Abstract
Studies of the continuum between geographic races and species provide the clearest insights into the causes of speciation. Here we report on mate choice and hybrid viability experiments in a pair of warningly colored butterflies, Heliconius erato and Heliconius himera, that maintain their genetic integrity in the face of hybridization. Hybrid sterility and inviability have been unimportant in the early stages of speciation of these two Heliconius. We find no evidence of reduced fecundity, egg hatch, or larval survival nor increases in developmental time in three generations of hybrid crosses. Instead, speciation in this pair appears to have been catalyzed by the association of strong mating preferences with divergence in warning coloration and ecology. In mate choice experiments, matings between the two species are a tenth as likely as matings within species. F1 hybrids of both sexes mate frequently with both pure forms. However, male F1 progeny from crosses between H. himera mothers and H. erato fathers have somewhat reduced mating success. The strong barrier to gene flow provided by divergence in mate preference is probably enhanced by frequency-dependent predation against hybrids similar to the type known to occur across interracial hybrid zones of H. erato. In addition, the transition between this pair falls at the boundary between wet and dry forest, and rare hybrids may also be selected against because they are poorly adapted to either biotope. These results add to a growing body of evidence that challenge the importance of genomic incompatibilities in the earliest stages of speciation.
Keywords: Heliconius, speciation, mating behavior, postmating isolation, interspecific hybridization
Empirical research on speciation has mostly involved either laboratory hybridization experiments on Drosophila or studies of natural hybrid zones in taxa as diverse as crickets, butterflies, frogs, birds, and mice. This work has yielded valuable insights into the nature of species and racial differences, but the proximal causes of speciation remain unclear. Many Drosophila studies, for example, have examined species pairs between which there are multiple behavioral and genetic incompatibilities, making it hard to distinguish causes of speciation from differences that have evolved subsequently (1). In addition, with the possible exception of Hawaiian and cactophilic Drosophila (2, 3), the behavior and ecology of wild Drosophila and its importance in speciation are still poorly understood (1). Many hybrid zones, on the other hand, have been studied intensively both in the field and via laboratory experiments (4–6). However, in most of these studies, genetic differences between pure forms break down in narrow zones composed mainly of hybrids, and a good argument can be made that speciation has not occurred. Indeed, as many of these zones are thought to represent secondary contact, these pairs may better highlight a failure to speciate rather than giving insight into speciation. Fewer studies have examined very closely related taxa that can maintain their genetic integrity despite persistent hybridization. Taxa in this intermediate stage have only recently acquired the ability to remain distinct and are of extreme interest because they are more likely to reveal the traits that actually initiated speciation.
Here we examine the incipient stages of speciation of a pair of Heliconius butterflies. Heliconius erato is a common, brightly colored butterfly of secondary and gallery forests throughout the New World tropics. Like other members of the genus, H. erato is unpalatable to predators and shows strong Müllerian mimicry to other unpalatable species (7–10). Racial diversification within this species has reached extraordinary proportions. Nearly 30 parapatric races are described with ranges spanning thousands to a few hundred square kilometers. These races correspond almost exactly to those of its usual Müllerian comimic, Heliconius melpomene (7–10). Races differ, often strikingly, in warning color patterns, although they mate randomly in narrow contact zones (11–13). Other than loci coding for wing pattern elements there is little genetic distinction between races: speciation has clearly not happened (13–15).
Heliconius himera represents an intermediate step in the transition from a race to a species in this group. It has variously been considered a race of H. erato, which it replaces in the dry thorn-scrub habitats of Andean valleys in southern Ecuador and northern Peru, or a separate species (16). Both genetic and morphological analyses confirm the close relationship between H. himera and H. erato (15, 17). mtDNA differences between H. himera and H. erato fall at the edge of the range of interracial differences and suggest a divergence time of approximately 1 million years (15). In contrast to interracial contact zones within H. erato, parental types predominate in the hybrid zones between H. erato and H. himera. In the contact zone between H. himera and H. erato cyrbia in Ecuador, hybrids (F1 and backcrosses) make up only 9% of the population (16). Similarly, hybrids are known to be rare in contact zones of H. himera/H. e. favorinus and H. himera/H. e. lativitta in Peru (13). In the Ecuador hybrid zone, morphologically “pure” erato and himera are also “pure” at allozyme and mtDNA loci (unpublished data). This pair has clearly reached the crucial stage in speciation where emerging forms can maintain multilocus genotypic differences when in contact. Genetic differences can continue to accumulate, opening the possibility for continued divergence that may eventually permit widespread coexistence of the two species.
