Abstract
Avian plumage has long been used to test theories of sexual selection, with humans assessing the colors. However, many birds see in the ultraviolet (<400 nm), to which humans are blind. Consequently, it is important to know whether natural variation in UV reflectance from plumage functions in sexual signaling. We show that female starlings rank males differently when UV wavelengths are present or absent. Principal component analysis of ≈1300 reflectance spectra (300–700 nm) taken from sexually dimorphic plumage regions of males predicted preference under the UV+ treatment. Under UV− conditions, females ranked males in a different and nonrandom order, but plumage reflectance in the human visible spectrum did not predict choice. Natural variation in UV reflectance is thus important in avian mate assessment, and the prevailing light environment can have profound effects on observed mating preferences.
Ever since the work of Darwin (1), avian plumage coloration has been a major focus for studies of sexual selection (2). Almost without exception, these colors have been assessed, quantified, or manipulated according to human standards (3) even though humans are not the receivers for which the signals evolved. Avian color vision differs from our own in several key ways: most birds see in the UV (to which humans are blind), have at least four spectrally distinct classes of retinal cone cells (compared with only three in humans), and have oil droplets that filter the light reaching the cone photopigments (4, 5). Recent experiments (6) show that female zebra finches (Taeniopygia guttata) avoid males whose plumage lacks UV reflectance and that their preference for symmetry in artificial ornaments (7) extends to leg bands arrangements that are discriminable only in the UV (6). A crucial question remains: Is naturally occurring individual variation in UV reflectance used in mate choice decisions, or is it redundant in information terms and thus ignored, being correlated with reflectance variation in the human visible waveband? We tested these possibilities by forcing female starlings (Sturnus vulgaris) to choose between the same males under conditions in which their natural UV reflectance was either present or absent.
METHODS
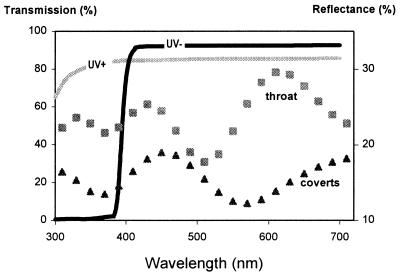
The experiment was conducted on 48 wild-caught adult starlings that were in breeding plumage during spring (8, 9). Male plumage at this time exhibits extensive areas of iridescence, especially on the throat and coverts (8). These regions show variation in reflectance at short wavelengths (Fig. 1), so potential UV cues exist. Mate choice preferences were investigated in an apparatus we have described (6, 7) that is similar to that used in numerous avian mate choice experiments. In brief, a single female visually assesses four males, with her preference indicated by the number of hops (or time) in front of each male (6, 7). By means of transparent filters mounted vertically between the female and each stimulus male, wavelengths available for mate choice decisions were manipulated such that UV wavelengths were available in some trials (UV+) but not others (UV−) (Fig. 1). We used eight pairs of females (n = 16) and eight quartets of males (n = 32), with each female of a pair viewing the same quartet of males in separate trials. All females viewed males under both UV+ and UV− treatments (Fig. 1), so each quartet of males was assessed four times (two females × two treatments). By comparing the preferences of females (within a pair) within filter treatments and across filter treatments, we could determine (i) if the females’ rankings of males were consistent within each filter treatment and (ii) whether they differed when UV wavelengths were removed.
Figure 1.
Left axis and solid lines, transmission spectra of the two filter types (UV+, UV−) used in the experiment. Spectra are the mean of five randomly located measurements on each filter taken with a Unicam Prism spectrophotometer. The two filter types were equalized for transmitted quantal flux (<1% difference in flux 300–700 nm) as measured for the spectral irradiance on a female viewing empty stimulus cages, with lights as described earlier (6). In this way, any preference for UV+ or UV− is unlikely to be a preference for higher or lower quantal flux. A hyper-Graeco Latin square design (10) was used to allocate individual filters to stimulus cages, stimulus cages to positions in the room, and males to individual filters; order of treatment was randomized in a balanced way. Right axis and dotted lines, reflectance spectra of iridescent feathers on two body regions (throat and wing coverts) of male starlings. Spectra are the mean of the 32 bird means taken across 10 randomly located measurements within each body region and are plotted at 20-nm intervals. Measurement details in legend of Fig. 3.
