Abstract
Autism is a profound disorder of neurodevelopment with poorly understood biological origins. A potential role for maternal autoantibodies in the etiology of some cases of autism has been proposed in previous studies To investigate this hypothesis, maternal plasma antibodies against human fetal and adult brain proteins were analyzed by western blot in 61 mothers of children with autistic disorder and 102 controls matched for maternal age and birth year (62 mothers of typically developing children (TD) and 40 mothers of children with non-ASD developmental delays (DD)). We observed reactivity to two protein bands at approximately 73kDa and 37kDa in plasma from 7 of 61 (11.5%) mothers of children with autism (AU) against fetal but not adult brain, which was not noted in either control group (TD; 0/62 p=0.0061 and DD; 0/40 p=0.0401). Further, the presence of reactivity to these two bands correlated with a diagnosis of behavioral regression in the child when compared to the TD (p=0.0019) and DD (0.0089) groups. Individual reactivity to the 37kDa band was observed significantly more often in the AU population compared with TD (p=0.0086) and DD (p=0.002) mothers, yielding a 5.69-fold odds ratio (95% confidence interval 2.09 - 15.51) associated with this band. The presence of these antibodies in the plasma of some mothers of children with autism, as well as the differential findings between mothers of children with early onset and regressive autism may suggest an association between the transfer of IgG autoantibodies during early neurodevelopment and the risk of developing of autism in some children.
Keywords: Autism, Maternal Antibodies, Regression, Autoantibodies
1. Introduction
The autism spectrum disorders (ASD), manifest as highly variable combined deficits in social interaction, verbal and non-verbal communication, and often include the presence of repetitive, stereotypical and overly restrictive behaviors (APA, 1994). Despite the lack of clear etiology for the large majority of ASD cases, evidence from twin studies (Bailey et al., 1995) and familial incidence (Lauritsen et al., 2005) rates support a view of ASD as a largely, but not exclusively, genetic disorder. The potential role of the immune system in ASD has been addressed in several studies. These include reports of neuroglial activation and neuroinflammation in the CNS (Pardo et al., 2005), as well as plasma antibodies reactive to rodent neuronal tissue (Singer et al., 2006) in children with autism. However, there have been no systematic case-based studies describing a direct relationship between maternal autoantibodies to human fetal neuronal proteins and the development of ASD.
The role of the maternal immune system in fetal neurodevelopment is an area of active research. It has long been known that in humans, maternal IgG isotype antibodies readily cross the placenta to equip the immunologically naïve fetus with a subset of the maternal adaptive humoral immune system proteins (Garty et al., 1994); these maternal IgG antibodies are known to persist for up to six months post-natal (Heininger et al., 2006). However, together with IgG antibodies that are immunoprotective, autoantibodies that react to fetal ‘self’-proteins can also cross the placenta. A recent report demonstrated maternal IgG antibody reactivity to rodent Purkinje cells in a mother of multiple children with ASD, as well as the presence of behavioral deficits in pups of a mouse injected during gestation with her serum (Dalton et al., 2003). In another study, mothers of children with autism and their affected children were found to have consistent patterns of antibody reactivity against rat prenatal (day 18) brain proteins. In contrast, unaffected children and control mothers had alternative patterns of reactivity (Zimmerman et al., 2006).
The preponderance of evidence suggests a pre-natal or early post-natal etiology for autism, potentially involving errant developmental cues. Advances in understanding the role of immune system components during fetal neurodevelopment combined with the cross-talk between the maternal and fetal immune systems, led us to investigate the profiles of autoantibody reactivity in mothers of children with autism and to compare them with profiles from mothers of typically developing children and from mothers of children with other developmental disorders excluding autism.