Rapid racial evolution has occurred repeatedly throughout Heliconius and especially in H. erato. This propensity could be an important factor contributing to the approximately 10-fold greater species diversity within Heliconius relative to ancestral heliconiine groups. Nonetheless, the link between racial evolution and speciation is poorly characterized in Heliconius. We report on mate choice and breeding experiments that, in combination with field data, examine the relative roles that mating behavior, hybrid inviability, sterility, and ecology play in early stages of the transition from race to a species.
MATERIALS AND METHODS
Mating and breeding experiments were performed in outdoor insectaries in Vilcabamba, Ecuador (altitude 1,600 m), a dry forest region within the range of H. himera, between August 1994 and March 1995.
Mating Behavior.
Two types of mate-choice experiments, “single-male” and “multiple-male,” were designed to study competition between females and males, respectively. The four types of males and females used in these experiments were designated as follows: E = erato, H = himera, EH = F1 progeny of erato female × himera male, and HE = the reciprocal F1 progeny. In each single-male experiment, a male was caged with two newly eclosed virgin females in 1 × 1 × 2 m3 outdoor insectaries. In multiple-male experiments a single newly eclosed virgin female was placed in a much larger (3 × 2 × 2 m3) cage containing three H, three E, and three F1 males. Mating experiments were monitored hourly. Most matings occurred within the first hour, but occasionally took until the following day. In single-male experiments, individuals were used only once. In multiple-male experiments, the mated pair and a randomly chosen male from each of the two classes that did not mate were removed and replaced with three new males (one H, one E, and one F1). Wild-caught and reared males (≥7 days old) were used in single-male experiments but only reared males (≥7 days old) were used in multiple-male experiments. Most of the 415 individual H and E used in these experiments were collected or raised from individuals collected at least 25 km from the hybrid zone. However, 43 pure individuals (19 male and 12 female E, and 4 male and 8 female H) were collected from the hybrid zone or were the offspring of one parent collected from hybrid zone populations. Because mate preference was very strong, even in individuals collected away from the hybrid zone, we used both sets of individuals to estimate mating probabilities (see below).
Estimation of Mating Probabilities.
We used a novel likelihood approach to estimate the relative probability of mating between pure species and F1 progeny. Likelihood gives a powerful means of estimating parameters and support intervals across multiple experiments, and our analysis could also be useful in many other studies of mating behavior between different strains or species. We estimated the probability, PI×J, of a mating between I-type females and J-type males relative to the probability of matings within erato, PE×E, setting PE×E to 1.00 (similarly, PH×H was set to 1.00 in multiple-male experiments involving himera females). These relative mating probabilities were then combined to give actual mating probabilities, which, in any experiment, must sum to one. For example, the actual probabilities of E males mating with E and EH females in the single-male experiments (see Table 1), were then: ΠE×E = 1/(1 + PEH×E) and ΠEH×E = PEH×E/(1 + PEH×E), respectively. Similarly, the actual probabilities that E females mate with E, EH, HE, and H males in multiple-male experiments (Table 1) were given by:
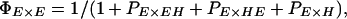 |
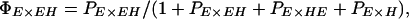 |
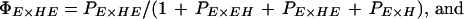 |
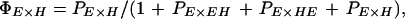 |
respectively. These probabilities were estimated using likelihood (18). For example, the likelihood of results in the two experiments described above were ΠE×E13 ΠEH×E5, and ΦE×E11 ΦE×EH2 ΦE×HE6 ΦE×H0, respectively (data are from Table 1). The likelihood over all experiments was maximized, and used to estimate all PI×J parameters and compare different models of mating. Twice the difference in maximum log-likelihood (G = 2ΔlogeL) between two models asymptotically follows a χ2 distribution, permitting comparison of models differing in numbers of parameters (18). The reliability of our estimates of mating behavior were given “support limits”—i.e., the parameter values at which the loge likelihood dropped two units below the maximum. Support limits are asymptotically equivalent to 95% confidence limits.
Table 1.
