Abstract
We report a case of acute lead poisoning in an adult female who had last been exposed to lead 7 years ago. She presented with abdominal pain, knee pain, and neurological symptoms, hypertension, chronic kidney disease, and anemia with basophilic stippling and lead gum lines. Compared to during her recent pregnancy, her lead level had almost tripled in 5 months to 81 mcg/dL. Chelation therapy was initiated and improved the patient’s symptoms and lead level significantly. In the absence of any new lead exposure or other reasons for increased bone turnover, this acute lead increase was likely due to skeletal mobilization caused by increased resorption from mineralized tissue during and after her pregnancy. This case report illustrates the seriousness of long-term health effects associated with lead poisoning at a multi-organ level, even years after the initial exposure. Thus, patient care should not be limited to the acute treatment of increased lead levels, but also include prevention of increased mobilization and bone turnover and appropriate patient education. In this context, we review various aspects of lead toxicity, especially during pregnancy and lactation.
KEY WORDS: lead, toxicity, pregnancy, mobilization, gum line
INTRODUCTION
Lead poisoning is a less common but serious health threat.1,2 Especially higher lead levels or exposure over longer periods can cause irreversible damage to the nervous systems and the kidneys. During pregnancy, even lower lead levels are of serious concern because of their adverse effects on the fetus, including developmental delays, low birth weight, and miscarriage.2,3 Here, we report the case of an adult female who had last been exposed to lead 7 years earlier but now presented with symptoms and findings of acute lead poisoning which we treated with chelation therapy. In the absence of an acute lead exposure, her increased lead levels were likely due to increased mobilization and redistribution from mineralized tissues during and after a recent pregnancy. Her case emphasizes the seriousness of long-term health effects associated with lead poisoning. The danger of lead redistribution from mineralized tissue during a variety of conditions including pregnancy even years after exposure underscores the importance of preventive measures to avoid the initial lead exposure or any trigger that may lead to an acute mobilization from bone stores after an exposure.
CASE PRESENTATION
A 22-year`old African-American woman presented with a 2-week history of sharp, constant and progressively worsening mid-abdominal pain and nausea/vomiting for 2 days without fever or chills. She also complained of bilateral knee pain, headache, and tingling in her fingers, and stated that she was more irritable lately. She had a longstanding history of lead poisoning starting at the age of 6, and then again at age 15, when she was hospitalized and treated for seizures and renal failure with lead levels as high as 145 mcg/dL (please note that lead levels are generally considered to be elevated above 9 mcg/dL) and a creatinine of 1.6 mg/dL. At that time, she received chelating treatment with succimer (2,3-dimercaptosuccinic acid)4 for 3 weeks.
During her pregnancy 8 months ago, she had a reported lead level in the 50s that decreased to 30 mcg/dL 5 months ago, at which time she lost the fetus due to a chorioamnionitis caused by gonorrhea. In addition, her past medical history was significant for hypertension, chronic kidney disease with creatinine levels between 1.3 and 2.8 mg/dL, and recurrent genitourinary infections for which she was recently started on metronidazole. According to our patient, the source of her previous episodes of lead poisoning was thought to be the apartment she had been living in. After her hospitalization at age 15, the patient and her family had moved to a new apartment, which was found to be free of lead by the city’s Health Department. Although not formally tested, her other family members denied any symptoms of lead poisoning. Our patient also denied any new exposure at home or at work.
On admission, her blood pressure was 190/110 mmHg with a regular heart rate of 110/min, a respiration rate of 20/min, and she was afebrile. Her abdomen was non-obese and soft, but showed diffuse tenderness on palpation, mostly in the epigastric region without guarding. Both knees were tender to palpation without appreciable effusions. Most notable were blue/grey lines across the upper and lower gum lines.5 Her physical exam was otherwise normal as were her abdominal x-rays. We admitted the patient overnight for further diagnostics and symptomatic treatment. Her lead level was 81 mcg/dL, and her red blood cell/zinc protoporphyrin was 1,047 mcg/dL. The patient also had chronic microcytic anemia with a hemoglobin of 9.6 g/dL, hematocrit of 29.6%, mean corpuscular volume of 74 fL, mean corpuscular hemoglobin of 24 pg, and mean corpuscular hemoglobin concentration 32.4 g/dL. Her white blood count was normal as was her basic metabolic panel with the exception of elevated blood urea nitrogen and creatinine of 28 and 2.6 mg/dL, respectively. Her TSH was normal at 1.55 mIU/L. We treated her hypertension with labetolol and initiated chelating treatment with oral succimer for 3 weeks. At a follow-up visit 5 months later, her lead level had decreased to 57 mcg/dL, and she showed none of her previous symptoms.
