Abstract
Background
Evidence-based practices designed for large urban clinics are not necessarily portable into smaller isolated clinics. Implementing practice-based collaborative care for depression in smaller primary care clinics presents unique challenges because it is often not feasible to employ on-site psychiatrists.
Objective
The purpose of the Telemedicine Enhanced Antidepressant Management (TEAM) study was to evaluate a telemedicine-based collaborative care model adapted for small clinics without on-site psychiatrists.
Design
Matched sites were randomized to the intervention or usual care.
Participants
Small VA Community-based outpatient clinics with no on-site psychiatrists, but access to telepsychiatrists. In 2003–2004, 395 primary care patients with PHQ9 depression severity scores ≥12 were enrolled, and followed for 12 months. Patients with serious mental illness and current substance dependence were excluded.
Measures
Medication adherence, treatment response, remission, health status, health-related quality of life, and treatment satisfaction.
Results
The sample comprised mostly elderly, white, males with substantial physical and behavioral health comorbidity. At baseline, subjects had moderate depression severity (Hopkins Symptom Checklist, SCL-20 = 1.8), 3.7 prior depression episodes, and 67% had received prior depression treatment. Multivariate analyses indicated that intervention patients were more likely to be adherent at both 6 (odds ratio [OR] = 2.1, p = .04) and 12 months (OR = 2.7, p = .01). Intervention patients were more likely to respond by 6 months (OR = 2.0, p = .02), and remit by 12 months (OR = 2.4, p = .02). Intervention patients reported larger gains in mental health status and health-related quality of life, and reported higher satisfaction.
Conclusions
Collaborative care can be successfully adapted for primary care clinics without on-site psychiatrists using telemedicine technologies.
KEY WORDS: depression, telepsychiatry, rural
INTRODUCTION
The chronic care model for depression, known as collaborative care, improves depression treatment outcomes in primary care (PC) settings1–12 in a cost-effective manner.13–19 The chronic care model uses patient self-management, delivery system redesign, decision support, and clinical information systems to maximize the effectiveness of interactions between informed activated patients and prepared, proactive care teams.20–22Practice-based collaborative care involves primary care providers (PCPs) working with an on-site depression care team comprising nonphysicians (e.g., nurses, pharmacists) and mental health specialists (e.g., psychiatrists).
The underlying problems of treating depression are similar in rural or other isolated practices and larger urban practices. However, the solutions are not necessarily the same because evidence-based practices designed for large urban practices may not be portable into smaller practices where it is typically not feasible to employ mental health specialists on site.23 Only 25% of PC practices nationwide have on-site mental health specialists.24 Unless collaborative care models can be successfully adapted for small practices without on-site mental health specialists, patients treated in these settings will not benefit from dissemination efforts.
The Institute of Medicine Defines telemedicine as “the use of electronic information and communications technologies to provide and support health care when distance separates the participants.”25 The purpose of the Telemedicine Enhanced Antidepressant Management (TEAM) study was to adapt the collaborative care model for small PC practices without on-site psychiatrists. Telemedicine technologies (e.g., telephone, interactive video, electronic medical records, and internet) were used to facilitate communication between a centrally located off-site depression care team and PCPs practicing in geographically diverse clinic locations. We chose to conduct this first telemedicine-based collaborative care trial in the Veterans Administration (VA) because of the widespread use of interactive video technology and electronic medical records. VA treats about one-half million veterans for depression annually and delivers higher quality depression care than private practices.26 We hypothesized that telemedicine-based collaborative care would improve antidepressant prescribing, medication adherence, depression outcomes, health status, quality of life, and satisfaction.
METHODS
Study Setting and Enrollment Procedures
The intervention and evaluation methods are described in detail in a companion article.27 The study was conducted in VA community-based outpatient clinics (CBOCs), which are satellite facilities of “parent” VA Medical Centers (VAMC). Eligible CBOCs had to have interactive video equipment dedicated to mental health, but no on-site psychiatrists. The 7 eligible CBOCs in the South Central Veterans Healthcare Network were matched by parent VAMC, and one CBOC within each pair was randomized to the intervention. Five of the CBOCs had on-site midlevel mental health specialists (e.g., social workers).
We sought to enroll all patients with depression that PCPs would be comfortable treating, and excluded those with serious mental illness (Fig. 1). Administrative data were used to identify 24,882 patients due for annual depression screening, and 73.6% (n = 18,306) were successfully screened by phone using the Patient Health Questionnaire depression scale (PHQ9).28 6.9% of these patients screened positive for depression, defined as a PHQ9 score ≥12. This definition has a 96% specificity and 97% sensitivity for detecting depression.28 Exclusion criteria included a diagnosis of schizophrenia, current suicide ideation, recent bereavement, pregnancy, a court-appointed guardian, substance dependence, bipolar disorder, cognitive impairment, or receiving specialty mental health treatment. Among eligible patients, 91.3% agreed to participate and were administered the baseline interview, and 91.9% attended their appointment and provided written consented. We enrolled 395 patients between April 2003 and September 2004.