Observed and expected number of matings between pure and F1 individuals in single-male and multiple-male experiments
| Females used | Males used
|
|||
|---|---|---|---|---|
| E | EH | HE | H | |
| Single-male experiments | ||||
| E | 20 (19.2) | 3 (3.0) | 4 (4.0) | 4 (2.4) |
| H | 1 (1.8) | 7 (7.0) | 4 (4.0) | 17 (18.6) |
| E | 13 (9.0) | 1 (2.4) | 1 (1.5) | |
| EH | 5 (9.0) | 3 (1.6) | 2 (1.5) | |
| E | 2 (2.5) | 3 (0.9) | 2 (1.5) | |
| HE | 3 (2.5) | 0 (2.1) | 1 (1.5) | |
| H | 13 (11.6) | 0 (1.5) | 10 (8.0) | |
| EH | 2 (3.4) | 3 (1.5) | 6 (8.0) | |
| H | 3 (2.5) | 2 (1.5) | 2 (1.0) | |
| HE | 2 (2.5) | 1 (1.5) | 0 (1.0) | |
| Multiple-male experiments | ||||
| E | 11 (10.6) | 2 (2.7) | 6 (4.3) | 0 (1.4) |
| H | 1 (1.0) | 4 (6.9) | 7 (4.1) | 11 (11.0) |
Expected numbers of matings (in parentheses) are based on the relative mating probabilities given in Table 2. H represents a H. himera individual and E represents a H. erato cyrbia individual. The female parent of F1 individuals is given first.
Hybrid Sterility and Inviability.
Our breeding experiments were conducted under ambient conditions. Each mated female was kept in an individual 1 × 1 × 2 m3 insectary that contained ample artificial nectar, pollen resources, and larval food plants. Artificial nectar consisted of a 10% sugar solution displayed in small red and yellow plastic cups. Sugar solution was checked daily and replenished as necessary. In addition, fresh nectar and pollen resources were provided by cut Lantana sp. flowers. Lantana flowers were replaced every 2–3 days. Potted Passiflora rubra and Passiflora punctata, each utilized by wild populations of both erato and himera (16), was provided for oviposition. Eggs were collected daily, and the larvae were raised individually in small plastic pots to prevent cannibalism. Developing larvae were fed ample new growth of P. rubra. Larval pots were cleaned daily, and new food was added as needed. A small twig taped to the top of each pot provided a suitable pupation site. Pupae were housed together by brood in larger plastic cages until emergence. We avoided inbreeding by crossing only unrelated individuals.
To control for environmental fluctuations in our breeding experiments, hybrid crosses were reared simultaneously with pure crosses as controls in complexes of six cages. Groups of hybrids and controls reared together in this way were designated as a replicate. The number of eggs laid per day, hatching success, larval survival, and developmental times of hybrid or hybrid-mated crosses could then be examined relative to pure crosses raised in the same cage complex and during the same period. We used hierarchical G tests to examine differences in egg hatch and larval survival (i) between different F1 crosses (H × E vs. E × H), (ii) between different pure crosses (H × H vs. E × E), and (iii) between hybrids and controls. The F2 crosses were not raised under this experimental design but were compared directly to pure broods of himera and erato females collected from the wild and reared simultaneously.
RESULTS
Mating Behavior.
Although the mating behavior of H. himera in the wild is still poorly researched, mating in H. erato is known to occur shortly after female eclosion (19). Male H. erato actively search for larvae and pupae on host plants and have been observed in greenhouses to “pupal-mate”—i.e., sit on pupae—and then mate with females as they eclose (20). Pupal-mating never occurred with himera and erato in our insectary, even when females were allowed to develop on host plants growing in all-male cages. Nonetheless, in our mating experiments with newly eclosed females, mating was often rapid and lacked obvious courtship elements. In both single- and multiple-male experiments, males flew aggressively toward females, sometimes hovering briefly, before landing and attempting to mate. Females could and often did reject males by spreading their wings and raising their abdomens out of reach of male claspers.
There were no differences in the relative probabilities of mating between single-male and multiple-male experiments (G = 4.68, P > 0.5, df = 6). Under both experimental designs interspecific matings were rare, occurring in only 8 of 84 mating experiments (Table 1). Because the type of mating experiment did not influence the relative probabilities of different mating combinations, we could reduce the number of PI×J mating probabilities to be estimated from 21 in the full model to 15. This 15-parameter model was reduced still further because all but 4 of the PI×J matings were not informatively different from PE×E (see Table 2). Under this final model, interspecific matings were approximately a tenth as likely as within-species mating (Table 2).
Table 2.
Estimates (with support limits) of the relative mating probabilities (PI×J) between erato, himera, and F1 individuals
| Females | Males
|
|||
|---|---|---|---|---|
| E | EH | HE | H | |
| E | [1] | 0.43 (0.17, 0.95) | 1 | 0.13 (0.04, 0.33) |
| EH | 1 | 0.28 (0.09, 0.80) | 1 | 1 |
| HE | 1 | 1 | 1 | 1 |
| H | 0.09 (0.02, 0.25) | 1 | 1 | 1 |
By convention, all probabilities were estimated relative to the probability of E × E matings, which was set to 1. To evaluate which PI×J matings were informative, they were first ranked in order of increasing information (i.e., ΔlogeL). Each PI×J was then set to 1 in turn in order of increasing rank, after which the likelihood was remaximized around the remaining parameters. PI×J parameters that, when set to 1, decreased the maximum log likelihood less than two units were judged noninformative and were set permanently to 1. The final reduced model with 4 mating parameters was justified because it resulted in only a small likelihood change from the complete 15-parameter model (G = 18.66, df = 11, P > 0.05).