To investigate the choice criteria used by females, we measured a variety of traits on males at the end of the mate choice experiment. Nine morphometric variables that we thought might be reliable indicators of size or body condition were measured: mass, fat score [a 6-point subjective scale (11)], length of tarsis (means of three measurements each on left and right leg), length of 8th primary feathers (mean of three measurements each on left and right wing), asymmetry of tarsi, asymmetry of 8th primary feathers, percentage of throat feathers with white spots, and percentage of covert feathers with white spots (methods as in ref. 12). Starling beaks change from black to yellow during the breeding season (8, 9), so the percentage of upper and lower mandible that was black was scored subjectively to the nearest 10%. The “color” of male plumage was assessed using ≈1300 reflectance spectra (300–700 nm) obtained from a sample of feathers which were removed at the completion of the experiment (details in legend to Fig. 3). The analysis of reflectance spectra has major advantages over traditional methods used for assessing avian coloration (3, 13). Reflectance spectra are the inherent properties of feathers that, with the ambient illumination, determine the raw signal perceived by the avian eye (13); reflectance spectra are not biased by human trichromacy nor humans’ narrow spectral range (3). Second, subtle variation in reflectance spectra can be detected statistically via principal component analysis (13), as used here. Feathers were selected randomly from the throat and wing coverts because both of these regions are revealed during male displays (8, 14) and throat feathers are sexually dimorphic (8).
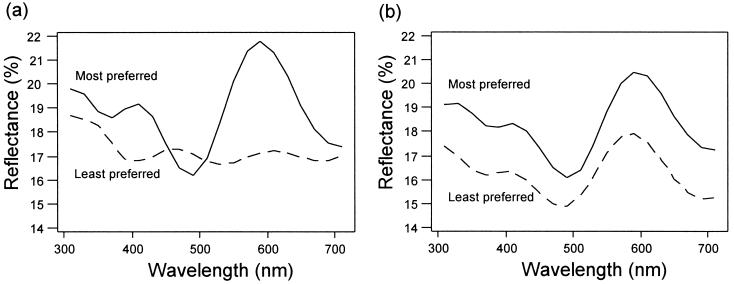
Figure 3.
Reflectance spectra of the iridescent throat feathers of the most preferred and least preferred males under (a) UV+ conditions and (b) UV− conditions. Each spectrum is the mean of the bird means for the eight respective males, calculated from 10 randomly located measurements on the iridescent throat feathers. All feathers were removed without cutting any innervated tissue, by snipping slightly above the base of the feather. Reflectances from these feathers were measured using a Zeiss MCS 230 diode array photometer, with illumination by a Zeiss CLX 111 Xenon lamp. Illumination and measuring fiber optics were held at 45° to the normal by a Zeiss GK 111 goniometer, with illumination from the proximal end of the feather. Spectra were recorded in 1-nm steps from 300 to 700 nm and were expressed relative to a Spectralon 99% white standard. All feathers were mounted on black velvet during measurement to eliminate stray reflections. Each measurement was taken from a 2-mm diameter spot, randomly chosen from within a uniform region of the exposed part of the main body of the feather. Because many feathers also had white or pale brown tips, most feathers were measured twice. To minimize measurement error, a dark current and reference calibration were taken immediately before measuring each feather. Within feathers, regions were randomly allocated for spectrophotometric measurements over time, and feathers from each individual were allocated over time in a randomized block design.
RESULTS AND DISCUSSION
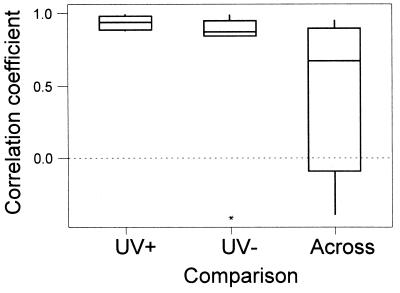
In both UV+ and UV− treatments, the preferences for particular males were highly correlated between females within a pair (Fig. 2). In contrast, the preferences across the two treatments were not significantly correlated. From these effects we can draw the important conclusion that different females rank males in the same way within any one illumination condition but that females rank males differently under UV+ and UV− conditions. The fact that females show consistent preferences under UV− conditions implies that removing UV wavelengths does not totally abolish species or sex recognition, or have a nonspecific stress effect. If removal of UV made the stimulus birds unrecognizable as male starlings or was stressful through creation of “unnatural” or novel viewing conditions, we would expect UV− preferences to tend toward random. Instead, removing the UV did not abolish preference but changed the criteria for choice.