2. Materials and Methods
2.1 Study Subjects
This case-control study examined 61 mothers of children with autism and 102 control mothers enrolled through the Center for Children's Environmental Health (CCEH) as part of the ongoing CHARGE (Childhood Autism Risks from Genetics and Environment) study at the M.I.N.D. Institute at the University of California at Davis (Hertz-Picciotto et al., 2006). The CHARGE study population was sampled from three strata: children considered to have autism (AU), children selected from the general population who were typically developing (TD), and children with developmental disabilities without autism (DD). The families were recruited for this study without bias for any medical or demographic factors.
To confirm and further detail the initial diagnosis, all children were assessed at the UC Davis M.I.N.D. Institute. The diagnosis of autism was confirmed for all cases using the Autism Diagnostic Interview-Revised (Lord et al., 1997) and the Autism Diagnostic Observation Schedule, modules 1, 2 or 3 (DiLavore et al., 1995; Joseph et al., 2002; Lord et al., 2001; Owley et al., 2001). The ADI-R provides a standardized, semi-structured interview and a diagnostic algorithm for the DSM-IV(APA, 1994) and the ICD-10 definitions of autism (Steinhausen and Erdin, 1992; WHO, 1992). The ADOS is a semi-structured, standardized assessment in which the researcher observes the social interaction, communication, play and imaginative use of materials for children suspected of having ASD. Final autism case diagnosis was defined as meeting criteria on the communication, social, and repetitive behaviors domains of the ADI-R and scoring at or above the cut-off for autistic disorder on the ADOS modules 1 or 2. The Social Communication Questionnaire was used to screen for behavioral and developmental characteristics of ASD among the subjects with developmental disabilities and among the general population typically developing controls; children who scored above the screening cut-off were fully assessed using the ADI-R and ADOS. Those who met criteria for autistic disorder were classified as AU; similarly any general population children who met criteria for DD based on the Vineland Scales of Adaptive Behavior (Sparrow et al., 1984) and the Mullen Scales of Early Learning (Mullen, 1995) were classified as DD. Controls who did not meet criteria for ASD or for DD were classified as typically developing (TD). Using clinical characteristics reported in the Early Development Questionnaire (Ozonoff et al., 2005) and answers to questions regarding loss of language (Q11) and social skills (Q25) of the ADI-R, the autism population was further divided into two groups based on the clinical onset of autistic symptoms; firstly, children with regression who initially developed normally, but subsequently lost acquired skills (n=36; (31 male, 5 female)) and secondly, children with early onset autism characterized by early deficits in the requisite behavioral domains (n=25; (24 male, 1 female)). The study protocol followed the ethical guidelines of the most recent Declaration of Helsinki (Edinburgh, 2000), and was approved by the Institutional Review Boards of the UC Davis School of Medicine and the State of California, and written informed consent was obtained for all participants enrolled in the study.
Following informed consent, plasma samples were collected from mothers of children meeting the above enrollment criteria. For this analysis we matched control maternal samples with AU case maternal samples for maternal age and parity as well as the age of offspring (Table 1). Mothers of AU children were considered without regard to sibling developmental status. TD mothers were excluded from this analysis if any of their children were diagnosed with a developmental disorder. Paternal half-siblings of case and control children were not included in total children (parity) and birth order demographics.
Table 1.
Demographics of study subjects.
| Primary Diagnosis | N = | Maternal Age (Yrs)* |
Child Age (Yrs) |
Mullens Score | Vineland Score | Language Level ** |
Parity |
|---|---|---|---|---|---|---|---|
| Autism (AU) Total | 61 | 31.1 ± 6.0 | 3.5 (2.1-5.0) | 56.9 ± 14.9 | 62.3 ± 12.9 | 1.2 ± 0.87 | 2.0 ± 1.0 |
| AU Regression | 36 | 30.7 ± 6.1 | 3.7 (2.2-5.0) | 57.6 ± 16.0 | 63.1 ± 12.9 | 1.0 ± 0.91 | 2.1 ± 1.1 |
| AU Early | |||||||
| Onset | 25 | 31.6 ± 6.1 | 3.2 (2.1-4.2) | 55.9 ± 13.2 | 61.1 ± 13.0 | 1.5 ± 0.71 | 1.8 ± 0.9 |
| Typically | |||||||
| Developing | 62 | 31.6 ± 6.2 | 3.3 (2.2-4.8) | 102.4 ± 22.5 | 104.9 ± 16.4 | NA | 2.3 ± 1.0 |
| Developmental | |||||||
| Delay | 40 | 28.4 ± 6.2 | 3.5 (2.0-4.9) | 57.4 ± 11.9 | 62.2 ± 15.2 | NA | 2.3 ± 1.1 |
All values represent Mean ± S.D., except age of child expressed as Mean (range).