Matings between hybrids and pure forms occurred more frequently in our experiments. As an example, F1 males mated pure females in 19 of 42 multiple-male experiments (Table 1). However, most of these matings involved HE males (progeny of himera females and cyrbia males). Mating models where the two reciprocal F1 types were considered the same were significantly worse than models where the two F1 types were considered separately (G = 19.69, P < 0.01, df = 7), indicating heterogeneity between the F1 hybrid classes. This heterogeneity was the result of reduced mating probabilities in only some crosses with EH males (Table 2). In particular, E × EH matings were less than half as probable as conspecific matings and the probability of EH × EH matings was reduced still further (Table 2). The reason for the mating asymmetry between F1 males was unclear. Because males are the homogametic sex in butterflies, the two F1 classes differ only at cytoplasmically inherited genes. Whatever the reason, the mating probabilities involving F1 males were, on average, much greater than those between erato and himera (Table 2).
Hybrid Sterility and Inviability.
Our experiment examined fitness parameters in over three generations of hybrid crosses. Nearly 4,500 eggs were laid over our 8-month breeding experiment generating 2,475 adults. Egg-to-adult developmental time was approximately 29 days and not significantly different between males and females. Mortality was generally highest in the early larval stages.
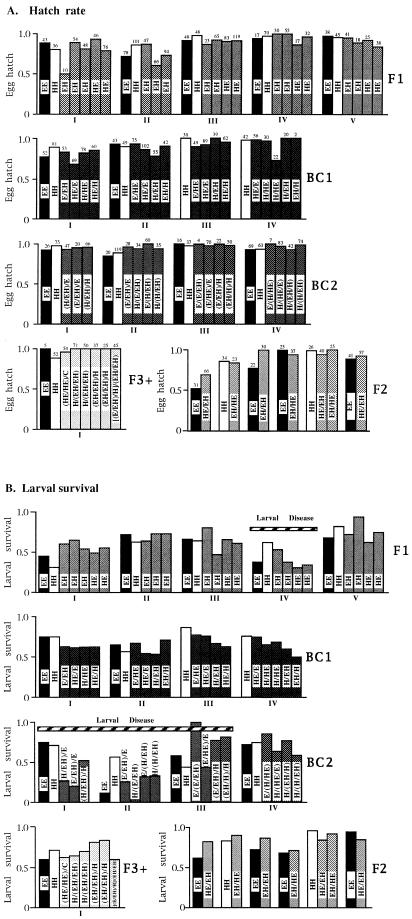
All hybrid crosses proved both as viable and fertile as pure crosses (Table 3). Hybrid crosses laid fertile eggs and, with the possible exception of F2 crosses, fecundity, as estimated by number of eggs laid per day, did not differ significantly among conspecific, interspecific, and hybrid matings (Table 3). Moreover, hybrid egg hatch was high, averaging 90%, and similar to controls (Table 3). There was strong brood-dependent variation in hatching success within F1, BC1, and both types of pure crosses (within F1 broods: G = 85.33, P < 0.001, df = 19; within BC1 broods: G = 70.07, P < 0.001, df = 16; within H × H broods: G = 32.70, P < 0.01, df = 13; within E × E broods: G = 33.38, P < 0.001, df = 11); however, in standardized comparisons the only significant differences in egg hatch between pure and hybrid crosses was between F3+ and their controls (Fig. 1A). In this case, egg hatch was significantly higher among the hybrid crosses (G = 13.48, P < 0.0001, df = 1).
Table 3.
