Abstract
BACKGROUND
Previous studies suggest that patients who are more involved in their medical care have better outcomes.
OBJECTIVES
We sought to compare health care processes and outcomes for patients with HIV based on their preferred level of involvement in health decisions.
DESIGN
Cross-sectional analysis of audio computer-assisted interviews with patients at an urban HIV clinic.
PATIENTS
One thousand and twenty-seven patients awaiting an appointment with their primary care provider.
MEASURES
Patients were asked how they preferred to be involved in decisions (doctor makes most or all decisions, doctor and patient share decisions, patient makes all decisions). We also asked patients to rate the quality of communication with their HIV provider, and their self-reported receipt of and adherence to HAART.
RESULTS
Overall, 23% patients preferred that their doctor make all or most decisions, 63% preferred to share decisions with their doctor, and 13% preferred to make all final decisions alone. Compared to patients who prefer to share decisions with their HIV provider, patients who prefer that their provider make all/most decisions were significantly less likely to adhere to HAART (OR [odds ratio] 0.57, 95% CI 0.38–0.86) and patients who preferred to make decisions alone were significantly less likely to receive HAART or to have undetectable HIV RNA in unadjusted analyses (OR 0.52, 95% CI 0.31–0.87 for receipt of HAART; OR 0.64, 95% CI 0.44–0.95 for undetectable HIV RNA). After controlling for potentially confounding patient characteristics and differences in patient ratings of communication quality, patients who preferred that their provider make all/most decisions remained significantly less likely to adhere to HAART (OR 0.58, 95% CI 0.38–0.89); however, the associations with receipt of HAART and undetectable HIV RNA were no longer significant (OR 0.60, 95% CI 0.34–1.05 for receipt of HAART; OR 0.80, 95% C.I 0.53–1.20 for undetectable HIV RNA).
CONCLUSIONS
Although previous research suggests that more patient involvement in health care decisions is better, this benefit may be reduced when the patient wants to make decisions alone. Future research should explore the extent to which this preference is modifiable so as to improve outcomes.
KEY WORDS: patient involvement in care, patient–provider relationship, HIV, medication adherence
BACKGROUND
The paternalism that characterized interactions between providers and patients in earlier years has evolved into a model of the patient–provider relationship in which the patient’s role in his or her own medical care is appreciated to a greater extent. Patient involvement in care has therefore become an increasingly important area of research in many chronic diseases as the roles of the patient and provider have evolved. Studies have found that patients who report greater involvement in medical care are more satisfied with their physicians1–3, report more understanding, reassurance, and perceived control over their illness1,2, and have improvements in medical conditions1. Furthermore, interventions to increase patient involvement have had beneficial effects on satisfaction and functional status4, blood glucose (in diabetic patients)5,6, quality of life6, and the frequency and length of hospitalizations7,8.
Less is known specifically about the impact of the patient’s preference for involvement in decisions on patient outcomes. We do know from previous studies that the benefits of being involved in one’s own health care are not influenced by the patient’s preference for involvement, such that patients seem to benefit from becoming more involved in their own health care regardless of how much they want to be involved9,10. A considerable amount of research is aimed at identifying which factors are related to patient preference for involvement in decisions11, and 1 study has found that patients who prefer a more active role are less satisfied when their physicians do not support their preference12.
Among patients with HIV, patient involvement in care has not been as well studied, but is particularly important for several reasons. As a whole, patients with HIV infection have been particularly active in gaining knowledge and advocating for treatment13. It is unclear, however, whether individual HIV-infected patients desire an involved role in decisions made with their providers, or what consequences their desire might have. With the advent of effective therapy, HIV has become a chronic disease with many different treatment options, which pose different benefits and risks, and therefore the involvement of the patient in treatment decisions is increasingly important. Furthermore, because antiretroviral medication regimens require that the patient be adherent to avoid resistance and disease progression, it is important that patients themselves be committed to the medication plans. We have designed this study to understand how health care processes and outcomes may differ based on preferred level of involvement in health decisions among patients with HIV infection.
METHODS
Design, Subjects, and Setting
We conducted a cross-sectional analysis of 1,027 interviews with HIV-infected patients to evaluate the association between patient decision-making role preference and 3 outcomes: receipt of HAART, adherence to HAART, and absence of detectable serum HIV-1 RNA. This study took place as part of the Johns Hopkins HIV Clinical Cohort Study,14 which routinely interviews patients every 6 months using an audio computer-assisted survey instrument (ACASI). All patients in the HIV clinic are eligible for recruitment into the HIV cohort study, and less than one-half of 1% refuse. Patients were interviewed while awaiting appointment with their primary care provider at an urban clinic specializing in HIV care, and were compensated $5 per interview. Data for this analysis were collected from December 2004 to January 2006.