Figure 2.
The similarity of preference of each pair of females for each of the four males within a quartet was assessed by Pearson correlation on the numbers of hops facing each male (actual durations show the same pattern). Whether any one pair of females shows a significant correlation is not of as much interest as whether females on average show consistent similarities of preferences. Thus, Wilcoxon one-sample tests (15) were used to compare if average correlation coefficients across pairs of females differed from 0; Friedman’s test (15) was used to see if the median correlation coefficients differed across treatments. Nonparametric tests were used because it was not possible to normalize the residuals. Correlation coefficients for similarity of preference are presented separately for (i) females of a pair under UV+, (ii) females of a pair under UV−, and (iii) females of a pair across viewing conditions. In the latter case, there are two possible sets of comparisons (i.e., correlating the UV− preferences of female A with the UV+ preferences of female B, and vice versa), so the mean of these two correlations (per pair of females) are graphed. Indicated are medians ± the interquartile range. ∗, an outlier in one of eight pairs, but results remain robust because rank orders are used for treatment comparisons. Similarity of preference differed significantly across the three types of comparison [UV−, UV+, across treatments; Friedman’s test, S = 9.0; df = 2; P = 0.011; multiple comparisons (15): within UV+ > within UV− > across treatments]. Under UV+ conditions, preferences of pairs of females were highly correlated (mean r = 0.94, median r = 0.94, W = 36, n = 8, P = 0.014). They also were highly correlated under UV− conditions, although less so than under UV+ conditions (mean r = 0.74, median r = 0.87, W = 35, n = 7, P = 0.021). Preferences across UV+ and UV− treatments were not correlated (for the two possible comparisons it is arbitrary which female is designated A and B; using Monte Carlo simulation to select one female as A and one as B from each pair, the median P = 0.080 from 1000 randomly selected sets of eight).
None of the morphological variables independently predicted which males were preferred under either UV+ or UV− conditions (Table 1). By contrast, the spectrophotometric variables did predict female preferences although only under UV+ conditions (Table 1). In that treatment, the shape of spectral reflections of both the iridescent region of throat feathers and the throat feather tips was correlated with male attractiveness. Of course, under UV− conditions, some other variable that we did not measure (such as display rate) may predict female choice; the crucial result is that spectral reflectance within the human visible waveband did not. Inspection of the principal components revealed which features of spectra predicted UV+ attractiveness (Table 1; Fig. 3). It is intriguing that the throat “color” predictors of preference have human visible correlates (preferred males should have more purple, and less greenish, throats), yet removal of UV changes the “normal” ranking. This suggests that, although, in information terms, one could consider the UV signal as being somewhat redundant (being correlated with human visible variation), it is nevertheless a component of the birds’ mating decisions. This may arise because the UV waveband is “hard-wired” to contribute to hue perception, irrespective of any value of the waveband in information terms. Alternatively, as variation in the UV is not perfectly correlated with variation in the human visible waveband, the UV may still provide useful (nonredundant) information. Precisely what information the UV waveband is signaling remains to be determined. Nevertheless, our results suggests that even though variation in the UV waveband is correlated with variation in the human visible waveband, the UV variation plays an important role in mate choice decisions.
Table 1.