Summary of measures of viability of pure and hybrid crosses
| Cross type (n) | Eggs/ brood (SD) | Eggs/day* | % eggs hatching | % larval survival | Development time,† (days) | Sex ratio (female/total) |
|---|---|---|---|---|---|---|
| H × H (14) | 61 (26.0) | 2.6 ± 0.8 | 92.7 ± 3.3 | 66.7 ± 8.1 | 28.5 ± 0.9 | 0.49 ± 0.05 |
| E × E (12) | 38 (22.1) | 1.9 ± 0.4 | 90.3 ± 5.5 | 57.5 ± 12.3 | 29.8 ± 1.0 | 0.48 ± 0.06 |
| EH (12) | 46 (23.6) | 1.8 ± 0.6 | 83.7 ± 9.8 | 64.1 ± 9.8 | 29.3 ± 0.8 | 0.48 ± 0.06 |
| HE (8) | 55 (35.0) | 2.4 ± 1.2 | 89.2 ± 4.7 | 53.8 ± 12.7 | 29.2 ± 1.1 | 0.45 ± 0.06 |
| BC1 (E) (7) | 70 (19.7) | 2.8 ± 1.2 | 87.3 ± 9.0 | 67.8 ± 7.8 | 30.0 ± 1.0 | 0.51 ± 0.06 |
| BC1 (H) (10) | 41 (23.1) | 2.2 ± 1.2 | 90.0 ± 7.1 | 62.3 ± 4.7 | 28.9 ± 1.2 | 0.53 ± 0.07 |
| BC2 (16) | 44 (24.2) | 2.0 ± 0.7 | 85.7 ± 12.3 | 53.8 ± 14.9 | 28.1 ± 0.6 | 0.44 ± 0.06 |
| F2 (7) | 40 (16.3) | 1.5 ± 0.7 | 92.0 ± 9.6 | 84.5 ± 6.7 | 30.2 ± 2.0 | 0.51 ± 0.08 |
| F3+ (6) | 46 (15.4) | 2.5 ± 1.2 | 99.3 ± 1.8 | 83.4 ± 10.0 | 28.1 ± 0.7 | 0.50 ± 0.08 |
The female parent of a cross is given first. Averages and 95% confidence limits of number of eggs/day, egg hatch, larval survival, developmental time, and sex ratio are taken over all hybrid and control crosses in each category. The two different F1 crosses (H × E and E × H) were examined separately. Similarly, backcross broods (BC1) were grouped into crosses between F1s and eratos [BC1 (E)] or between F1s and himeras [BC1 (H)]. All BC2 (crosses between BC1 and pure individuals) and F3+ (crosses between F2s and pure types) broods were evaluated together. Egg hatch and larval survival for all individual crosses is presented in Fig. 1.
In comparisons of hybrid and control broods reared together, only F2 crosses laid significantly fewer eggs/day relative to erato and himera (t = 2.547, P = 0.04, df = 6).
Egg-adult developmental time for each cross was estimated using the mean developmental time of five male and five female butterflies.
Figure 1.
Proportion of egg-hatch (A) and larval survival (B) for hybrid and pure matings. Cross type is given within each bar. Hybrid individuals are designated by a combination of letters, where the female parent of each cross is listed first. The number of eggs laid per female is listed above the proportion hatching bar in A. Individuals raised together are designated by roman numerals.
Hybrid larval survival was also similar to that of controls. Only H × E crosses showed a slight reduction of larval survival relative to H × H crosses when averaged over the entire experimental period (Table 3). This apparent difference, as well as an apparent increase in survival of F2 and F3+ crosses, however, was not obvious in comparisons between hybrid and pure crosses raised together. As with egg-hatch, there was significant variation in survival among broods in some hybrid and control crosses (within F1 broods: G = 65.64, P < 0.001, df = 19; within BC2 broods: G = 139.35, P < 0.001, df = 15; within H × H broods: G = 42.32, P < 0.001, df = 13; within E × E broods: G = 40.06, P < 0.001, df = 11). This variation was particularly marked because of a disease outbreak. The epidemic was localized to individual hybrid and control broods (Fig. 1B). Largely because of this disease, there were strong differences in larval survival between controls and hybrids in F1 and BC2 replicates (G = 15.69, P < 0.001, df = 5; G = 54.85, P < 0.0001, df = 4, respectively). However, differences between F1 hybrids and controls were most strongly influenced by higher hybrid larval survival in replicate I (G = 5.86, P < 0.05, df = 1) and a higher control survival in replicate IV (G = 8.57, P < 0.001, df = 1) (Fig. 1B). Lower larval survival of BC2 hybrids compared with their controls was chiefly due to diseased hybrid broods in replicates I and II (Fig. 1B) (G = 32.4, P < 0.0001, df = 1, and G = 18.73, P < 0.0001, df = 1, respectively). BC2 replicates that were largely unaffected by disease did not differ significantly in larval survival. With the exception of a barely significant reduction in the number of females among BC2 hybrids overall (G = 4.9, P < 0.05, df = 1), all crosses produced approximately equal numbers of males and females (Table 3).