DISCUSSION
Lead Toxicity
Lead is an electropositive heavy metal that can affect various organ systems in humans including the peripheral and central nervous system, the GI tract, joints, and muscles, kidneys, and the hemopoetic system.1,6 Neurological symptoms can range from fatigue, headache, and lethargy to peripheral neuropathy, severe convulsions, encephalopathy, and coma. Gastrointestinal symptoms include anorexia and abdominal cramps. While arthralgia and muscle pain can be early signs of milder lead exposure, anemia and renal failure usually indicate more severe lead poisoning.
Lead competitively interferes with divalent cations such as calcium, magnesium, and zinc.7 Subsequent impairment of mitochondrial oxidative phosphorylation and the intracellular messenger system affects endocrine and neuronal function and smooth muscle contraction. Hematologic complications result from its high affinity for sulfhydryl groups in enzymes that are essential for heme biosynthesis.7 Inhibition of 5-aminolevulinic acid dehydratase (Fig. 1) prevents the formation of porphobilinogen and leads to an accumulation of 5-amino-levulinic acid like in acute intermittent porphyria. By inhibiting mitochondrial ferrochelatase, lead prevents the incorporation of iron into protoporphyrin IX, which then accumulates and forms a metal chelate with zinc (Fig. 1). Lead-related anemia is a late complication when blood lead levels exceed 50 mcg/dL.6 The anemia is typically hypochromic and microcytic with basophilic stippling of the erythrocytes like it was the case in our patient. In addition, lead increases errors by DNA and RNA polymerases, potentially resulting in somatic and germline mutations.8
Figure 1.
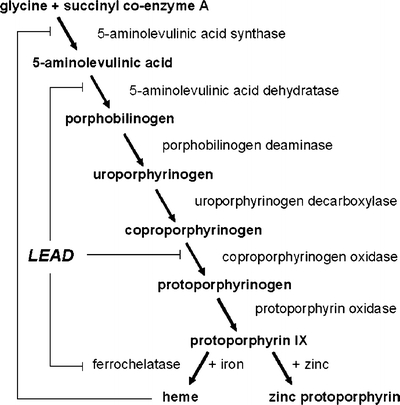
Effect of lead on heme synthesis. Lead inhibits (┤) 5-aminolevulic acid dehydratase, coproporphyrinogen oxidase, and ferrochelatase. Inhibition of ferrochelatase increases free protophorphyrin IX which chelates with zinc and forms zinc protoporphyrin within the erythroctes, a marker of lead exposure within the past 3 months.
After absorption, lead is stored in three major pools. Blood contains approximately 2% of the body’s lead with a relatively short half-life of 30 to 40 days.9 The remainder is distributed between soft tissue and a more stable pool in mineralized tissue which contains over 95% of the body’s lead load. There, lead can have a biological half-life of several decades.10 Lead is excreted through the kidneys.
