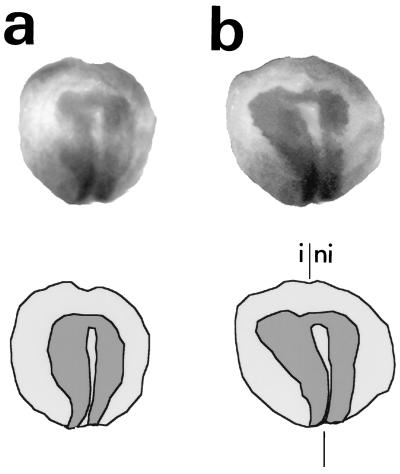
Xenopus embryos injected with Relax/XE12 RNA and uninjected controls were allowed to develop until the neural plate stage (stage 14), when the neural folds first rise. At this stage the neural plate was clearly visualized by in situ hybridization with an N-CAM probe (Fig. 1a). In the injected embryos the neural plate was much larger on the injected side, spreading out laterally (Fig. 1b). N-CAM expression starts at this stage and, thus, could not be detected by immunocytochemistry in the uninjected embryos. Only the area corresponding to the lateral enlargment of the neural plate was labeled by the N-CAM antibody (data not shown). A similar expansion of the neural plate at the expense of the lateral ectoderm was observed in embryos injected in parallel with Xash-3/XE12, a vertebrate proneural gene (data not shown).
Figure 1.
Phenotype of Relax/XE12-injected Xenopus embryos at stage 14. (Top) In situ hybridization of an N-CAM digoxygenin-labeled riboprobe in noninjected (a) and Relax/XE12-RNA-injected (b) embryos. (Bottom) Schematic representation of both embryos; dark gray represents the N-CAM label. The injected side (i) and the noninjected side (ni) of the embryo are indicated. The symmetric axis is represented by two vertical lines. All injected embryos presented a phenotype similar to those shown here.
The Relax/XE12 RNA injection mainly affected the anterior part of the neural plate (Fig. 1b), whereas the posterior part of the neural plate did not seem to be affected. Injection of Relax RNA alone promoted a similar but less pronounced enlargement of the anterior neural plate (data not shown), suggesting a synergy between Relax and XE12 for the induction of neural gene expression. The injection of XE12 alone at the dose used in the Relax/XE12 experiments did not cause any expansion of the neural plate (data not shown). Thus, Relax induces an enlargment of the anterior part of the neural plate.
The Excess Neuronal Tissue Persists During Neurulation.
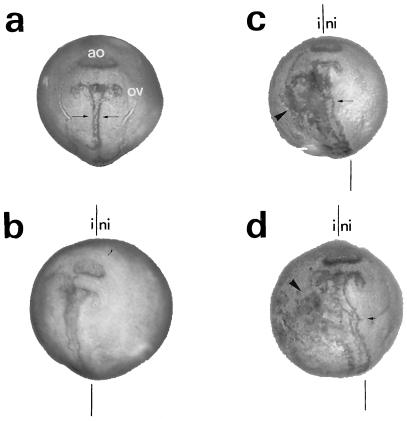
We then examined whether the neural hyperplasia described above persisted throughout neurulation and whether the overexpression of Relax affected a specific neural cell fate. Thus, Relax/XE12 RNA-injected embryos were examined at developmental stage 20. At this stage, the neural tube is almost completely closed, and the primordia of the optic vesicles and of the adhesion organ can be clearly visualized by N-CAM immunostaining (Fig. 2). Large amounts of N-CAM have previously been described at this developmental stage in the primordia of the adhesion organ (16). Similar to stage 14, the N-CAM immunoreactivity was detected at the lateral edge of the neural ectoderm (Fig. 2c). No modification of the primordia of the adhesion organ was observed. β-Tubulin immunostaining revealed that the extra neural tissue was predominantly neuronal (Fig. 2d). In control experiments in which embryos were either not injected or injected with XE12, the neural tube was clearly labeled by N-CAM and β-tubulin antibodies, whereas the lateral margins were not labeled (Fig. 2 a and b; data not shown). In addition, a major distortion of the neural tube was observed following Relax/XE12 RNA injection (Fig. 2 c and d). In summary, neural hyperplasia persisted during neurulation, suggesting that ectopic expression of Relax leads to an irreversible change in cell fate, producing mainly neurons.
Figure 2.
Persistence of the ectopic expression of Relax at the late neurula stage. Immunocytochemistry with N-CAM (a–c) and β-tubulin (d) antibodies (1:100) of noninjected (a), XE12 RNA-injected (b), and Relax/XE12 RNA (c and d) embryos at development stage 20. The adhesion organ (ao), the optic vesicle (ov), and the injected (i) and the noninjected sides (ni) of the embryo are indicated. Arrows indicate neural tube labeling; arrowheads indicate ectopic labeling. The symmetric axis is represented by two vertical lines. All the injected embryos presented a phenotype similar to those shown here.