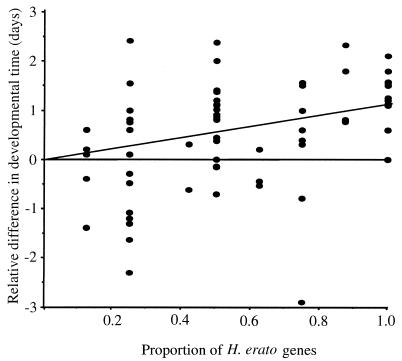
Egg-to-adult developmental times, potentially sensitive to incompatibilities within hybrid genomes, were also similar between hybrids and controls (Table 3). In standardized comparisons, there were significant differences in developmental time between himera and erato, and hybrid larvae developed at a roughly intermediate rate. The observed difference in egg-to-adult developmental time between himera and erato probably reflects an underlying adaptive difference. H. himera and H. erato live in markedly different biotopes: H. himera is found in drier forests at altitudes up to 400 m higher that those of H. erato (16). All larvae were reared under ambient conditions within the himera biotope in Vilcabamba. In this environment, EE larvae took 1.3 ± 0.4 days longer than HH larvae to reach adulthood (n = 11, brood comparisons). In general, the more erato genes in a hybrid cross the longer the egg-to-adult developmental time and there was a significant regression of the extra development time (in days, relative to H × H controls) against the proportion of erato genes (slope = 1.10, r = 0.56, P = 0.001, df = 66) (Fig. 2). Heliconius himera was also more active in the early morning and late evening suggesting that physiological adaptation to different environmental conditions extends to adults (A. Davison, W.O.M., A. S. Griffin, C.D.J., and J.M., unpublished data).
Figure 2.
Excess larval developmental time (in days) as a function of the proportion of erato genes. Each point represents the mean egg-to-adult developmental time of five females and five males from a single cross. Differences in mean developmental time were calculated relative to the developmental time of H × H crosses reared at the same time. The regression line was calculated forcing the y-intercept through the origin.
DISCUSSION
Today, H. W. Bates is remembered as the discoverer of mimicry. However, his original treatise (7), in which he describes mimetic patterns in Amazonian butterflies, is primarily concerned with speciation. He believed that species and geographic races form a continuum and that heliconiine and ithomiine butterflies best demonstrated the transition from geographic variation to speciation. Here we show that for himera and erato, the step most critical for this transition is the evolution of strong mate preference and that classical hybrid inviability or sterility plays, at best, a minor role. Our breeding experiments show virtually no reductions of fecundity, egg hatch, larval survival, or developmental time of hybrids. Mating preferences, in contrast, provide an approximately 90% effective barrier to the production of hybrids. The only possible evidence of classical hybrid incompatibility was the lower mating propensity of EH hybrid males in some crosses. The very strong barrier to the production of hybrids tally with the observation that hybrids make up only 9% of individuals in the zones of overlap between the two species (16). Further indication of strong mating preferences comes from females collected (n = 35) in the contact zones. In these collections of the four “pure-species” females that were not mated by conspecific only one produced an F1 progeny. The remaining three hybrid matings involved F1 males.
It is tempting to attribute the divergence in mating preferences we observe to changes in warning coloration. Vision is well developed in butterflies and colors can attract Heliconius from distances of 20 m or more (21); however, differences in wing patterns, as great as those that exist between himera and erato, have not resulted in assortative mating among the many geographic races of erato (11–13). In addition, as Bates discovered over a hundred years ago, within any given area of the neotropics an extraordinary number of different species share nearly identical color patterns. This convergence would seem to make color pattern a poor signal for sexual communication in mimetic butterflies like Heliconius. Changes in chemically mediated signals, such as pheromones or cuticular hydrocarbons, are thought to be important in mate-selection in other butterflies (22). Divergence in these molecules may similarly underlie the strong differences in mate preference we observe in erato and himera and this pair will provide a tractable model for future study of the cues responsible for the evolution of mate choice.
Even if change in warning coloration does not directly cause divergence in mate choice, it is probably a very important component of speciation in this pair. This is because mating preferences alone cannot fully explain the continued maintenance of genetic differences between erato and himera in areas of overlap. Ten percent hybridization per generation would quickly erode the distinctness of these two species in areas of contact unless hybrids were eliminated from the population. Strong frequency-dependent selection by predators (s ≈ 0.5) removes rare color pattern phenotypes in interracial hybrid zones of H. erato (13), and is probably important in the erato/himera hybrid zone as well. Where assortative mating limits hybrid numbers, as in this case, strong frequency-dependent selection becomes an even more potent source of “post-mating isolation.” In addition, the transition from erato to himera occurs in the boundary between wet and dry forests and hybrids may be poorly adapted to either biotope. The very narrow transition (≈5 km) between species, is further evidence for extremely strong selection coefficients (s ≈ 1.0) (16).