Diagnosis
If suspected from a patient’s history and presenting clinical symptoms, the diagnosis of inorganic lead exposure is made by measuring blood lead concentrations. Organic lead exposure is best assessed by measuring urinary lead excretion. Urinary excretion of 5-amino-levulinic acid can also be used to assess lead exposure. As zinc protoporphyrin remains in the erythrocytes for their entire lifetime, it is a useful indicator of lead exposure during the past 3 months. Urinary lead excretion after a dose of sodium calcium ethylenediaminetetraacetic acid (EDTA) is helpful in estimating total body lead.11 However, this test requires an injection and a quantitative urine collection and is therefore often impractical. An alternative is L-line x-ray fluorescence, which measures cortical bone lead noninvasively.12
Treatment
Apart from removing the patient from the source of exposure, the mainstay of treatment is the administration of chelating agents. They form complexes with lead and are eliminated through the kidneys. For example, sodium calcium EDTA exchanges calcium for lead and is capable of mobilizing lead from mineralized tissue.13 Succimer, a dimercaprol analogue, is used for heavy metal poisoning with lead, arsenic, and mercury.14 Its advantages are a high affinity for lead and oral administration. Ten to 30 mg per kg per day are given for 1 week, followed by a reduced dose for two more weeks.4
Acute Lead Poisoning by Redistribution
Because of its long half-life in mineralized tissues, mobilization and redistribution from the skeleton under certain physiological and pathophysiological conditions can also cause acute increases in blood lead levels that mimic lead poisoning by acute exposure and pose an equally serious health threat. These conditions include prolonged immobilization,15 hyperthyroidsm,16 chemotherapy,17 tumor infiltration of the bones, menopause,18 and breast-feeding.19,20 In the absence of any of these conditions (normal TSH, no immobilization, etc.) and without evidence to suggest new or increased lead exposure, lead mobilization from the skeleton during and after her recent pregnancy is the most likely cause of the increase in lead level in our patient.19,21,22
Lead Toxicity and Pregnancy
Resorption from bone rather than dietary absorption usually determines changes in blood lead levels. In pregnancy, however, the resorption of trabecular bone is dissociated from the resorption of cortical bone. In early pregnancy, only trabecular bone with lower lead content is resorbed, whereas in late pregnancy, resorption of cortical bone with higher lead content leads to an increase in blood lead levels. During lactation, the entire skeleton participates in resorption leading to further increased blood lead levels that reach a maximum approximately 8 months after delivery. In bottle feeders, the cortical bone resorption ceases after delivery. Nevertheless, the expected potential decrease in blood lead levels is compensated by the hemoconcentrating effect of postpartum plasma volume loss.23
Besides the associated risks of lead poisoning for the mother’s health and therefore indirectly the fetus like, e.g., chronic kidney disease, hypertension,24 behavioral changes or neurological complications, elevated lead levels during pregnancy increase the risk for miscarriage, preterm delivery, and low birth weight.3 Starting at approximately 12 weeks of gestation, lead can cross the placenta so that the levels in mother and baby become identical. Although only a few pregnant women in the United States are exposed to very high lead levels, the lack of a sufficient brain barrier for lead in the developing fetus causes even lower levels to have adverse effects on neurodevelopment25–27 with subsequent behavioral and learning problems later in life.
The Role of Calcium Supplementation During Pregnancy and Lactation
During pregnancy, increased fetal calcium demands lead to increased maternal bone resorption, which is usually aggravated by insufficient calcium intake. This increased demand (about 30 g) is met by increased gastrointestinal absorption caused by increased vitamin D. During lactation, vitamin D levels decline, and gastrointestinal calcium absorption returns to normal. Calcium is retained by the kidneys and resorbed from the skeleton secondary to falling estrogen levels. Overall, breast-feeding may lead to up to 10% loss of bone, which may take 1 to 2 years to fully recover.28 Adequate calcium supplementation (1,200 mg calcium per day)29 has been found to be an important means of limiting fetal and maternal lead toxicity especially during the second half of the pregnancy.2 Lead-induced decreases in birth weight and length are nevertheless unlikely to be prevented.30 During lactation, however, calcium supplementation is evidently less effective in minimizing the lead mobilization from the skeleton.31,32
Lead Toxicity and Breast-Feeding
Although lead levels in the breast milk are similar to the mother’s blood, lead exposure during breast feeding has a lower impact on the child than exposure during fetal development. During breast feeding, the child’s risk of lead toxicity is small because of a lower intestinal lead absorption33 compared to the amount of lead crossing the placenta. Moreover, lead can be frequently found in infant formulas, which lack the psychological and nutritional advantages of breast-feeding so that a recommendation against breast-feeding should not be made.34
CONCLUSION
Our case report illustrates the seriousness of the long-term health effects associated with lead poisoning at a multi-organ level. Our patient’s neurologic, renal, orthopedic, hematologic, and gastrointestinal systems and her reproductive system were affected even years after exposure. Thus, prevention of lead exposure is imperative. However, patient care for individuals who previously have been exposed to lead should not be limited to adequate treatment in case of an acute increase in lead levels. It should also be geared towards preventing increased mobilization and bone turnover by treating any possible triggering event in time and by sufficient calcium intake. Treatment should be accompanied by appropriate patient education, especially with regard to the interaction between pregnancy and lead mobilization.