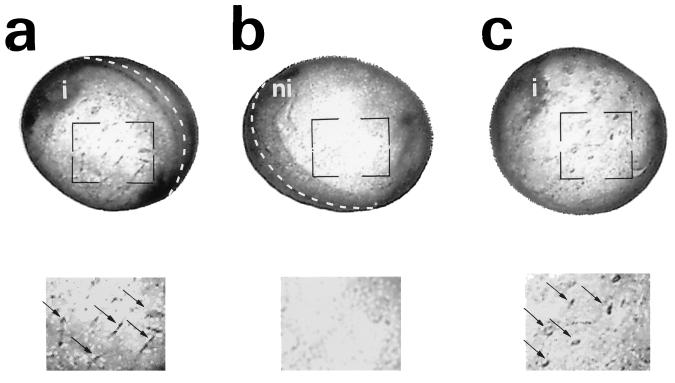
Differentiation of Neural Precursor Cells in the Epidermis at the Late Neurula Stage by Ectopically Expressed Relax.
The stage 20 embryos injected with Relax, as above, at the four-cell stage also showed ectopic N-CAM immunostaining in the lateral (Fig. 3a) and ventral epidermis (Fig. 3c). N-CAM-positive cells generated in the epidermis were scattered (Fig. 3 a and c), and this pattern was first observed in the late neurula stage (stage 20). Similar results were obtained with β-tubulin immunostaining (data not shown), indicating that these ectopic neural cells were mostly neurons. In contrast, no N-CAM-positive cells were observed among epidermal cells in negative controls, including the uninjected side of the embryo, as well as in uninjected and XE12-injected embryos (Fig. 3b; data not shown). Thus, the overexpression of Relax in Xenopus embryos fosters ectopic neurogenesis both in the neuroectoderm and in the lateral and ventral epidermis. Hence, the capacity of Relax to promote a stable overproduction of neural tissue suggests that it has a fate determination function.
Figure 3.
Ectopic N-CAM expression in Xenopus embryos injected with Relax/XE12. Immunocytochemistry with N-CAM antibody (1:100) of Relax-XE12-injected embryos at development stage 20: the injected left side (a), the noninjected right side (b), and the ventral side (c). (Lower) Twofold magnification of the marked area shown in a, b, and c, respectively. The injected (i) and the noninjected (ni) sides of the embryo are indicated. The dotted lines indicate the dorsal midline of the embryo. Open arrows indicate ectopic N-CAM-positive cells. All the injected embryos presented a phenotype similar to those shown here.
DISCUSSION
We have isolated a novel rat bHLH transcription factor named Relax that exhibits the expected features of a neural fate determination gene (10). Relax transcripts are detected in discrete regions of the CNS and only during neuron production. In addition, Relax-expressing cells are strictly localized in the ventricular zone of the neural tube, where neural progenitors originate. In this work we tested the neural fate-determination function of Relax by overexpressing it in Xenopus embryos. Ectopic expression in amphibian embryos is a powerful approach to investigating the role of bHLH transcription factors in early neurogenesis. We show that Relax is able to affect ectodermal cell fate in this model by causing a differentiation of neurons.
At the early neurula stage (stage 14), molecular markers for neural and neuronal cells show that the anterior neural plate expands laterally in Relax-injected embryos. The effect was stronger when Relax was coinjected with the ubiquitious bHLH XE12, whereas injection of XE12 alone had no significant effect. The lateral enlargment of the neural plate induced by the ectopic expression of Relax/XE12 was similar to that resulting from Xash-3/XE12. Turner and Weintraub (5) have shown by in situ hybridization with an N-CAM probe that unilateral overexpression of Xash-3 induces the enlargement of the CNS at the expense of the epidermis in Xenopus embryos at stage 14. This increased number of neural cells is not due to an additional proliferation of neural precursors but rather to Xash-3, which converts additional cells to a neural fate. In our experiment, we cannot exclude that the enlargment of the neural plate subsequent to the Relax injection is not due to an increased proliferation of neuroectodermal cells, although the effect of Relax injection is similar to that of Xash-3.
The effect of ectopic expression of Relax/XE12 at stage 14 persists at least until the late neurula stage (stage 20). In addition, there was a differentiation of neural cells in the entire injected side of the developing embryo, and the whole epidermis contained scattered ectopic neural cells. Similar results have been described after overexpression of either NeuroD (4) or X-ngnr-1 (8). The effect of these two genes on ectopic expression of neural genes in the epidermis was observed at the early neurula stage, whereas Relax induces this phenotype only at a later stage. The ectopic production of neural cells inside the epidermis induced by NeuroD or X-ngnr-1 results from fate conversion rather than an aberrant neural cell migration. In this experiment, the fate conversion affects only dispersed epidermal cells. These cells acquire a phenotype which resembles that generated by Relax/XE12. Although we cannot exclude the possibility that Relax may induce an aberrant cell migration, the common phenotype in the epidermis of Relax, NeuroD, and X-ngnr-1 ectopic expressions suggest that Relax induces a fate conversion. Our data indicate that Relax produces a mixed phenotype that combines the Xash-3, NeuroD, and X-ngnr-1 features. First, ectopically expressed Relax, like Xash-3, causes the neural plate to expand laterally. The second important feature, characteristic of Relax, is its ability to convert the fate of epidermal cells into neurons, as do NeuroD and X-ngnr-1, although the effect is not as pronounced as that of X-ngnr-1. The phenotype obtained after Relax/XE12 overexpression clearly indicates that Relax is able to function in the Xenopus embryo as a neuronal fate–determination gene.