ADIR language level score (0-2), where a higher score indicates more severe language deficit.
NA indicates that this evaluation was not performed in a particular subject population.
Detailed information regarding current autoimmune disease status for first-degree relatives was collected at the clinic visit by questionnaire (Hertz-Picciotto et al., 2006). A list of autoimmune conditions was reviewed with the parent by the clinician and descriptions were provided where needed.
2.2 Sample Collection
Maternal blood was collected in yellow top acid citrate dextrose tubes (BD Diagnostic, Franklin Lakes, NJ). Plasma was separated from cells, coded, and aliquoted to minimize freeze/thaw cycles and stored at −80°C until use.
2.3 Western blot analysis
Western blots were performed as described elsewhere (Cabanlit et al., 2007 (In Press)). Briefly, 300 μg human fetal brain protein medley (Clontech, Mountain View, CA), prepared from a pooled sample of 63 spontaneously aborted male and female fetuses 20-40 weeks gestation, was separated under reducing conditions on 4-15% SDS-polyacrylamide prep gels (Bio-Rad, Hercules, CA) and transferred electrophoretically to 0.2μm-pore nitrocellulose membranes (Whatman, Florham Park, NJ) at 35V for 14hrs. Magic mark XP molecular weight marker (Invitrogen, Carlsbad, CA) was used in the single marker lane allowing chemiluminescent visualization of marker bands from 20 to 220kDa. After transfer, the blots were blocked in 10% Casein Block (Pierce Biotechnology, Rockford, IL) and then cut into strips including the MW marker and 24 fetal brain strips. Strips were placed in mini-incubation trays (Bio-Rad, Hercules, CA) on a rocking platform with 700μl of 1:400 maternal plasma diluted in PBS/0.05% Tween 20/0.5% Casein Block (PBSTC) for 1.5hrs and then washed in PBS/0.05% Tween 20 (PBST) 5 times for 5 minutes each. Zymax horseradish peroxidase conjugated Goat anti-Human IgG (Invitrogen, Carlsbad, CA) diluted 1:25,000 in PBST was added and strips were incubated for 30 minutes with rocking. Following secondary antibody incubation, strips were washed 5 times for 5 minutes with PBST and subsequently incubated with SuperSignal West Pico (Pierce Biotechnology, Rockford, IL) chemiluminescent substrate for 5 minutes. The strips were then removed from the incubation trays and arranged on a glass plate for imaging using a FluorChem 8900 with AlphaEaseFC software (Alpha Innotech, San Leandro, CA) with a 1, 3, 5 minute stacked movie acquisition. Reactivity against proteins from control tissues including human adult brain (Clontech, Mountain View, CA), duodenum (Clontech, Mountain View, CA) and human kidney (Clontech, Mountain View, CA), was assessed using 300 μg/gel as described in the above technique.
Band presence and apparent molecular weight were determined using the image analysis capabilities of the AlphaEaseFC software. After defining the loading well position (protein migration start-point) and the dye front (end-point), relative migration (Rf) of each of the molecular weight markers was calculated. A point-by-point curve fit was applied to the Rf of the molecular weight markers and was used to determine the molecular weight of bands of maternal immunoreactivity to fetal brain. The presence of IgG heavy- and light-chain bands at approximately 25kDa and 50 kDa, arising from reactivity of the secondary antibody to endogenous IgG present in the protein preparations, provided an internal reference for each sample strip and was used to verify uniform protein migration. Blots were analyzed completely before revealing the diagnosis of the child. Bands were considered to be the same between samples when <4% difference was observed in Rf. The threshold for assigning the presence of a band was a two-fold higher densitometry reading above background on the strip.