Measures
To measure the patients’ decision-making role preference, we used a single item developed by Brody et al.1, which asks patients to indicate what role they would like to play during their visit with their doctor. Possible response categories are: “the doctor takes the initiative and decides what is best for me”; “the doctor considers some of my ideas but still makes most, if not all, of the final decisions”; “the doctor and I make the final decisions together”; and “I make all of the final decisions.” We modified this item such that the term “HIV provider” was used instead of the term “doctor.” In our analysis, we collapsed the first 2 responses, as both indicate the patient’s preference that the provider make the decisions.
For our primary analyses, the association of the above measure was determined for the following 3 outcome measures: receipt of HAART among eligible patients, adherence to HAART among those on therapy, and the absence of detectable HIV-1 RNA. Receipt of HAART was measured by patient self-report and confirmed by chart review. Patients were considered to be taking HAART if they were on any regimen that met national guidelines15 for antiretroviral therapy relevant to that date. Patients were considered eligible for HAART either if they were on HAART or if they had a CD4 count that was less than 350 cells/μL. For the patients receiving HAART, adherence to HAART was measured by self-report using a validated survey16 in which patients are asked to report how many doses they have missed using a 24-hour, 3-day, and 2-week recall. In this study, we considered patients adherent to HAART if they had not missed any doses in the past 3 days. Serum HIV-1 RNA was measured using the Roche Amplicor assay within 4 weeks of the patient interview. Patients with less than 50 copies/mL were considered for this study to have undetectable serum HIV RNA.
We measured additional patient characteristics, using the ACASI, including patient age, sex, race/ethnicity, any illicit drug use in the previous 6 months, and the presence of depressive symptoms using the 5-item CES-D depression screening instrument17. For illicit drug use, patients were first asked about the use of illicit substances generally, and then asked specifically about cocaine, heroin, and marijuana separately. A positive response to the more general statement or to any of these drugs was considered illicit drug use. In addition, we measured the quality of patient–provider communication using patient ratings on the ACASI. Three particular aspects of communication were considered relevant to this study (theoretically or previously shown to be related to either decision-making preference or outcomes) and were measured using previously validated items. The degree to which patients believed they were known “as a person” was measured with an item that asked patients to respond “yes,” “no,” or “don’t know” to the statement “My HIV provider really knows me as a person.”18 The extent to which patients felt they were actually involved in decisions was measured by asking patients to complete the statement “Does your HIV provider involve you in decisions about your care...” with “as much as you wanted,” “almost as much as you wanted,” “less than you wanted,” or “a lot less than you wanted”19,20. Finally, the degree to which patients understand their providers’ explanations was measured by asking patients to respond “never,” “sometimes,” “usually,” or “always” to the statement “My HIV provider explains things in a way I can understand.”21
Analysis
Our analysis was cross-sectional, and guided by the conceptual framework presented in the Figure 1. Our independent variable was patient self-reported decision-making preference: we compared patients who preferred that their HIV provider make most/all decisions and patients who preferred that they make all decisions alone to patients who preferred that they share decisions with their HIV provider. We used analysis of variance for continuous variables and chi-squared tests for categorical variables to compare patient characteristics and communication quality across decision-making role preference.
Figure 1.
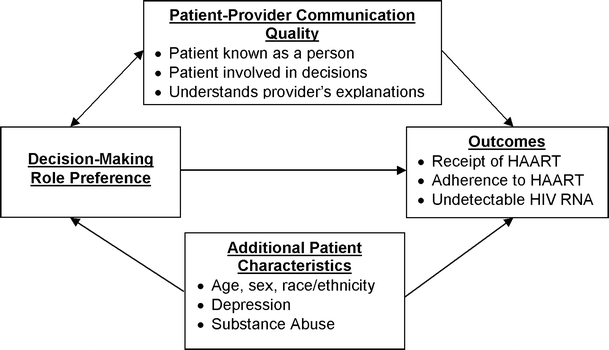
Conceptual framework for associations between decision-making role preference and outcomes
Our 3 outcome measures (receipt of HAART among eligible patients, adherence to HAART among those on therapy, and the absence of detectable HIV-1 RNA) were dichotomous as described above. We used chi-squared and logistic regression analyses to examine the associations between decision-making preference and each of the 3 outcome measures in unadjusted and adjusted analyses controlling for patient characteristics and communication quality. Analyses examining associations between decision-making role preference and receipt of HAART were limited to a subset of our sample who were designated as eligible for HAART. Analyses examining associations between decision-making role preference and adherence to HAART were limited to a subset of patients who were receiving HAART. Finally, analyses examining associations between decision-making role preference and HIV RNA levels were limited to a subset of our sample on whom we had data available. Data were analyzed using Stata Version 7.0.