Thus, the transition from a race to a species in these Heliconius appears to have been catalyzed by the association of strong mating preferences with divergence in warning color and ecology. Divergence in mate preference in this pair probably evolved as a by-product of ecological or sexual selection rather than through direct selection against hybridization [i.e., reinforcement, sensu Dobzhansky (23)]. There are at least three reasons to doubt reinforcement in this case. First, many strongly selected hybrid zones between races of H. erato are stable over thousands of generations but have not resulted in assortative mating (11). Second, the two species hybridize over only a very narrow region relative to the total geographic range of each species, a situation that makes reinforcement theoretically difficult (ref. 24, but see also ref. 25). Finally, in most of our experiments strong mate preference occurred between erato collected >25 km, and himera collected >60 km from the hybrid zone.
Taxa that have evolved the ability to remain distinct in the face of hybridization offer ideal systems in which to identify proximal causes of speciation. Interspecific hybridization of this kind is common in taxa as diverse as butterflies, birds, and coral reef fishes (26–28). Whether divergence in mate choice and ecology precedes the evolution of sterility and inviability, as in this case, needs to be tested in many more pairs of incipient species. However, a growing number of studies demonstrate that genomic incompatibilities leading to hybrid sterility or inviability have been relatively unimportant in the early stages of speciation (29–34). Rapid divergence of mate preference through sexual selection (35) may account for the extraordinary diversity of Hawaiian Drosophila (29) and may have driven the divergence in pollinators in monkeyflowers (30). In other groups, ecological divergence also seems to be necessary for the maintenance of genetic distinctiveness in sympatry (31, 32). Indeed, long-term study of Darwin’s finches underscores the importance of adaptation in speciation. Despite strong mating preferences, when disruptive ecological selection was relaxed because of climatic changes following the 1983/84 El Niño, hybrids of three species of finches (Geospiza fortis, Geospiza scandens, and Geospiza fuliginosa) increased in abundance leading to an erosion of morphological distinctions (36). Even in Drosophila, where hybrid inviability and sterility seem to evolve rapidly, strong mating discrimination has preceded any genomic incompatibility (I = 0) in at least 7 of 17 closely related sympatric pairs (Nei’s D < 0.3) (1).
The idea that the evolution of hybrid sterility and inviability is important in speciation dates to the very beginning of the modern synthesis (37, 38) and has become entrenched in theories of speciation largely because of the wide spread application of the biological species concept (38). Our findings, together with other recent studies, however, challenge the generalization that genomic incompatibilities are important causes of speciation. Inviability and sterility may be the eventual result of cladogenesis (39), but their relevance as initiators of speciation may have been overemphasized.
Acknowledgments
We thank the government of Ecuador, El Instituto Ecuatoriano Forestal y de Areas Naturales y Vida Silvestre, Museo Ecuatoriano de Ciencias Naturales, Peter Wilson, and Fundaciōn Arcoiris. Help in raising butterflies was provided by Angus Davison, Ashleigh Griffin, José Carpio Ayala, Carlos Roberto Carpio Ayala, María del Carmen Cabrera, and María Soledad Arias Díaz. We thank Jeff Feder, Kevin Fowler, and Andrew Pomiankowski for comments on earlier drafts. This research was funded by the Biotechnology and Biological Sciences Research Council (United Kingdom).