Acknowledgments
Funding was provided exclusively by institutional support.
Conflict of Interest None disclosed.
References
- 1.Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM. Lead toxicity update. A brief review. Med Sci Monit. 2005;11:RA329–36. [PubMed]
- 2.Bellinger DC. Teratogen update: lead and pregnancy. Birth Defects Res A Clin Mol Teratol. 2005;73:409–20. [DOI] [PubMed]
- 3.McMichael AJ, Vimpani GV, Robertson EF, Baghurst PA, Clark PD. The Port Pirie cohort study: maternal blood lead and pregnancy outcome. J Epidemiol Community Health. 1986;40:18–25. [DOI] [PMC free article] [PubMed]
- 4.Mann KV, Travers JD. Succimer, an oral lead chelator. Clin Pharm. 1991;10:914–22. [PubMed]
- 5.Saito T, Ikezoe T, Taguchi H, Miyoshi I. Plumbism. Br J Haematol. 2004;124:2. [DOI] [PubMed]
- 6.Gordon JN, Taylor A, Bennett PN. Lead poisoning: case studies. Br J Clin Pharmacol. 2002;53:451–8. [DOI] [PMC free article] [PubMed]
- 7.Garza A, Vega R, Soto E. Cellular mechanisms of lead neurotoxicity. Med Sci Monit. 2006;12:RA57–65. [PubMed]
- 8.Zelikoff JT, Li JH, Hartwig A, Wang XW, Costa M, Rossman TG. Genetic toxicology of lead compounds. Carcinogenesis 1988;9:1727–32. [DOI] [PubMed]
- 9.Barltrop D, Smith AM. Kinetics of lead interaction with human erythrocytes. Postgrad Med J. 1975;51:770–3. [DOI] [PMC free article] [PubMed]
- 10.Rabinowitz MB, Wetherill GW, Kopple JD. Kinetic analysis of lead metabolism in healthy humans. J Clin Invest. 1976;58:260–70. [DOI] [PMC free article] [PubMed]
- 11.Piomelli S, Rosen JF, Chisolm JJ Jr, Graef JW. Management of childhood lead poisoning. J Pediatr 1984;105:523–32. [DOI] [PubMed]
- 12.Rosen JF, Markowitz ME, Bijur PE, et al. L-line x-ray fluorescence of cortical bone lead compared with the CaNa2EDTA test in lead-toxic children: public health implications. Proc Natl Acad Sci USA. 1989;86:685–9. [DOI] [PMC free article] [PubMed]
- 13.Hammond PB. The effects of chelating agents on the tissue distribution and excretion of lead. Toxicol Appl Pharmacol. 1971;18:296–310. [DOI] [PubMed]
- 14.Aposhian HV. DMSA and DMPS-water soluble antidotes for heavy metal poisoning. Annu Rev Pharmacol Toxicol. 1983;23:193–215. [DOI] [PubMed]
- 15.Markowitz ME, Weinberger HL. Immobilization-related lead toxicity in previously lead-poisoned children. Pediatrics. 1990;86:455–7. [PubMed]
- 16.Goldman RH, White R, Kales SN, Hu H. Lead poisoning from mobilization of bone stores during thyrotoxicosis. Am J Ind Med. 1994;25:417–24. [DOI] [PubMed]
- 17.el-Sharkawi AM, Morgan WD, Cobbold S, et al. Unexpected mobilisation of lead during cisplatin chemotherapy. Lancet. 1986;2:249–50. [DOI] [PubMed]
- 18.Silbergeld EK, Schwartz J, Mahaffey K. Lead and osteoporosis: mobilization of lead from bone in postmenopausal women. Environ Res. 1988;47:79–94. [DOI] [PubMed]
- 19.Gulson BL, Mahaffey KR, Jameson CW, et al. Mobilization of lead from the skeleton during the postnatal period is larger than during pregnancy. J Lab Clin Med. 1998;131:324–9. [DOI] [PubMed]