RESULTS
Study Sample
Characteristics of the study sample are presented in Table 1. The study population was young (mean age 39.0 years), mostly male (64.9%), and predominantly African American (83.4%). There was a high prevalence of substance abuse and depressive symptoms: 20.9% used illicit drugs in the past 6 months and 23.9% of patients reported current depressive symptoms with a score ≥10 on the 5-item CES-D screening questionnaire. In our sample, the majority of illicit drug use was cocaine (12%), heroin (9%), and marijuana (6%) (categories were not mutually exclusive). Patients generally reported good communication with their primary HIV providers: 82.2% believed that they were known “as a person” by their HIV provider, 86.0% felt they were involved in decisions to the extent that they wanted, and 82.9% reported that their provider always explains things in a way they can understand.
Table 1.
Characteristics of Study Sample Associated with Decision-Making Role Preference
| Total sample | Decision-making role preference | Overall p value* | |||
|---|---|---|---|---|---|
| Provider and patient share decisions N = 651 | Provider makes decisions N = 240 | Patient makes decisions alone N = 136 | |||
| Patient Characteristics | |||||
| Age, Mean (SD) | 39.0 (8.7) | 39.3 (8.6) | 39.4 (7.7) | 36.8 (10.1)† | 0.007 |
| Sex, % Male | 64.9 | 64.7 | 65.4 | 64.7 | 0.978 |
| Race, % African American | 83.4 | 81.7 | 85.8 | 86.8 | 0.178 |
| Drug Use, % Active | 20.9 | 19.2 | 25.4 | 21.3 | 0.128 |
| Depression, %> = 10 CES-D | 23.9 | 22.7 | 24.7 | 28.2 | 0.384 |
| Communication Quality | |||||
| % Known As a Person | 82.2 | 81.4 | 89.6† | 72.8† | <0.001 |
| % Involved in Decisions | 86.0 | 88.8 | 81.3† | 80.9† | 0.003 |
| % Always Understand Explanations | 82.9 | 86.5 | 77.1‡ | 75.7† | <0.001 |
*Using analysis of variance for continuous variables and chi-squared tests for dichotomous variables
†p ≤ 0.01 compared with shared decisions
‡ p ≤ 0.001 compared with shared decisions
Characteristics Associated with Decision-making Preference
Overall, 240 patients (23%) preferred a more passive role in decision-making, responding either that “the doctor takes the initiative and decides what is best for me” or “the doctor considers some of my ideas but still makes most, if not all, of the final decisions”; 651 patients (63%) preferred that “the doctor and I make all final decisions together”; and 136 (13%) preferred that “I make all final decisions.”
Characteristics associated with decision-making role preference are also shown in Table 1. There were no significant differences in decision-making preferences based on patient sex, race, depressive symptoms, or drug use. Compared to patients who wanted to share decisions, patients who preferred that their provider make most or all decisions were significantly more likely to feel known as a person, less likely to have been involved in decisions to the extent that they wanted in the past, and less likely to understand their provider’s explanations. Patients who preferred that they make all decisions alone were significantly younger, less likely to feel known as a person by their HIV provider, less likely to have been involved in decisions to the extent that they wanted in the past, and less likely to understand their provider’s explanations.
Associations between Decision-making Preference and Outcomes
Overall, 687 (84%) of the 821 patients eligible for HAART were receiving it, and 544 (79%) of the 687 patients taking HAART were adherent to it. Among all patients, 429 (46%) of the 948 for whom laboratory data were available had undetectable HIV-1 RNA. Unadjusted associations between decision-making preference, communication quality, additional patient characteristics, and HIV outcomes are shown in Table 2. Patients who preferred that their HIV provider make most/all decisions were equally likely to receive, significantly less likely to adhere to HAART, and equally likely to have undetectable HIV-1 RNA as patients who preferred to share decisions with their provider. Patients who preferred to make all decisions themselves were significantly less likely to receive HAART, equally likely to adhere to HAART, and less likely to have undetectable HIV-1 RNA than those who preferred to share decisions.