Figure 1.
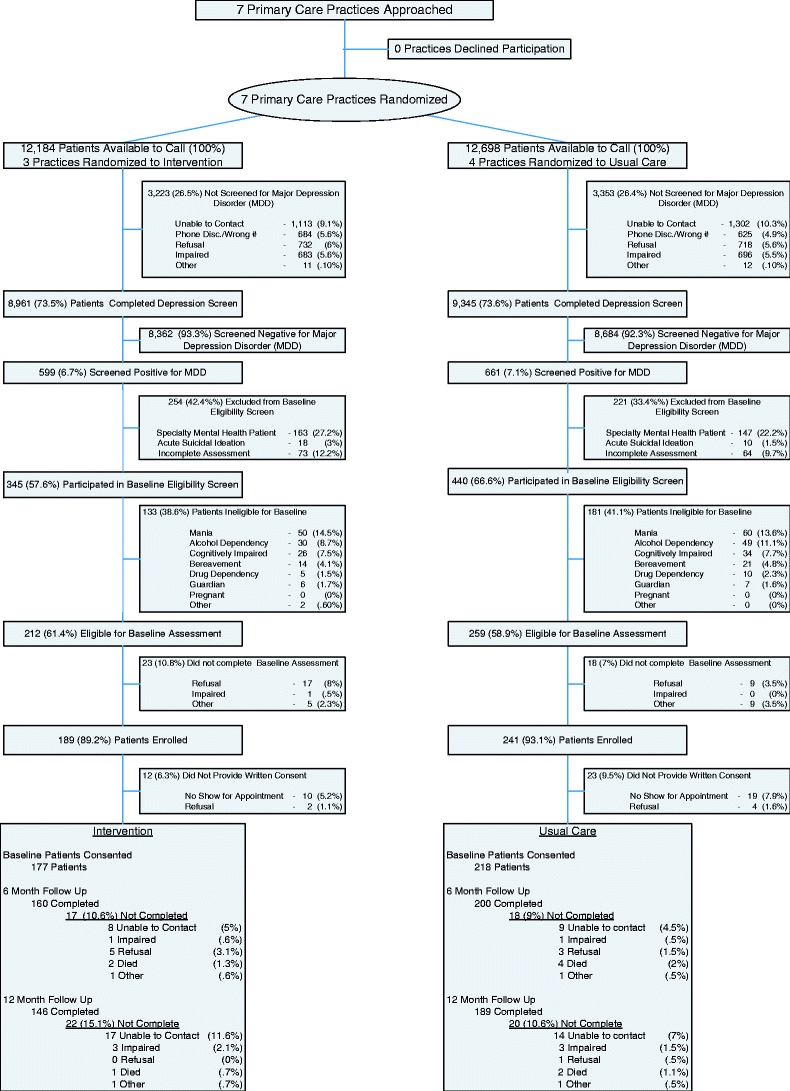
Enrollment of patients from 7 eligible primary care practices. Among 24,882 patients due for annual depression screening, 18,306 (74%) were screened.
TEAM Intervention
Provider education (via interactive video and website) and patient education (via mail and website) were provided to both intervention and usual care sites. Depression screening results were entered into the electronic medical record at both intervention and usual care sites. These intervention components were deemed necessary, but not sufficient, to improve outcomes.
Patients at intervention sites received a stepped-care model of depression treatment for up to 12 months. Treatment intensity was increased for patients failing to respond to lower levels of care by involving a greater number of intervention personnel with increasing mental health expertise. The intervention involved 5 types of providers: (1) PCPs located at CBOCs; 2) consult telepsychiatrists located at parent VAMCs; (3) an off-site depression nurse care manager (RN); (4) an off-site clinical pharmacist (PharmD); and 5) an off-site supervising psychiatrist. The consult-telepsychiatrist accepted consultations or referrals from PCPs. The supervising psychiatrist provided clinical supervision to the care manager and clinical pharmacist via weekly face-to-face meetings.
Patients and providers could choose either watchful waiting or antidepressant treatment (Step 1). Psychotherapy was available for all patients, but facilitating access to evidence-based psychotherapy was not an intervention component. Nurse care manager encounters were conducted via telephone and were scripted to enhance standardization and reproducibility. All scripts and instruments were administered using WinCATI software. During the initial care management encounter, patients were: (1) administered the PHQ9 symptom monitoring tool; (2) educated and activated using a semistructured script4; and 3) assessed for treatment barriers using semistructured scripts for endorsed barriers.4 Follow-up encounters to monitor symptoms, medication adherence, and side-effects were scheduled every 2 weeks during acute treatment and every 4 weeks during watchful waiting or continuation treatment. Non-adherent patients or those experiencing severe side effects were administered semistructured scripts.3 A trial was considered to have failed in the acute phase if the patient: (1) was nonadherent to the medication, (2) experienced severe side effects, (3) experienced ≥5-point increase in their PHQ9 score, or (4) did not respond (50% decrease in PHQ9 score) after 8 weeks of antidepressant therapy. All feedback was provided to PCPs using the electronic medical record. Progress notes reporting failed trials requested an electronic co-signature from the PCP.