2.4 Statistical Analysis
Statistical analysis was carried out with SAS statistical analysis software (SAS Institute Inc. Cary, NC). Comparisons of experimental groups were made using a Fisher's exact test applied to all bands individually and in all possible combinations to determine individual as well as grouped associations with diagnosis. Differences were considered significant at p < 0.05. Because of the presence of zero values, an odds ratio and 95% confidence interval could only be calculated for the 37kDa band.
3. Results
3.1 Band Prevalence
Autoreactivity to a protein at approximately 37kDa was observed in the plasma of 16/61 mothers of AU children (26.2%) (Figure 1 and Table 2) compared with 1/40 mothers of DD children (2.5%; p=0.0023), and 5/62 mothers of TD children (8.1%; p=0.0086) (Table 2). Furthermore, the presence of the 37kDa band yielded a significantly elevated odds ratio of 5.69 (95% confidence interval: 2.09 – 15.51) when compared with the TD group. Of particular note, reactivity against proteins at both 37kDa and 73kDa was observed only in mothers of AU children, yielding highly significant statistical differences between mothers of AU children and mothers of TD children (7/61 vs. 0/62; p=0.0061) and mothers of DD children (0/40; p=0.0401, Table 2). The presence of these bands did not correlate with maternal age or history of autoimmune disease, nor with child birth order or child IQ (data not shown).
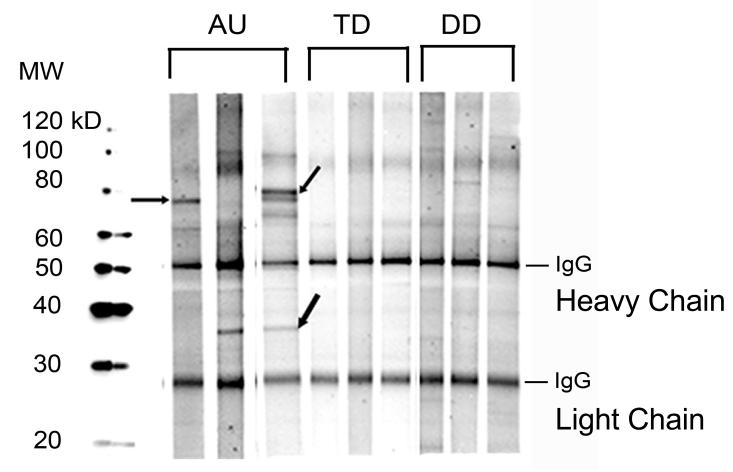
Figure 1.
Reactivity of maternal IgG against human fetal brain proteins is illustrated by western blot. Depicted are representative samples from the mothers of children with autism (AU) demonstrating typical patterns of reactivity against human fetal brain extract. Shown are the predominant bands at 73 kDa (upper arrows), 37 kDa (middle AU strip) and the 73 and 37 kDa (upper and lower arrows) bands, which are most specific for a diagnosis of autism. Plasma from three representative mothers of typically developing children from the general population lacks a response to human fetal brain. Similarly, the mothers of children with developmental delay but not autism (DD) do not express reactivity to the proteins recognized by the mothers of AU children.
Table 2.