Table 2.
Characteristics of Study Sample Associated with Receipt of HAART, Adherence to HAART, and Undetectable HIV RNA
| Overall sample | On HAART N = 821 (patients eligible for HAART) | Adherent to HAART N = 687 (patients on HAART) | Undetectable HIV RNA N = 948 (patients with available data) |
|---|---|---|---|
| 687 (83.7%) | 544 (79.2%) | 429 (46.3%) | |
| Decision-making role preference | |||
| Shares decisions (reference) | 85.8% | 81.7% | 47.6% |
| Provider decides | 82.1% | 71.8%† | 43.8% |
| Patient decides | 75.8%* | 81.3% | 36.9%* |
| Additional patient characteristics | |||
| Age | |||
| ≤35 (reference) | 79.7% | 78.0% | 36.2% |
| 35–50 | 84.2% | 78.4% | 47.9%‡ |
| >50† | 93%† | 87.1% | 57.9%‡ |
| Sex | |||
| Male | 84.9% | 80.7% | 45.8% |
| Female | 81.3% | 76.1% | 44.3% |
| Race | |||
| African American | 82.3% | 77.1% | 43.0% |
| White | 89.8%* | 87.9%† | 56.8%† |
| Drug use | |||
| Active | 66.9% | 71.4% | 27.0% |
| Inactive | 87.6%‡ | 80.6%* | 50.0%‡ |
| Depression | |||
| ≥10 CES-D | 77.0% | 68.1% | 39.4% |
| <10 CES-D | 85.5%† | 82.3%‡ | 47.4%* |
| Communication quality | |||
| Known as a person | |||
| Yes | 85.1% | 78.7% | 47.2% |
| No/Don’t know | 76.3%* | 82.0% | 36.0%† |
| Involvement in decisions | |||
| To the extent that patient wants | 84.8% | 80.2% | 47.0% |
| Less than patient wants | 76.8%* | 72.1% | 34.6%† |
| Provider explanations | |||
| Always understandable | 83.3% | 80.4% | 45.7% |
| Not Always understandable | 85.1% | 73.8% | 43.6% |
*p ≤ 0.05 compared with reference category
†p ≤ 0.01 compared with reference category
‡p ≤ 0.001 compared with reference category
In terms of communication quality, patients who believed that they were known “as a person” were significantly more likely to be on HAART when it was indicated, and to have undetectable HIV-1 RNA. Patients who were involved in decisions to the extent that they wanted were significantly more likely to receive HAART and to have undetectable HIV-1 RNA. Patients who reported that they always understood their provider’s explanations were no more or less likely to receive HAART, to adhere to it, or have undetectable HIV-1 RNA. In terms of additional patient characteristics related to HIV outcomes, younger patients, African-American patients, patients who actively used drugs (either “hard” drugs such as cocaine and heroin or “soft” drugs such as marijuana), and patients who had depressive symptoms tended to have worse outcomes than older patients, white patients, patients who were not actively using drugs, and patients who were not depressed, respectively (see Table 2).
Unadjusted and adjusted associations between decision-making preference and HIV outcomes are shown in Table 3. In both unadjusted and adjusted analyses, patients who prefer that their HIV provider make all/most decisions were significantly less likely to adhere to HAART than patients who preferred to share decisions with their provider. Patients who preferred to make decisions alone were significantly less likely to receive HAART or to have undetectable HIV-1 RNA in unadjusted analyses; however, this association was no longer significant after adjustment for other patient characteristics and communication quality.
Table 3.
Unadjusted and Adjusted Associations between Patients’ Decision-making Preference and Outcomes
| Patient prefers... | Unadjusted | Adjusted for patient characteristics* | Adjusted for patient characteristics and communication quality | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Receipt of HAART | To share decisions | ||||||
| Provider to decide | 0.76 | (0.49–1.17) | 0.83 | (0.53–1.31) | 0.83 | (0.52–1.31) | |
| To decide alone | 0.52 | (0.31–0.87) | 0.57 | (0.33–0.99) | 0.60 | (0.34–1.05) | |
| Adherence to HAART | To share decisions | ||||||
| Provider to decide | 0.57 | (0.38–0.86) | 0.56 | (0.36–0.85) | 0.58 | (0.38–0.89) | |
| To decide alone | 0.98 | (0.52–1.83) | 1.11 | (0.58–2.13) | 1.18 | (0.61–2.27) | |
| Undetectable HIV RNA | To share decisions | ||||||
| Provider to decide | 0.86 | (0.63–1.17) | 0.92 | (0.67–1.28) | 0.94 | (0.67–1.30) | |
| To decide alone | 0.64 | (0.44–0.95) | 0.74 | (0.49–1.11) | 0.80 | (0.53–1.20) | |
*Adjusted for patient age, race, drug use, and depression
DISCUSSION
As far as we are aware, this study is the first to examine decision-making preferences in HIV-infected adults. We found that patients who preferred to share decisions with their HIV provider had better outcomes than those who wanted their HIV provider to make decisions and those who wanted to make decisions alone. This is seemingly in contrast to research in other settings, which suggests that more patient involvement in health care decisions leads to better outcomes. In our study, the benefit of the patients being involved in health decisions was reduced when the patient preferred to make decisions alone.