If the patient did not respond to the initial antidepressant, the pharmacist conducted a medication history and provided pharmacotherapy recommendations to PCPs via an electronic progress note (Step 2). The pharmacist also provided nonscripted medication management over the phone to patients experiencing severe side-effects or problems with nonadherence. If the patient did not respond to 2 antidepressants trials, the protocol was to recommend a telepsychiatry consultation followed by additional treatment recommendations to the PCP (Step 3).
Data Collection
Data were collected via blinded telephone interview. At baseline, demographics and depression history were measured using the Depression Outcomes Module.29,30 Psychiatric comorbidity was measured using the Mini International Neuro-psychiatric Interview.31,32 Social support was measured using the Duke Social Support and Stress Scale.33,34 Acceptability of antidepressant treatment was measured using an item developed for the Quality Improvement for Depression studies.4,5 The Depression Health Beliefs Inventory was used to measure perceptions about depression treatment including barriers, need, and effectiveness.35 Follow-up telephone interviews were completed for 91.1% (n = 360) of the study participants at 6 months and 84.8% (n = 335) at 12 months (Fig. 1). The primary outcomes were antidepressant prescribing, medication adherence, and treatment response, and remission. Secondary outcomes included health status, quality of life, and satisfaction.
Antidepressant prescribing was determined from the active medications list in the electronic medical record. Patients with an active prescription were categorized as adherent if item responses indicated they took the full dosage ≥80% of the days in the previous month. This cutoff was chosen to facilitate comparison with other studies.36–38 Patients without an active prescription or who reported stopping antidepressants because of PCP instruction were excluded from the adherence analysis.
Depression severity was measured using the Hopkins Symptom Checklist (SCL-20).39,40 Response is measured dichotomously as a 50% improvement in depression severity between baseline and follow-up. Remission is defined dichotomously as SCL-20 < 0.5. Improvement in health status was measured by the change in the physical health and mental health component scores (PCS and MCS) of the Short Form (SF12V) between baseline and follow-up.41,42 Improvement in health-related quality of life was measured by the change in the Quality of Well Being (QWB) score.43–46 Satisfaction was measured dichotomously using the total behavioral health satisfaction measure from the Experience of Care and Health Outcomes Survey.47
Statistical Analysis
Patients were the unit of the intent-to-treat analysis. We did not adjust standard errors for potential nesting of patients within CBOCs or parent VAMCs as the intraclass coefficients were close to zero at the CBOC level (0.015) and the VAMC level (0.004) with respect to changes in SCL-20 scores. Independent variables with missing values were imputed using multiple imputation. Sampling and attrition weights were calculated from administrative and baseline data, respectively, to adjust for the potential bias associated with nonparticipation and/or loss to follow-up. Because of the large number of available casemix variables, only those found to significantly predict dependent variables at the p ≤ .2 level in bivariate analyses were included in multivariate analyses. Logistic and linear regression analyses were used to estimate intervention effects for dichotomous and continuous outcomes, respectively. Separate regression analyses were conducted to examine the 6- and 12-month outcomes. Intervention effect sizes were calculated using Cohen’s d statistic for continuous variables and the number needed to treat (NNT) statistic for dichotomous outcomes. The study was approved by the Research and Development Committees of the Central Arkansas Veterans Healthcare System in Little Rock, AR, the Overton Brooks VA Medical Center in Shreveport, LA, and the G.V. (Sonny) Montgomery VA Medical Center in Jackson, MS and their affiliated Institutional Review Boards at the University of Arkansas for Medical Sciences, and University of Louisiana Health Sciences Center at Shreveport.
RESULTS
Baseline Characteristics
At baseline, most (82.0%) met diagnostic criteria for major depressive disorder (Table 1). Virtually all patients reported having at least 1 serious chronic health condition and the average number was 5.5 (e.g., diabetes [32.9%], heart disease [32.2%], lung disease [20.3%], stroke [18.2%], and cancer [12.7%]). PCS and MCS scores of study participants were much lower than the general population and lower than typical veterans using VA PC services.48 Over half (57.2%) of the study participants reported that pain impaired their functioning. Psychiatric comorbidity was common, with 56.5% having at least 1 current anxiety disorder. Study participants averaged 3.7 prior depression episodes, 66% had received prior depression treatment, and 41% were receiving depression treatment at baseline.
Table 1.