Summary and significant associations of maternal autoantibody reactivity patterns for human fetal brain proteins.
| Prevalence (%) | 37kD & 73kD | 37kD | 73kd |
|---|---|---|---|
| AU (n=61) | 7 (12%)* | 15 (25%)* | 10 (17%) |
| AU Reg (n=36) | 6 (17%)* | 10 (28%)* | 9 (25%)* |
| AU EO (n=25) | 1 (4%) | 5 (21%) | 1 (4%) |
| TD (n=62) | 0 (0%) | 5 (8%) | 6 (9%) |
| DD (n=40) | 0 (0%) | 2 (5%) | 7 (17%) |
| Significance (p-value) | |||
| AU vs TD | 0.0061* | 0.0086* | 0.2 |
| AU vs DD | 0.0401* | 0.002* | 0.79 |
| AU Reg vs. AU EO | 0.223 | 0.777 | 0.106 |
| AU Reg vs TD | 0.0019* | 0.0174* | 0.078 |
| AU Reg vs DD | 0.0089* | 0.0023* | 0.388 |
| AU EO vs. TD | 0.287 | 0.0703 | 1 |
| AU EO vs. DD | 0.385 | 0.0109* | 0.4711 |
AU= autism; TD= typically developing; DD= developmental delay
3.2 Band reactivity and clinical onset of autism
When band prevalence was analyzed based on the pattern of clinical onset of autistic behaviors in children, 6/7 (86%) of the AU mothers that exhibited reactivity to the pair of bands at 37kDa and 73kDa had children with the regressive phenotype (Table 2). Moreover, this trend was also evident regarding reactivity to the 37kDa and 73kDa bands separately (Table 2). In contrast, the antibody response of the mothers of early onset children was only significantly different from the TD and DD groups for the 37kDa band alone (Table 2). Finally, no association was observed between the presence of autoreactivity to fetal brain antigens and history of autoimmune disease in the maternal populations at the time of blood draw.
3.3 Maternal antibody reactivity to control proteins
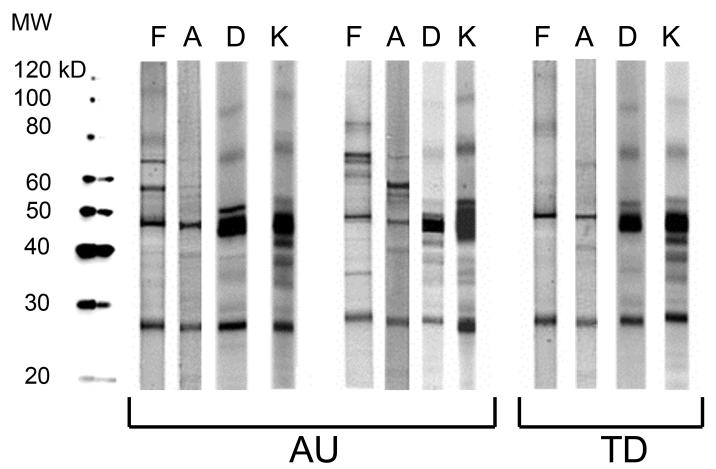
As a control for tissue specificity of these maternal antibodies, we analyzed several other tissue protein extracts for reactivity patterns. Duodenum, a highly innervated compartment of the GI tract, was chosen as a non-CNS tissue with substantial levels of neuronal proteins. We also examined reactivity of maternal IgG to kidney protein extract, and found, as with duodenum, an absence of the 37kDa and 73kDa protein pattern of reactivity. Similarly, although faint bands were sometimes observed at 73kD, the specific pattern of both the 37kDa and 73kDa bands was not seen for human adult brain protein extract (Figure 2).
Figure 2.
Tissue specificity of maternal antibody reactivity. Plasma antibodies of mothers of children with autism and controls were assessed for reactivity against human proteins from tissues other than fetal brain. Note that the significant bands visualized for fetal brain are absent in adult brain, duodenum, and kidney. F= Fetal Brain; A= Adult Brain; D= Duodenum; K= Kidney.
4. Discussion
Herein we present a detailed analysis of maternal antibodies to human fetal brain in a large cohort of mothers for whom detailed familial information is available. The presence of autoantibodies to proteins at 37kDa and 73kDa occurred significantly more often in AU mothers when compared with two distinct control populations. Bands that were shown to be different between autism and controls were seen in more than a quarter of mothers who had children with autism. The fact that these bands were not found in all mothers of children with autism further emphasizes the heterogeneity that is widely reported in autism, and the variety of etiologic mechanisms that likely exist (Hertz-Picciotto et al., 2006). Furthermore, while none of the individual bands were noted exclusively in mothers of children with AU, the simultaneous presence of the 37kDa and 73kDa protein bands was unique to the AU group.