The overexpression of X-ngnr-1 induces ectopic neurogenesis and ectopic expression of endogenous NeuroD mRNA. There is a sequential temporal expression of the two endogenous genes in Xenopus, X-ngnr-1 expression preceding that of NeuroD. Based on these results, Ma et al. (8) conclude that both genes are part of a common regulatory cascade in which X-ngnr-1 is upstream from NeuroD. Furthermore, endogenous X-ngnr-1 expression is strictly localized in the areas where primary neurogenesis occurs in the developing Xenopus embryo. X-ngnr-1 expression also becomes restricted to subsets of cells by lateral inhibition mediated by X-Delta-1 and X-Notch. The results of overexpression studies combined with those obtained by the characterization of the endogenous X-ngnr-1 expression suggest that this gene functions as a vertebrate neuronal determination factor.
Functional analysis in the Xenopus embryo of Relax, NeuroD, and X-ngnr-1 gene products gives information pivotal to our understanding of their functions in the developing rat nervous system. In the rat neural tube, Relax, neurogenin, and NeuroD are all expressed during a similar period. Only Relax and neurogenin are expressed in the ventricular zone, whereas NeuroD is expressed in postmitotic cells contained in the subventricular zone. Furthermore, neurogenin and NeuroD are both localized in the same dorso-ventral domains within the spinal cord and the forebrain. This indicates that neurogenin and NeuroD may participate in a similar regulatory cascade in the developing rat embryo as they do in the frog. Relax-expressing compartments in the CNS are restricted to the ventricular zone, suggesting a similar function in neural fate determination. Our studies on neural gene expression in Xenopus embryos after ectopic expression of Relax clearly demonstrate that Relax acts as a neuronal fate determination factor. As in the case of the neurogenin/NeuroD cascade, the regulatory genes downstream from Relax remain to be identified.
Acknowledgments
We gratefully acknowledge the contribution of Dr. F. Broders to the embryo injection experiments and Dr. V. A. Liepkalns for critical reading of the manuscript. The pSP64Xβm-XE12, XASH3, and N-CAM plasmids were generous gifts from Drs. J. B. Gurdon, W. A. Harris, and J.-F. Riou, respectively. The N-CAM antibody was kindly provided by Dr. G. Levi. This work was supported by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, Rhône-Poulenc Rorer, and the Institut de Recherche sur la Moelle Epinière.
ABBREVIATIONS
- CNS
central nervous system
- bHLH
basic helix–loop–helix
References
- 1.Alonso M C, Cabrera C V. EMBO J. 1988;7:2585–2591. doi: 10.1002/j.1460-2075.1988.tb03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarman A P, Grau Y, Jan L Y, Jan Y N. Cell. 1993;73:1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- 3.Johnson J E, Birren S J, Anderson D J. Nature (London) 1990;346:858–861. doi: 10.1038/346858a0. [DOI] [PubMed] [Google Scholar]
- 4.Lee J E, Hollenberg S M, Snider L, Turner D L, Lipnick N, Weintraub H. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- 5.Turner D L, Weintraub H. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman K, Shih J, Bars J, Collazo A, Anderson D J. Development (Cambridge, UK) 1993;119:211–232. doi: 10.1242/dev.119.1.221. [DOI] [PubMed] [Google Scholar]
- 7.Guillemot F, Lo L C, Johnson J E, Auerbach A, Anderson D J. Cell. 1993;75:1245–1258. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- 8.Ma Q, Kintner C, Anderson D J. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- 9.Ferreiro B, Kintner C, Zimmerman K, Anderson D J, Harris W A. Development (Cambridge, UK) 1994;120:3649–3655. doi: 10.1242/dev.120.12.3649. [DOI] [PubMed] [Google Scholar]
- 10.Ravassard P, Chatail F, Mallet J, Icard-Liepkalns C. J Neurosci Res. 1997;48:146–158. doi: 10.1002/(sici)1097-4547(19970415)48:2<146::aid-jnr7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Sommer L, Ma Q, Anderson D J. Mol Cell Neurosci. 1996;8:221–241. doi: 10.1006/mcne.1996.0060. [DOI] [PubMed] [Google Scholar]
- 12.Dent J A, Polson A G, Klymkowsky M W. Development (Cambridge, UK) 1989;105:61–74. doi: 10.1242/dev.105.1.61. [DOI] [PubMed] [Google Scholar]
- 13.Harland R M. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- 14.Murre C, McCaw P S, Vaessin H, Caudy M, Jan L Y, Jan Y N, Cabrera C V, Buskin J N, Hauschka S D, Lassar A B, Weintaub H, Baltimore D. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 15.Rashbass J, Taylor M, Gurdon J. EMBO J. 1992;11:2981–2990. doi: 10.1002/j.1460-2075.1992.tb05368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi G, Crossin K L, Edelman G M. J Cell Biol. 1987;105:2359–2372. doi: 10.1083/jcb.105.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]