Baseline Socioeconomic and Clinical Characteristics by Practice-Randomized Group Assignment
| Variables | Overall n = 395 | Intervention group n = 177 | Usual care group n = 218 | |
|---|---|---|---|---|
| Mean (SD) or Percentage | pvalue | |||
| Sociodemographic | ||||
| Age | 59.2 (12.2) | 58.4 (12.2) | 59.8 (12.1) | .24 |
| Male | 91.7% | 93.8% | 89.9% | .17 |
| Race | ||||
| White | 74.7% | 76.3% | 73.4% | .39 |
| Black | 18.2% | 15.3% | 20.6% | |
| Native American | 3.% | 4.0% | 3.2% | |
| Other | 3.6% | 4.4% | 2.8% | |
| Annual household income <$20,000 | 51.7% | 52.1% | 51.4% | .54 |
| Married | 62.3% | 62.7% | 61.9% | .87 |
| High school graduate | 76.0% | 74.6% | 77.1% | .57 |
| Employed | 21.9% | 24.4% | 20.0% | .32 |
| Social support (0–1) | 0.42 (0.22) | 0.42 (0.2) | 0.42 (0.2) | .79 |
| Perceived barriers (0-9) | 4.14 (1.87) | 3.99 (1.89) | 4.26 (1.84) | .16 |
| Perceived need (0–6) | 2.91 (1.45) | 2.79 (1.46) | 3.00 (1.45) | .16 |
| Perceived treatment effectiveness (0–2) | 1.22 (0.82) | 1.16 (0.85) | 1.26 (0.79) | .26 |
| Clinical | ||||
| PHQ9 (Depression Screen score) | 16.4 (3.4) | 16.3 (3.4) | 16.4 (3.4) | .77 |
| SCL20 (Depression Severity score) | 1.8 (0.7) | 1.9 (0.7) | 1.8 (0.7) | .76 |
| PCS (Physical Component score) | 30.0 (13.0) | 30.4 (13.5) | 29.7 (12.5) | .62 |
| MCS (Mental Component score) | 36.5 (12.3) | 36.1 (12.2) | 36.9 (12.4) | .56 |
| QWB (Quality of Well-Being score ) | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.1) | .65 |
| Chronic physical illnesses | 5.5 (2.8) | 5.3 (2.7) | 5.7 (2.8) | .16 |
| Family history of depression | 45.2% | 46.6% | 44.2% | .64 |
| Age depression onset <18 | 17.2% | 15.6% | 19.2% | .34 |
| Prior depression episodes | 3.7 (1.8) | 3.7 (1.8) | 3.6 (1.8) | .79 |
| Prior depression treatment | 65.7% | 66.5% | 65.1% | .78 |
| Current depression Treatment | 40.9% | 35.2% | 45.4% | .04 |
| Antidepressants acceptable | 79.4% | 79.9% | 78.9% | .28 |
| Current major depressive disorder | 82.0% | 83.1% | 81.2% | .63 |
| Current dysthymia | 4.1% | 2.8% | 5.1% | .27 |
| Current panic disorder | 9.6% | 9.6% | 9.6% | .99 |
| Current generalized anxiety disorder | 50.7% | 45.9% | 54.1% | .69 |
| Current post traumatic stress disorder | 23.8% | 24.9% | 22.9% | .66 |
| Current at-risk drinking | 12.9% | 13.0% | 12.8% | .96 |
Intervention Fidelity
For patients in the intervention group (n = 177), PCPs usually signed the positive depression screen note before the appointment (70.7%), although some signed it after the appointment (12.9%), or not at all (16.4%). The care manager completed initial encounters with 96.6% (n = 171) of patients. Average time to the initial encounter was 21.4 days (SD = 41.3) and the average duration of initial encounter was 37.2 minutes (SD = 13.0). For patients completing the initial encounter (n = 171), the average number of follow-up encounters during the acute stage was 7.3 (SD = 4.9) and the average duration was 23.0 minutes (SD = 7.4). PCPs signed 95% of the progress notes requiring an electronic signatures in the acute stage of treatment. Three quarters of intervention patients (73.7%, n = 126) had at least 1 medication trial and 59.5% (n = 75) failed the first trial. Of those with at least 1 medication trial, 27.0% had a second medication trial and 79.4% failed this trial. Two-thirds of patients (64.9%, n = 111) eventually entered the continuation phase of treatment, although 36% (n = 40) subsequently relapsed. Of those failing the first medication trial (n = 75), the pharmacist conducted medication histories for 98.7%, but only recommended specific medication changes for 20.0%. The depression care team never recommended a telepsychiatry consultation for patients failing a second antidepressant trial. The low number of recommended medication changes and telepsychiatry consultations was because of the fact that either the patient had already been referred to a mental health specialist or patient preference. In fact, 43.4% of intervention patients reported an encounter with a VA mental health specialist (including 30.7% who reported an encounter with a VA psychiatrist or telepsychiatrist).
Six- and 12-month Outcomes
The proportion of patients with an active antidepressant prescription was 70.0% at 6 months and 77.6% at 12 months, and multivariate analyses indicated no significant difference in the likelihood of having an active prescription between the groups at 6 (OR = 1.2, p = .52) and twelve months (OR = 1.3, p = .40). There was no significance difference in the number of PC visits between intervention (3.8) and usual care (3.9) patients.