Previous studies have also suggested a role for maternal antibodies in the etiology of some cases of autism (Dalton et al., 2003). Moreover, Zimmerman et al. (2006) recently reported differing patterns of serum immunoreactivity to pre-natal rat brain between mothers of children with autism and mothers of control children. Furthermore, the authors demonstrated that immunoreactivity persisted in maternal circulation for up to 18 years post-delivery (Zimmerman et al., 2006). Interestingly, the group differences in brain reactivity patterns were observed only with pre-natal rat brain protein and not post-natal (day 8) rat brain protein. The patterns described in the Zimmerman study differ from those presented in the current report, which is possibly due to disparities between rat and human brain proteins, or differences in sample processing. However, the presence of maternal antibody reactivity against neuronal protein associated with an outcome of autism in the child, is consistent across the two studies.
The transplacental passage of maternal IgG isotype antibodies has long been known as a mechanism for fetal immune instruction (Garty et al., 1994) and protection (Harris et al., 2006; Simister, 2003). A recently described organelle in the placental epithelium that expresses the low affinity IgG receptor, FcγRIIb, as well as the IgG receptor and transport protein FcRn, appears to provide a dedicated transport mechanism for maternal IgG to enter fetal circulation (Mishima et al., 2006). Detectable levels of maternal IgG are present in fetal circulation as early as 18 weeks gestation, and by 38 weeks gestation, fetal levels are comparable with maternal levels. Interestingly, neonatal IgG, which is overwhelmingly maternal in origin, is seen at levels exceeding the maternal concentration at delivery and persists at detectable levels up to 6 months post-delivery (Garty et al., 1994). Further, the presence of IgG heavy- and light-chain bands in the fetal brain immunoblots supports the transport of maternal IgG into the fetal brain during gestation. Thus the window of exposure to maternal IgG coincides substantially with critical periods of early neurodevelopment.
Despite the beneficial nature of the majority of maternal IgG received by the fetus, a number of neonatal autoimmune diseases have been demonstrated to result from pathogenic maternal IgG. Notably, the presence of maternal anti-Ro/SS-A and anti-La/SS-B antibodies cause neonatal lupus syndrome, often leading to congenital heart block (Tincani et al., 2006) In addition, cases of neonatal anti-phospholipid syndrome (APS), mediated through maternal autoantibodies, have been observed in the newborn infants of mothers with primary APS (Soares Rolim et al., 2006). Finally, abnormal thyroid function is often noted in infants born to mother with Hashimoto's thyroiditis or Graves' disease, caused by placental transfer of maternal anti-thyroid antibodies(Fu et al., 2005). Typically, symptoms of neonatal thyroiditis resolve as maternal antibodies are cleared from the circulation of the infant.
Our data suggest that the presence of maternal autoantibodies to fetal brain proteins of approximately 37kDa and 73kDa molecular weight confers an elevated risk for autism. We performed western blots using several other protein sources in order to determine the tissue specificity of these autoantibodies. Interestingly, the reactivity observed towards fetal brain protein was not seen among kidney, duodenum or adult brain proteins. This finding suggests the possibility that the autoreactivity described herein is targeted towards proteins expressed exclusively, or at substantially higher levels, during fetal development.
Maternal plasma collection for the current retrospective study occurred on average 3.5 years after the birth of the affected child, and approximately 18 months after a diagnosis of autism. As circulating antibody titers are known to vary over time based on the immunological state of the individual (Toptygina et al., 2005), the maternal antibody profile observed at the time of the registration of her child into the CHARGE study may be slightly different than during gestation. However, it has been demonstrated that antibodies, such as those generated in response to vaccination, can persist for many years due to the maintenance and subsequent polyclonal reactivation of memory B cells (Shinefield et al., 2002). It is currently unknown whether or not successive children from those mothers with reactivity to fetal brain will have autism. A longitudinal analysis of subsequent offspring from these control mothers will allow us to resolve this issue.