Characteristics of males that predicted their rankings by females under UV+ and UV− conditions, shown separately for morphometric variables and plumage reflectance spectra
| Morphometrics | ||||
|---|---|---|---|---|
| Trait | UV+ ranking predictor
|
UV− ranking predictor
|
||
| Statistic | P | Statistic | P | |
| Mass | F = 0.58 | NS | F = 1.00 | NS |
| Fat score | S = 2.33 | NS | S = 1.39 | NS |
| Length of tarsus | F = 0.27 | NS | F = 1.12 | NS |
| Length of 8th primary | F = 2.75 | NS | F = 0.11 | NS |
| Asymmetry of tarsus | S = 0.28 | NS | S = 1.88 | NS |
| Asymmetry of 8th primary | K-W H = 3.15 | NS | K-W H = 1.19 | NS |
| Throat feathers with white tips, % | S = 3.12 | NS | S = 4.86 | NS |
| Covert feathers with white tips, % | S = 3.67 | NS | S = 3.67 | NS |
| Bill black, % | S = 0.19 | NS | S = 0.10 | NS |
| Spectrophotometrics | |||||||
|---|---|---|---|---|---|---|---|
| Trait | UV+ ranking predictor
|
Summary | |||||
| Wilk’s λ | P | mean | PC1 | PC2 | PC3 | ||
| Throat | 0.308 | <0.05 | 1.08 | 1.62 | 7.58‡ | 0.60 | preferred males have high “violet” and “red” relative to UV and “green” |
| Throat tips | 0.071 | <0.01 | 2.72 | 4.03* | 0.22 | 5.48† | preferred males “whiter” |
| Coverts | 0.430 | NS | 3.92* | 1.15 | 0.79 | 0.80 | NS |
| Covert tips | 0.616 | NS | 0.40 | 0.58 | 2.47 | — | NS |
| UV− ranking predictor | |||||||
| Throat | 0.331 | NS | 0.81 | 1.83 | 1.30 | 0.53 | NS |
| Throat tips | 0.512 | NS | 0.68 | 1.11 | 0.55 | 0.79 | NS |
| Coverts | 0.725 | NS | 0.28 | 0.59 | 0.35 | 0.97 | NS |
| Covert tips | 0.585 | NS | 0.10 | 0.07 | 2.31 | — | NS |
Throat and coverts refer to the main iridescent region of the throat and wing covert feathers, respectively; tips are their white/brown margins. In all cases, the independent variable is rank, with four levels (1 = most preferred, 4 = least preferred). Morphometrics: Variables are compared by univariate tests. S refers to Friedman’s nonparametric repeated-measures ANOVA (15), with the males’ quartet as the blocking factor. Because primary feather asymmetry had too many missing values (due to abrasion of tips) for Friedman’s test, Kruskal–Wallis’ nonparametric ANOVA was used (15) as if males were independent. For repeated-measures ANOVA F tests, df = 3,21; for Friedman S and Kruskal–Wallis H, df = 3. Spectrophotometrics: Plumage variables are compared by repeated-measures MANOVA and ANOVA with 10 replicate feathers per region per bird (measurement details in legend of Fig. 3). For the UV+ rankings, mean reflectance and principal components (PC) are calculated from the full avian visible range (300-700 nm); for the UV− rankings, they are calculated for the human visible range (400-700 nm). Principal components were calculated from standardized reflectance measurements (mean reflectance subtracted) such that PC1 is the first component of spectral shape rather than a correlate of mean reflectance. Only PCs explaining >5% of the spectral variation are included. Wilk’s λ tests the mean reflectance and main PCs together by repeated-measures MANOVA, with ∗, P < 0.05;
, P < 0.01; and
, P < 0.001. Only where Wilk’s λ is significant should the univariate F tests for each dependent variable be consulted; for Wilk’s λ, df = 12,47.
Hamilton (16), in relation to the parasite theory of sexual selection (17), suggested that it might be worthwhile paying attention to UV reflectances from birds such as starlings, which had high parasite loads but appeared dull to humans. Although our results might at first sight seem to support Hamilton’s proposition and so add to the tentative support for the hypothesis from a comparative study (18), our results in fact showed that preferred males had relatively less UV reflectance than nonpreferred males (Fig. 3). Moreover, it is probably unwise to think of particular wavebands as being particularly “colorful” or “conspicuous”; colors are the perceptual representation of particular comparisons of reflectance at certain wavelengths (3, 13). Our results strongly suggest that (relative) UV reflectance of starling plumage affects male attractiveness but that it is low UV and green reflectance, in combination with high violet and red wavelengths, that females prefer (Fig. 3). How preference translates to starling colors, as opposed to human colors, will only be clear when a fuller understanding of starling color cognition is available (3).
The fact that removal of the UV cues did not abolish preferences of females (i.e., to random levels) suggests that UV plumage cues are not used exclusively for species recognition. This conclusion is also supported by our finding that intraspecific variation (including the UV waveband) predicted female choice. We make no claims that UV cues are not used in avian species recognition, simply that our evidence points to finer-tuned discrimination. These results, and those from zebra finches (6), do not support the hypothesis (19) that UV will tend to be used in mate choice decisions when plumage reflects “purely” in the UV; in neither species are reflectances purely (or even predominantly) in the UV, yet in both species UV plays a clear role in conspecific ranking.