Table 2 presents unadjusted outcomes along with multivariate results. Most patients in both groups reported taking the full dosage of their antidepressant ≥80% of days. Patients in the intervention group had significantly greater odds of being adherent than those in usual care at both 6 (OR = 2.1, p = .04) and 12 months (OR = 2.7, p = .01). At 6 months, patients in the intervention group were significantly more likely to respond (OR = 1.9, p = .02), but not to remit (OR = 1.8, p = .14) compared to usual care. By 12 months, the intervention group had significantly greater odds of remitting (OR = 2.4, p = .02), but not responding (OR = 1.4, p = .18). Most patients were satisfied with care, with 70.9% of the intervention group reporting that they were very or somewhat satisfied with their care for emotional problems at the 12-month follow-up compared to 61.4% in the usual care group. Patients in the intervention group had greater odds of being satisfied than usual care patients at both 6 (OR = 1.8, p = .01) and 12 months (OR = 1.7, p = .03).
Table 2.
Medication Adherence, Depression Outcomes, and Satisfaction
| Unadjusted estimates No. (%) | Adjusted analysis for intervention vs usual care | ||||
|---|---|---|---|---|---|
| Intervention | Usual Care | OR (95% CI) | P value | NNT* | |
| Medication Adherence† | |||||
| 6-month follow-up | 80 (74.5%) | 87 (68.3%) | 2.11 (1.02–4.36) | .04 | 8 |
| 12-month follow-up | 84 (76.4%) | 88 (66.2%) | 2.72 (1.36–5.44) | <.01 | 6 |
| Response | |||||
| 6-Month follow up‡ | 38 (23.8%) | 31 (15.5%) | 1.94 (1.09–3.45) | .02 | 11 |
| 12-Month follow-up§ | 53 (36.3%) | 51 (27.0%) | 1.42 (0.85–2.37) | .18 | – |
| Remission | |||||
| 6-Month follow up‡ | 22 (13.8%) | 17 (8.5%) | 1.79 (0.82–3.88) | .14 | – |
| 12-Month follow-up§ | 35 (24.0%) | 24 (12.7%) | 2.39 (1.13–5.02) | .02 | 11 |
| Satisfaction | |||||
| 6-Month follow up‡ | 110 (71.4%) | 111 (58.1%) | 1.83 (1.14–2.93) | .01 | 8 |
| 12-Month follow-up§ | 100 (70.9%) | 113 (61.4%) | 1.71 (1.06–2.77) | .03 | 9 |
*NNT = Number of patients needed to treat to achieve 1 additional successful outcome
†Analysis conducted on the subsample of patients with an active antidepressant prescription, and not reporting antidepressant discontinuation as a result of PCP instruction: (n = 229) at the 6-month follow-up and (n = 243) at the 12-month follow-up
‡Analysis conducted on patients completing the 6-month follow-up interview (n = 360)
§Analysis conducted on patients completing the 12-month follow-up interview (n = 335)
Patients in both groups experienced little change in PCS scores and the intervention did not significantly impact PCS (Table 3). However, patients in both groups experienced improvements in MCS scores and intervention patients experienced significantly greater increases in MCS scores at 12 (p = .01), but not 6 months (p = .07). QWB scores improved significantly more in the intervention group than in the usual care group by 6 months (p < .01), but not at 12 months (p = .70).
Table 3.
Health Status and Health-related Quality of Life
| Unadjusted estimates No. (SD) | Adjusted analysis for intervention vs usual care | ||||
|---|---|---|---|---|---|
| Intervention | Usual care | Grp diff (95% CI) | Pvalue | Effect size | |
| Change in PCS | |||||
| 6-month follow-up* | 0.074 (9.27) | −0.087 (9.42) | 0.31 (−1.61–2.24) | .75 | – |
| 12-month follow-up† | −0.34 (10.17) | −1.38 (10.31) | 1.09 (−0.94–3.12) | .29 | – |
| Change in MCS | |||||
| 6-month follow up* | 5.666 (14.03) | 2.686 (12.87) | 2.46 (−0.20–5.12) | 0.07 | – |
| 12-month follow-up† | 9.39 (15.18) | 4.69 (14.55) | 3.90 (0.97–6.83) | <0.01 | 0.46 |
| Change in QWB | |||||
| 6-month follow up* | 0.039 (0.118) | 0.003 (0.118) | 0.037 (0.01–0.06) | <0.01 | 1.43 |
| 12-month follow-up† | 0.039 (0.134) | 0.032 (0.128) | 0.005 (−0.02–0.03) | 0.70 | – |
*Analysis conducted on patients completing the 6-month follow-up interview (n = 360)
†Analysis conducted on patients completing the 12-month follow-up interview (n = 335)
DISCUSSION
Our primary finding is that telemedicine technologies can be used successfully to adapt the collaborative care model for implementation in small PC clinics lacking on-site psychiatrists. The TEAM intervention significantly improved medication adherence, depression severity, mental health status, health-related quality of life, and satisfaction. Our telemedicine-based collaborative care intervention had similar effect sizes compared to practice-based collaborative care interventions included in a recent meta analysis.49 The intervention’s impact on response (intermediate treatment goal) narrowed over time, whereas the impact on remission (ultimate treatment goal) increased over time. This suggests that symptoms improved more rapidly in the intervention group compared to the usual care group. By 6 months, this increased rate of symptom improvement led to a significant difference in response rates, although the difference in remission rates was not yet statistically detectable. By 12 months, this increased rate of symptom improvement resulted in a significant difference in remission rates, whereas usual care patients caught up to intervention patients in terms of response.