One possible reason for why our findings seem to differ from those of previous studies is that we examined multiple outcomes, allowing us to distinguish the effect of decision-making preference on appropriate receipt of HAART from adherence to HAART. Our finding related to adherence is similar to findings in other studies: patients who want to share decisions with providers and those who want to make decisions alone are similarly likely to adhere, whereas those who want their provider to decide are less likely to adhere. In this regard, a higher degree of patient preference for involvement was associated with a better outcome, and it makes intuitive sense that patients who rely on their provider may have more difficulty with commitment to treatment plans when the provider is not present. Our finding related to receipt of HAART, however, adds a new dimension to previous studies. This new finding may also be intuitive: patients who preferred to make decisions alone were less likely to be on HAART when it was clinically indicated. Although we do not know exactly why these patients are less likely to take HAART, 1 possible explanation is that these patients are so independent that they may sometimes reject the advice or expertise of the HIV provider. Conversely, our finding that these patients are as adherent to HAART as decision sharers suggests that they are well able to take control of their medication regimen once they have committed to it.
We also found that most patients (63%) preferred to share decisions with their HIV provider. This is at the high end of the range (20–63%) found by a recent review of reported preferences for a shared decision-making role11, and indicates that patients with HIV tend to prefer levels of involvement that may be higher than those found among other groups. Perhaps this enhanced desire for involvement in decisions is related to the fact that patients with HIV have historically been activist, or because decisions for HIV treatments are complex, with substantial benefits, but also side effects and risks. Similar to studies on other groups of patients11, we found an association between decision-making preference and age, such that younger patients were more likely to prefer a more involved role than older patients. In contrast to other studies that have consistently found that women prefer a more involved role11, we did not find any association between gender and decision-making preference.
Finally, we found that patients who preferred to share decisions with their HIV provider reported better communication with their HIV providers. This suggests that providers may be able to influence the decision-making preferences of their patients through good communication. For example, patients who wanted to share decisions were more likely than patients in either of the other 2 groups to report that they understand their provider’s explanations and that they had been involved in decisions in the past. Therefore, providers who want to motivate patients toward a shared decision-making role must be able to explain complicated material well to patients, and engage them in their own health care. In addition, we found that patients who wanted to share decisions were also more likely to feel they were known “as a person” by their HIV provider than patients who wanted to make all decisions alone. This further suggests that 1 way for providers to assist highly independent patients in gaining trust and accepting advice might be to build a more personal relationship with them.
Several limitations are also worth noting. First, as with any observational study, there is the potential for unmeasured confusion, and causality cannot be determined. For example, a study of patients with cancer found that patients preferred more provider involvement in decisions as their cancer advanced and they became more ill22. If the same was true among patients with HIV, we might be suspicious of a cross-sectional association between a preference for a more provider involvement and greater illness as it could be inferred that a preference for more provider involvement caused the patient to become sicker. However, such an association was not found in our study, as it was patients who wanted the least provider involvement who were the least likely to have suppressed HIV RNA. Instead, we found an association between patient preference for more provider involvement and not taking antiretroviral medications, something that is not likely to be confounded by disease status. One other possible source of confounding is that patient preference for involvement in decision-making may be related to some global personality trait that is also associated with patient self-care, or the degree to which patients and providers are similar (i.e., concordant) with respect to race, ethnicity, gender, or sexual orientation. Finally, there is uncertain generalizability of our results from a single clinic to other settings.