Our study also demonstrates how behavioral experiments conducted under UV-deficient conditions can produce statistically robust conclusions that are, nevertheless, spurious in a natural context. Further experiments will be required to determine if the UV is in any way a “special” waveband for avian sexual signaling (4, 19) or is predisposed for signaling condition-dependent rather than aesthetic traits (2). Clearly, though, one should be very cautious about ignoring the UV waveband when considering avian mate choice experiments (see also ref. 20), plumage coloration, or display behavior in natural environments. Like fish (21–23), and consistent with recent studies on other avian species (6, 20, 24), birds are sensitive to the light conditions under which they choose mates.
Acknowledgments
We are very grateful to D. Burkhardt and R. H. Douglas for loans of equipment and to D. Burkhardt, R. H. Douglas, J. A. Endler, E. Finger, A. R. Goldsmith, W. D. Hamilton, E. Maier, N. J. Marshall, D. Osorio, A. Pomiankowski, J. Radwan, and an anonymous referee for stimulating discussions and helpful suggestions. This research was funded by the Biotechnology and Biological Sciences Research Council and the Nuffield Foundation.
References
- 1.Darwin C. The Descent of Man and Selection in Relation to Sex. London: Murray; 1871. [Google Scholar]
- 2.Andersson M. Sexual Selection. Princeton, NJ: Princeton Univ. Press; 1996. [Google Scholar]
- 3.Bennett A T D, Cuthill I C, Norris K J. Am Nat. 1994;144:848–860. [Google Scholar]
- 4.Bennett A T D, Cuthill I C. Vision Res. 1994;34:1471–1478. doi: 10.1016/0042-6989(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 5.Partridge J C. J Comp Physiol A. 1989;165:415–426. [Google Scholar]
- 6.Bennett A T D, Cuthill I C, Partridge J C, Maier E J. Nature (London) 1996;380:433–435. [Google Scholar]
- 7.Swaddle J P, Cuthill I C. Nature (London) 1994;367:165–166. [Google Scholar]
- 8.Feare C. The Starling. Oxford: Oxford Univ. Press; 1984. [Google Scholar]
- 9.Nicholls T J, Goldsmith A R, Dawson A. Physiol Rev. 1988;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- 10.Winer B J, Brown D J, Michels K M. Statistical Principles in Experimental Design. 3rd Ed. New York: McGraw–Hill; 1991. [Google Scholar]
- 11.Helms C W, Drury W H. Bird Band. 1960;31:1–40. [Google Scholar]
- 12.Swaddle J P, Witter M S. Proc R Soc London Ser B. 1994;255:147–152. [Google Scholar]
- 13.Endler J A. Biol J Linn Soc Lond. 1990;41:315–352. [Google Scholar]
- 14.Eens M, Pinxten R, Verheyen R F. Behavior. 1991;116:210–238. [Google Scholar]
- 15.Seigel S, Castellan N J. Nonparametric Statistics for the Behavioral Sciences. 2nd Ed. New York: McGraw–Hill; 1988. [Google Scholar]
- 16.Hamilton W D. Am Zool. 1990;30:341–352. [Google Scholar]
- 17.Hamilton W D, Zuk M. Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- 18.Radwan J. Acta Ornith. 1993;27:125–130. [Google Scholar]
- 19.Andersson S. Proc R Soc London Ser B. 1996;263:843–848. [Google Scholar]
- 20.Hunt, S., Cuthill, I. C., Swaddle, J. P. & Bennett, A. T. D. (1997) Anim. Behav., in press. [DOI] [PubMed]
- 21.Endler J A. Anim Behav. 1987;35:1376–1385. [Google Scholar]
- 22.Milinski M, Bakker T C M. Nature (London) 1990;344:330–333. [Google Scholar]
- 23.Endler J A. Vision Res. 1991;31:587–608. doi: 10.1016/0042-6989(91)90109-i. [DOI] [PubMed] [Google Scholar]
- 24.Endler J A, Thery M. Am Nat. 1996;148:421–452. [Google Scholar]