References
- 1.Coyne J A, Orr H A. Evolution. 1989;43:362–381. doi: 10.1111/j.1558-5646.1989.tb04233.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaneshiro K Y, Boake C R B. Trends Ecol Evol. 1987;2:207–212. doi: 10.1016/0169-5347(87)90022-X. [DOI] [PubMed] [Google Scholar]
- 3.Etges W J. Evolution. 1992;46:1945–1950. doi: 10.1111/j.1558-5646.1992.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 4.Barton N H, Hewitt G M. Annu Rev Ecol Syst. 1985;16:113–148. [Google Scholar]
- 5.Barton N H, Hewitt G M. Nature (London) 1989;341:497–503. doi: 10.1038/341497a0. [DOI] [PubMed] [Google Scholar]
- 6.Harrison R G. In: Oxford Survey of Evolutionary Biology. Futuyma D, Antonovics J, editors. Vol. 7. Oxford: Oxford Univ. Press; 1990. pp. 69–128. [Google Scholar]
- 7.Bates H W. Trans Linn Soc London. 1862;23:495–566. [Google Scholar]
- 8.Brown K S, Sheppard P M, Turner J R G. Proc R Soc London B. 1974;187:369–378. [Google Scholar]
- 9.Turner J R G. Annu Rev Ecol Syst. 1981;12:99–121. [Google Scholar]
- 10.Sheppard P M, Turner J R G, Brown K S, Benson W W, Singer M C. Phil Trans R Soc London B. 1985;308:433–613. [Google Scholar]
- 11.Turner J R G. Evolution. 1971;25:471–482. doi: 10.1111/j.1558-5646.1971.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 12.Mallet J, Barton N H. Evolution. 1989;43:421–431. doi: 10.1111/j.1558-5646.1989.tb04237.x. [DOI] [PubMed] [Google Scholar]
- 13.Mallet J. In: Hybrid Zones and the Evolutionary Process. Harrison R G, editor. New York: Oxford Univ. Press; 1993. pp. 226–260. [Google Scholar]
- 14.Turner J R G, Johnson M S, Eanes W F. Proc Natl Acad Sci USA. 1979;76:1924–1928. doi: 10.1073/pnas.76.4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brower A V Z. Proc Natl Acad Sci USA. 1994;91:6491–6495. doi: 10.1073/pnas.91.14.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiggins C, McMillan W O, Neukirchen W, Mallet J. Biol J Linn Soc. 1996;59:221–242. [Google Scholar]
- 17.Emsley M G. Zoologica. 1965;50:191–254. [Google Scholar]
- 18.Edwards A W F. Likelihood. Cambridge: Cambridge Univ. Press; 1972. [Google Scholar]
- 19.Mallet J. Oecologia. 1986;68:210–217. doi: 10.1007/BF00384789. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert L E. Proc Natl Acad Sci USA. 1972;69:1403–1407. doi: 10.1073/pnas.69.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallet J, Barton N, Lamas G, Santisteban J, Muedas M, Eeley H. Genetics. 1990;124:921–936. doi: 10.1093/genetics/124.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vane-Wright R I, Boppré M. Philos Trans R Soc London B. 1993;340:197–205. [Google Scholar]
- 23.Dobzhansky T. Am Nat. 1940;74:312–321. [Google Scholar]
- 24.Butlin R. In: Speciation and Its Consequences. Otte D, Endler J A, editors. Sunderland, MA: Sinauer; 1989. pp. 158–179. [Google Scholar]
- 25.Kelly J K, Noor M A F. Genetics. 1996;143:1485–1497. doi: 10.1093/genetics/143.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant P R, Grant B R. Science. 1992;256:193–197. doi: 10.1126/science.256.5054.193. [DOI] [PubMed] [Google Scholar]
- 27.Pyle R L, Randel J E. Environ Biol Fishes. 1994;41:127–145. [Google Scholar]
- 28.Guillaumin M, Descimon H. In: Les Problèmes de l’Espèce dans le Regne Animal. Bocquet C, Génermont J, Lamotte M, editors. Vol. 1. Paris: Société Zoologique de France; 1976. pp. 129–201. [Google Scholar]
- 29.Hoy R R, Hoikkala A, Kaneshiro K. Science. 1988;240:217–219. doi: 10.1126/science.3127882. [DOI] [PubMed] [Google Scholar]
- 30.Bradshaw H D, Wilbert S M, Otto S G, Schemske D W. Nature (London) 1995;376:762–765. [Google Scholar]
- 31.Schluter D. Science. 1994;266:798–801. doi: 10.1126/science.266.5186.798. [DOI] [PubMed] [Google Scholar]
- 32.Grant B R, Grant P R. Biol J Linn Soc. 1987;32:247–270. [Google Scholar]
- 33.Feder J L, Opp S B, Wlazlo B, Reynolds K, Go W, Spisak S. Proc Natl Acad Sci USA. 1994;91:7990–7994. doi: 10.1073/pnas.91.17.7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bush G L. Trends Ecol Evol. 1994;9:285–288. doi: 10.1016/0169-5347(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 35.Andersson M. Sexual Selection. Princeton: Princeton Univ. Press; 1994. [Google Scholar]
- 36.Grant B R, Grant P R. Ecology. 1996;77:500–509. [Google Scholar]
- 37.Dobzhansky T. Genetics and the Origin of Species. New York: Columbia Univ. Press; 1937. [Google Scholar]
- 38.Mayr E. Systematics and the Origin of Species. New York: Columbia Univ. Press; 1942. [Google Scholar]
- 39.Turelli M, Orr H A. Genetics. 1995;140:389–402. doi: 10.1093/genetics/140.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]