- 20.Ettinger AS, Tellez-Rojo MM, Amarasiriwardena C, et al. Levels of lead in breast milk and their relation to maternal blood and bone lead levels at one month postpartum. Environ Health Perspect. 2004;112:926–31. [DOI] [PMC free article] [PubMed]
- 21.Thompson GN, Robertson EF, Fitzgerald S. Lead mobilization during pregnancy. Med J Aust. 1985;143:131. [DOI] [PubMed]
- 22.Tellez-Rojo MM, Hernandez-Avila M, Lamadrid-Figueroa H, et al. Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy. Am J Epidemiol. 2004;160:668–78. [DOI] [PubMed]
- 23.Manton WI, Angle CR, Stanek KL, Kuntzelman D, Reese YR, Kuehnemann TJ. Release of lead from bone in pregnancy and lactation. Environ Res. 2003;92:139–51. [DOI] [PubMed]
- 24.Rothenberg SJ, Kondrashov V, Manalo M, et al. Increases in hypertension and blood pressure during pregnancy with increased bone lead levels. Am J Epidemiol. 2002;156:1079–87. [DOI] [PubMed]
- 25.Tong S, Baghurst P, McMichael A, Sawyer M, Mudge J. Lifetime exposure to environmental lead and children’s intelligence at 11–13 years: the Port Pirie cohort study. BMJ. 1996;312:1569–75. [DOI] [PMC free article] [PubMed]
- 26.Hu H, Tellez-Rojo MM, Bellinger D, et al. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect. 2006;114:1730–5. [DOI] [PMC free article] [PubMed]
- 27.Schnaas L, Rothenberg SJ, Flores MF, et al. Reduced intellectual development in children with prenatal lead exposure. Environ Health Perspect 2006;114:791–7. [DOI] [PMC free article] [PubMed]
- 28.Hopkinson JM, Butte NF, Ellis K, Smith EO. Lactation delays postpartum bone mineral accretion and temporarily alters its regional distribution in women. J Nutr. 2000;130:777–83. [DOI] [PubMed]
- 29.Hernandez-Avila M, Gonzalez-Cossio T, Hernandez-Avila JE, et al. Dietary calcium supplements to lower blood lead levels in lactating women: a randomized placebo-controlled trial. Epidemiology. 2003;14:206–12. [DOI] [PubMed]
- 30.Han S, Pfizenmaier DH, Garcia E, et al. Effects of lead exposure before pregnancy and dietary calcium during pregnancy on fetal development and lead accumulation. Environ Health Perspect. 2000;108:527–31. [DOI] [PMC free article] [PubMed]
- 31.Prentice A. Micronutrients and the bone mineral content of the mother, fetus and newborn. J Nutr. 2003;133:1693S–9S. [DOI] [PubMed]
- 32.Gulson BL, Mizon KJ, Palmer JM, Korsch MJ, Taylor AJ, Mahaffey KR. Blood lead changes during pregnancy and postpartum with calcium supplementation. Environ Health Perspect. 2004;112:1499–507. [DOI] [PMC free article] [PubMed]
- 33.Manton WI, Angle CR, Stanek KL, Reese YR, Kuehnemann TJ. Acquisition and retention of lead by young children. Environ Res. 2000;82:60–80. [DOI] [PubMed]
- 34.Dorea JG, Donangelo CM. Early (in uterus and infant) exposure to mercury and lead. Clin Nutr 2006;25:369–76. [DOI] [PubMed]