These results suggest that practicing clinicians ought to encourage patients toward a shared decision-making role. This means not just activating patients who are disengaged, but also building trust and rapport with patients who are highly independent. Perhaps an explicit discussion between patients and their providers regarding decision-making roles would be helpful. However, it may not be easy to modify decision-making preferences, and it is unknown whether modifying these preferences can lead to improvements in patient outcomes. Future work is needed to determine the most effective way of modifying patients’ preference for involvement in decisions, and exploring which characteristics and behaviors of clinicians are most supportive of patients developing a shared decision-making preference.
Acknowledgments
Dr. Beach is a Robert Wood Johnson Generalist Physician Faculty Scholar and a recipient of a K-08 grant from the Agency for Healthcare Research and Quality. Other grant support: National Institutes of Health R01 DA11602, K24 DA11602, R21 AA105032.
Conflict of Interest None disclosed.
References
- 1.Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care: relationship to illness attitudes and outcomes. J Gen Intern Med. 1989;4(6):506–11. [DOI] [PubMed]
- 2.Lerman CE, Brody DS, Caputo GC, Smith DG, Lazaro CG, Wolfson HG. Patients’ perceived involvement in care scale: relationship to attitudes about illness and medical care. J Gen Intern Med. 1990;5(1):29–33. [DOI] [PubMed]
- 3.Kaplan SH, Gandek B, Greenfield S, Rogers W, Ware JE, Jr. Patient and visit characteristics related to physicians’ participatory decision-making style. Results from the Medical Outcomes Study. Med Care. 1995;33(12):1176–87. [DOI] [PubMed]
- 4.Greenfield S, Kaplan SH, Ware JE, Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520–8. [DOI] [PubMed]
- 5.Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24(4):695–700. [DOI] [PubMed]
- 6.Greenfield S, Kaplan SH, Ware JE, Jr., Yano EM, Frank HJ. Patients’ participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3(5):448–57. [DOI] [PubMed]
- 7.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256–62. [PubMed]
- 8.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37(1):5–14. [DOI] [PubMed]
- 9.Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psychooncology. 2006;15(1):9–19. [DOI] [PubMed]
- 10.Gattellari M, Butow PN, Tattersall MH. Sharing decisions in cancer care. Soc Sci Med. 2001;52(12):1865–78. [DOI] [PubMed]
- 11.Say R, Murtagh M, Thomson R. Patients’ preference for involvement in medical decision making: a narrative review. Patient Educ Couns. 2006;60(2):102–14. [DOI] [PubMed]
- 12.Harvey RM, Kazis L, Lee AF. Decision-making preference and opportunity in VA ambulatory care patients: association with patient satisfaction. Res Nurs Health. 1999;22(1):39–48. [DOI] [PubMed]
- 13.Wachter RM. AIDS, activism, and the politics of health. N Engl J Med. 1992;326(2):128–33. [DOI] [PubMed]
- 14.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(Suppl 1):S38–S41. [DOI] [PubMed]
- 15.U.S.Department of Health and Human Services (DHHS). Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. URL: http://aidsinfo.nih.gov (accessed 13 Apr 2005).
- 16.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000 12(3):255–66. [DOI] [PubMed]
- 17.Bohannon RW, Maljanian R, Goethe J. Screening for depression in clinical practice: reliability and validity of a five-item subset of the CES-Depression. Percept Mot Skills. 2003;97(3 Pt 1):855–61. [DOI] [PubMed]
- 18.Beach MC, Keruly J, Moore RD. Is the quality of the patient–provider relationship associated with better adherence and improved health outcomes for patients with HIV? J Gen Intern Med 2006 (in press). [DOI] [PMC free article] [PubMed]
- 19.Beach MC, Sugarman J, Johnson R, Arbelaez JJ, Duggan PS, Cooper LA. Do patients treated with dignity report more satisfaction, adherence, and preventive care? Ann Fam Med. 2005;3(4):331–8. [DOI] [PMC free article] [PubMed]
- 20.Collins KS, Hughes DL, Doty MM, Ives BL, Edwards JN, Tenney K. Diverse Communities, Common Concerns: Assessing health care quality for minority Americans. New York: The Commonwealth Fund; 2002.
- 21.Hays RD, Shaul JA, Williams VS, et al. Psychometric properties of the CAHPS 1.0 survey measures. Consumer Assessment of Health Plans Study. Med Care. 1999;37(3 Suppl):MS22–MS31. [DOI] [PubMed]
- 22.Mallinger JB, Shields CG, Griggs JJ, et al. Stability of decisional role preference over the course of cancer therapy. Psychooncology. 2006;15(4):297–305. [DOI] [PubMed]