Increasing attention has been given to the notion that autism, as a spectrum of disorders, likely encompasses numerous, etiologically distinct behavioral phenotypes. Our observation of maternal reactivity to two protein bands at 37kDa and 73kDa more frequently in mothers of AU children exhibiting behavioral regression than in those with early onset AU may help to elucidate biologic mechanisms contributing to phenotypic variance in ASD. Assuming that the observed maternal autoantibody reactivity was also present during the prenatal and/or early postnatal period, the association of autoantibodies to neural antigens with delayed onset autism appears paradoxical. While beyond the scope of the present study, this could be explained by a pathogenic mechanism involving the interference of maternal autoantibodies with neurodevelopmental pathways for which compensatory mechanisms exist, but are ultimately overwhelmed, leading to disease symptoms. Such a pathogenesis is noted in Rett syndrome, where mutations in the gene Mecp2 manifest in behavioral regression around 18 months of age (Williamson and Christodoulou, 2006). Finally, it is important to note that the presence of maternal autoantibodies to both the 37 kDa and 73 kDa proteins does not provide an etiologic mechanism for all cases of regressive autism, and their presence is strongly associated with the regressive phenotype only in a sub-population of individuals.
These data provide evidence for an association between the presence of maternal immune system biomarkers and a diagnosis of autism in a subset of children. The presence of specific anti-fetal brain antibodies in the circulation of mothers during pregnancy may be a potential trigger that, when paired with genetic susceptibility, is sufficient to induce a downstream effect on neurodevelopment leading to autism. At present, we are investigating maternal plasma reactivity against fetal brain in a prospective cohort to determine the effect of the gestational autoantibody profile as it relates to an outcome of autism. Furthermore, work is currently under way to determine the protein targets of these antibodies, the identification of which will allow us to better understand potential pathogenic mechanisms as well as create specific screening assays.
Acknowledgments
Grant support: NIEHS 1 P01 ES11269-01, the U.S. Environmental Protection Agency (U.S. EPA) through the Science to Achieve Results (STAR) program (Grant R829388), the UC Davis M.I.N.D. Institute,
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- APA . Diagnostic and statistical manual of mental disorders: DSM-IV. American Psychiatric Association; Washington, DC: 1994. p. 886. [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific Autoantibodies in the Plasma of Subjects with Autistic Spectrum Disorders. PNYAS. 2007 doi: 10.1196/annals.1381.010. In Press. [DOI] [PubMed] [Google Scholar]
- Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, Styles P, Vincent A. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53:533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- DiLavore PC, Lord C, Rutter M. The pre-linguistic autism diagnostic observation schedule. J Autism Dev Disord. 1995;25:355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- Fu J, Jiang Y, Liang L, Zhu H. Risk factors of primary thyroid dysfunction in early infants born to mothers with autoimmune thyroid disease. Acta Paediatr. 2005;94:1043–1048. doi: 10.1111/j.1651-2227.2005.tb02043.x. [DOI] [PubMed] [Google Scholar]
- Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1:667–669. doi: 10.1128/cdli.1.6.667-669.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NL, Spoerri I, Schopfer JF, Nembrini C, Merky P, Massacand J, Urban JF, Jr., Lamarre A, Burki K, Odermatt B, Zinkernagel RM, Macpherson AJ. Mechanisms of neonatal mucosal antibody protection. J Immunol. 2006;177:6256–6262. doi: 10.4049/jimmunol.177.9.6256. [DOI] [PubMed] [Google Scholar]