Based on the fidelity data, we speculate that the active intervention component was telephone-based supervised nurse care management and the resultant impact on medication adherence. This speculation is supported by the observation that a large proportion of intervention patients was referred from PC to mental health for ongoing treatment before the pharmacist or telepsychiatrist could recommend medication changes to the PCP. This finding highlights the importance of targeting referral policy when implementing collaborative care models in integrated systems of care. Where clinically appropriate, cost-effective referral policies should be developed to encourage mental health consultations (i.e., assessment and treatment recommendations). The lack of clinical collaboration (e.g., consultations) highlights the difficulty of facilitating team-based care among providers in geographically different locations. The importance of the nurse care manager suggests that outcomes can be modestly improved by implementing a nurse care management model without investing in interactive video equipment or reorganizing practices to provide team-based care in a virtual environment. However, to improve outcomes more substantially, we suspect that a greater degree of collaboration between PC and mental health will be needed.
Although intervention patients had significantly better outcomes than usual care patients, a large majority in both groups were nonresponsive to treatment. Our 12-month response rate (36.3%) is higher than VA patients receiving practice-based collaborative care (18.1% at 9 months)9 and public sector outpatients receiving education and algorithm-based antidepressant treatment (26.3.% at 12 months).50 However, taken together, these findings suggest that the high response rates reported in antidepressant efficacy trials do not necessarily generalize to public sector patients. Unlike the participants of antidepressant efficacy trials, the patients in this study had significant comorbidities, and many were receiving depression treatment before enrollment. The high prevalence of comorbidities and treatment resistance may explain the low response and remission rates observed in this study. It may be that collaborative care programs designed for public sector clinics need to be more intensive or comprehensive than those designed for private sector clinics. In addition to focusing on antidepressant management, it may be necessary to strongly emphasize patient self-management techniques (e.g., encouraging patients to exercise, participate in social activities, and pursue hobbies) and facilitate access to evidence-based psychotherapy (e.g., psychotherapy via interactive video or telephone).51,52 Finally, collaborative care programs targeting public sector patients should probably target common comorbidities such as pain, anxiety, and substance abuse.
Intervention studies are rarely conducted in small isolated PC practices owing to difficulties implementing a standardized protocol in geographically dispersed clinics and enrolling enough patients to have sufficient statistical power. Thus, a major strength of the TEAM study is the practice setting in which it was conducted. Although the VA is the largest managed care organization in the U.S., our results may not generalize to nonintegrated systems of care. Likewise, because VA patients are different from private sector patients, our results may not generalize to private health care settings. However, the advantage of conducting this first evaluation of telemedicine-based collaborative care in the VA was the widespread use interactive video and electronic medical record technology. Although the VA has been an early adopter of these technologies, interactive video and electronic medical records are being adopted rapidly in the private sector. Moreover, the care manager encounters were conducted by telephone, a technology that is widely available. Whereas technology itself may not pose a significant barrier to the diffusion of telemedicine-based collaborative care in the future, identifying organizations offering contractual arrangements for off-site depression care may present a substantial challenge in nonintegrated health care systems. However, if available, telemedicine-based collaborative care should be considered an evidence-based alternative to reallocating scarce internal resources to deliver practice-based collaborative care.
Acknowledgments
This research was supported by VA IIR 00-078-3 grant to Dr. Fortney, VA NPI-01-006-1 grant to Dr. Pyne, the VA HSR&D Center for Mental Health and Outcomes Research, and the VA South Central Mental Illness Research Education and Clinical Center. Drs. Pyne and Edlund were supported by VA HSR&D Research Career Awards. Dr. Mittal was supported by the VISN 16 South Central Network Research/Career Development Grant Program and VA South Central Mental Illness Research Education and Clinical Center. We would like to gratefully acknowledge all of the research staff who worked on the project, the veterans who participated, and the staff of the VA Community-Based Outpatient Clinics in Meridian, MS; Hattiesburg, MS; Mountain Home, AR; Hot Springs, AR; El Dorado, AR; Monroe, LA; and Longview, TX. In addition, we would like to recognize Dr. Raymond Kimble who took over as site-PI at the G.V. (Sonny) Montgomery VAMC after Dr. Mittal left.
Conflicts of Interest None disclosed.
References
- 1.Katon W, Von Korff M, Lin E, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry. 1999;56(12):1109–15. [DOI] [PubMed]
- 2.Katon W, Robinson P, Von Korff M, et al. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry. 1996;53(10):924–32. [DOI] [PubMed]
- 3.Simon GE, VonKorff M, Rutter C, Wagner E. Randomised trial of monitoring, feedback, and management of care by telephone to improve treatment of depression in primary care. Br Med J. 2000;320(7234):550–4. [DOI] [PMC free article] [PubMed]
- 4.Rost K, Nutting P, Smith J, Werner J, Duan N. Improving depression outcomes in community primary care practice: a randomized trial of the QuEST intervention. Quality enhancement by strategic teaming. J Gen Intern Med. 2001;16(3):143–9. [DOI] [PMC free article] [PubMed]
- 5.Wells KB, Sherbourne C, Schoenbaum M, et al. Impact of disseminating quality improvement programs for depression in managed primary care: a randomized controlled trial. J Am Med Assoc. 2000;283(2):212–20. [DOI] [PubMed]
- 6.Finley PR, Rens HR, Pont JM, et al. Impact of collaborative care model upon depression in primary care: a randomized controlled trial. Pharmacotherapy. 2003;23:1175–85. [DOI] [PubMed]
- 7.Adler DA, Bungay KM, Wilson IB, et al. The impact of a pharmacist intervention on 6-month outcomes in depressed primary care patients. Gen Hosp Psychiatry. 2004;26(3):199–209. [DOI] [PubMed]
- 8.Unutzer J, Katon W, Callahan CM, et al. Improving mood-promoting access to collaborative treatment: collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. J Am Med Assoc. 2002;288(22):2836–45. [DOI] [PubMed]
- 9.Hedrick SC, Chaney EF, Felker B, et al. Effectiveness of collaborative care depression treatment in Veteran’s Affairs primary care. J Gen Intern Med. 2003;18(1):9–16. [DOI] [PMC free article] [PubMed]
- 10.Alexopoulos GS, Katz IR, Bruce ML, et al. Remission in depressed geriatric primary care patients: a report from the PROSPECT Study. Am J Psychiatry. 2005;162(4):718–24. [DOI] [PMC free article] [PubMed]
- 11.Bruce ML, Ten Have TR, Reynolds CF, III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. J Am Med Assoc. 2004;291(9):1081–91. [DOI] [PubMed]
- 12.Dobscha SK, Corson K, Hickam DH, Perrin NA, Kraemer DF, Gerrity MS. Depression decision support in primary care: a cluster randomized trial. Ann Intern Med (in press). [DOI] [PubMed]
- 13.Pyne JM, Rost KM, Farahati F, et al. One size fits some: the impact of patient treatment attitudes on the cost-effectiveness of a depression primary-care intervention. Psychol Med. 2005;35:839–54. [DOI] [PubMed]
- 14.Pyne JM, Smith J, Fortney J, Zhang M, Williams DK, Rost K. Cost-effectiveness of a primary care intervention for depressed females. J Affect Disord. 2003;74(1):23–32. [DOI] [PubMed]
- 15.Von Korff M, Katon W, Bush T, et al. Treatment costs, cost offset and cost-effectiveness of collaborative management of depression. Psychosom Med. 1998;60:143–9. [DOI] [PubMed]
- 16.Schoenbaum M, Unutzer J, Sherbourne C, et al. Cost-effectiveness of practice-initiated quality improvement for depression: results of a randomized controlled trial. J Am Med Assoc. 2001;286(11):1325–30. [DOI] [PubMed]
- 17.Simon GE, Manning WG, Katzelnick DJ, Pearson SD, Henk HJ, Helstad CP. Cost-effectiveness of systematic depression treatment for high utilizers of general medical care. Arch Gen Psychiatry. 2001;58(2):181–7. [DOI] [PubMed]
- 18.Simon GE, Von Korff M, Ludman EJ, et al. Cost-effectiveness of a program to prevent depression relapse in primary care. Med Care. 2002;40(10):941–50. [DOI] [PubMed]
- 19.Liu CF, Hedrick SC, Chaney EF, et al. Cost-effectiveness of collaborative care for depression in a primary care veteran population. Psychiatr Serv. 2003;54(5):698–704. [DOI] [PubMed]
- 20.Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness. Manag Care Q. 1996;4(2):12–25. [PubMed]
- 21.Wagner EH. Managed care and chronic illness: health services research needs. Health Serv Res. 1997;32(5):702–14. [PMC free article] [PubMed]
- 22.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–44. [DOI] [PubMed]
- 23.Rost K, Fortney J, Fischer E, Smith J. Use, quality and outcomes of care for mental health: the rural perspective. Med Care Res Rev. 2002;59(3):231–65. [DOI] [PubMed]
- 24.Williams JW, Jr., Rost K, Dietrich AJ, Ciotti MC, Zyzanski SJ, Cornell J. Primary care physicians’ approach to depressive disorders. Effects of physician specialty and practice structure. Arch Fam Med. 1999;8:58–67. [DOI] [PubMed]
- 25.Institute of Medicine. Telemedicine: A Guide to Assessing Telecommunications in Health Care. Washington, D.C.: National Academies Press; 1996. [PubMed]
- 26.Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141(12):938–45. [DOI] [PubMed]
- 27.Fortney JC, Pyne JM, Edlund MJ, Robinson DE, Mittal D, Henderson KL. Design and implementation of the Telemedicine-Enhanced Antidepressant Management Study. Gen Hosp Psych. 2006;28(1):18–26. [DOI] [PubMed]
- 28.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32(9):509–15.