- Heininger U, Desgrandchamps D, Schaad UB. Seroprevalence of Varicella-Zoster virus IgG antibodies in Swiss children during the first 16 months of age. Vaccine. 2006;24:3258–3260. doi: 10.1016/j.vaccine.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. J Child Psychol Psychiatry. 2002;43:807–821. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen MB, Pedersen CB, Mortensen PB. Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. J Child Psychol Psychiatry. 2005;46:963–971. doi: 10.1111/j.1469-7610.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Leventhal BL, Cook EH., Jr. Quantifying the phenotype in autism spectrum disorders. Am J Med Genet. 2001;105:36–38. [PubMed] [Google Scholar]
- Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, Fombonne E, Leboyer M, Minshew N. Diagnosing autism: analyses of data from the Autism Diagnostic Interview. J Autism Dev Disord. 1997;27:501–517. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- Mishima T, Kurasawa G, Ishikawa G, Mori M, Kawahigashi Y, Ishikawa T, Luo SS, Takizawa T, Goto T, Matsubara S, Takeshita T, Robinson JM, Takizawa T. Endothelial Expression of Fc Gamma Receptor IIb in the Full-term Human Placenta. Placenta. 2006 doi: 10.1016/j.placenta.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Mullen E. The Mullen Scales of Early Learning. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- Owley T, McMahon W, Cook EH, Laulhere T, South M, Mays LZ, Shernoff ES, Lainhart J, Modahl CB, Corsello C, Ozonoff S, Risi S, Lord C, Leventhal BL, Filipek PA. Multisite, double-blind, placebo-controlled trial of porcine secretin in autism. J Am Acad Child Adolesc Psychiatry. 2001;40:1293–1299. doi: 10.1097/00004583-200111000-00009. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Williams BJ, Landa R. Parental report of the early development of children with regressive autism: the delays-plus-regression phenotype. Autism. 2005;9:461–486. doi: 10.1177/1362361305057880. [DOI] [PubMed] [Google Scholar]
- Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17:485–495. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- Shinefield HR, Black SB, Staehle BO, Matthews H, Adelman T, Ensor K, Li S, Chan I, Heyse J, Waters M, Chan CY, Vessey SJ, Kaplan KM, Kuter BJ. Vaccination with measles, mumps and rubella vaccine and varicella vaccine: safety, tolerability, immunogenicity, persistence of antibody and duration of protection against varicella in healthy children. Pediatr Infect Dis J. 2002;21:555–561. doi: 10.1097/00006454-200206000-00014. [DOI] [PubMed] [Google Scholar]
- Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006 doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Soares Rolim AM, Castro M, Santiago MB. Neonatal antiphospholipid syndrome. Lupus. 2006;15:301–303. doi: 10.1191/0961203306lu2295cr. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales. American Guidance Service; Circle Pines, MN: 1984. [Google Scholar]
- Steinhausen HC, Erdin A. Abnormal psychosocial situations and ICD-10 diagnoses in children and adolescents attending a psychiatric service. J Child Psychol Psychiatry. 1992;33:731–740. doi: 10.1111/j.1469-7610.1992.tb00908.x. [DOI] [PubMed] [Google Scholar]
- Tincani A, Nuzzo M, Motta M, Zatti S, Lojacono A, Faden D. Autoimmunity and pregnancy: autoantibodies and pregnancy in rheumatic diseases. Ann N Y Acad Sci. 2006;1069:346–352. doi: 10.1196/annals.1351.032. [DOI] [PubMed] [Google Scholar]
- Toptygina AP, Pukhalsky AL, Alioshkin VA. Immunoglobulin G subclass profile of antimeasles response in vaccinated children and in adults with measles history. Clin Diagn Lab Immunol. 2005;12:845–847. doi: 10.1128/CDLI.12.7.845-847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . ICD-10: international statistical classification of diseases and related health problems, 10th revision. World Health Organization; Geneva: 1992. [Google Scholar]
- Williamson SL, Christodoulou J. Rett syndrome: new clinical and molecular insights. Eur J Hum Genet. 2006;14:896–903. doi: 10.1038/sj.ejhg.5201580. [DOI] [PubMed] [Google Scholar]
- Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, Pearce DA. Maternal antibrain antibodies in autism. Brain Behav Immun. 2006 doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]