- 29.Smith GR, Jr., Burnam A, Burns BJ, Cleary P, Rost KM. Depression outcomes module (DOM). In: American Psychiatric Association, ed. Handbook of Psychiatric Measures. Washington, DC; 2000:213–5.
- 30.Kramer TL, Smith GR, D’Arezzo KW, Card-Higginson P. Depression outcomes module. The guide to behavioral health outcomes management systems. University of Arkansas for Medical Sciences, Little Rock AR; 2000:71–83.
- 31.Lecrubier Y, Sheehan DV, Weiller E, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatr. 1997;12(5):224–31. [DOI]
- 32.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatr. 1997;12(5):232–41. [DOI]
- 33.Parkerson GR, Jr., Michener JL, Wu LR, et al. Associations among family support, family stress, and personal functional health status. J Clin Epidemiol. 1989;42(3):217–29. [DOI] [PubMed]
- 34.Parkerson GR, Jr., Broadhead WE, Tse CJ. Quality of life and functional health of primary care patients. J Clin Epidemiol. 1992;45(11):1303–13. [DOI] [PubMed]
- 35.Edlund M, Reaves-Powell CM, Fortney JC, Pyne JM, Mittal D. Beliefs about depression and depression treatment among depressed veterans. Working paper; 2006. [DOI] [PubMed]
- 36.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97. [DOI] [PubMed]
- 37.Revicki DA, Brown RE, Palmer W, et al. Modelling the cost effectiveness of antidepressant treatment in primary care. Pharmacoeconomics. 1995;8(6):524–40. [DOI] [PubMed]
- 38.Thompson C, Peveler RC, Stephenson D, McKendrick J. Compliance with antidepressant medication in the treatment of major depressive disorder in primary care: a randomized comparison of fluoxetine and a tricyclic antidepressant. Am J Psychiatry. 2000;157(3):338–43. [DOI] [PubMed]
- 39.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins symptom checklist (HSCL): a measure of primary symptom dimensions. Pharmacopsychiatry. 1974;7:79–110. [DOI] [PubMed]
- 40.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins symptom checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19:1–15. [DOI] [PubMed]
- 41.Jones D, Kazis L, Lee A, et al. Health status assessments using the Veterans SF-12 and SF-36: methods for evaluating outcomes in the Veterans Health Administration. J Ambul Care Manage. 2001;24(3):68–86. [DOI] [PubMed]
- 42.Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626–32. [DOI] [PubMed]
- 43.Kaplan RM, Anderson JP. The general health policy model: an integrated approach. In: Spiker B, ed. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia: Lippincott-Raven Publishers; 1996:309–21.
- 44.Kaplan RM, Bush JW. Health-related quality of life measurement for evaluation research and policy analysis. Health Psychol. 1982;1(1):61–80. [DOI]
- 45.Pyne JM, Patterson TL, Kaplan RM, Gillin JC, Koch WL, Grant I. Assessment of the quality of life of patients with major depression. Psychiatr Serv. 1997;48(2):224–30. [DOI] [PubMed]
- 46.Pyne JM, Patterson TL, Kaplan RM, et al. Preliminary longitudinal assessment of quality of life in patients with major depression. Psychopharmacol Bull. 1997;33(1):23–9. [PubMed]
- 47.Beebe TJ, Harrison PA, McRae JA, Jr., Asche SE. Evaluating behavioral health services in Minnesota’s Medicaid population using the Experience of Care and Health Outcomes (ECHO) Survey. J Health Care Poor Underserved. 2003;14(4):608–21. [DOI] [PubMed]
- 48.Kazis LE, Miller DR, Skinner KM, et al. Patient-reported measures of health: the Veterans Health Study. J Ambul Care Manage. 2005;27(1):70–83. [DOI] [PubMed]
- 49.Badamgarav E, Weingarten SR, Henning JM, et al. Effectiveness of disease management programs in depression: a systematic review. Am J Psychiatry. 2003;160(12):2080–90. [DOI] [PubMed]
- 50.Rush AJ, Trivedi M, Carmody TJ, Biggs MM, Shores-Wilson K. One-year clinical outcomes of depressed public sector outpatients: a benchmark for subsequent studies. Biol Psychiatry. 2004;56(1):46–53. [DOI] [PubMed]
- 51.Simon GE, Ludman EJ, Tutty S, Operskalski B, Von Korff M. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: a randomized controlled trial. J Am Med Assoc. 2004;292(8):935–42. [DOI] [PubMed]
- 52.Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62:1007–14. [DOI] [PubMed]


