Abstract
Background
Vitamin D deficiency, an important risk factor for osteoporosis and other chronic medical conditions, is epidemic in the United States. Uninsured women may be at an even higher risk for vitamin D deficiency than others owing to low intake of dietary and supplemental vitamin D and limited sun exposure.
Objective
Our goal was to determine the prevalence of vitamin D deficiency in this vulnerable population.
Setting and Participants
We enrolled 145 uninsured women at a County Free Medical Clinic in urban Michigan. Questionnaires were used to obtain information about demographics, medical history, vitamin supplementation, sunlight exposure, and dietary vitamin D intake.
Results
The 96 women who were tested for vitamin D status ranged in age from 21 to 65 years (mean 48 ± 11), and 67% were vitamin D deficient as indicated by a 25-hydroxyvitamin D [25(OH)D)] level <50 nmol/L (20 ng/mL). Non-Caucasians were 3 times more likely than Caucasians to be vitamin D deficient (P = .049). Mean dietary vitamin D intake was low (125 ± 109 IU/d) and only 24% of the participants used any supplemental vitamin D. Participants with total vitamin D intake <400 IU/day from diet and supplements were 10 times more likely to be vitamin D deficient than others (P < .001).
Conclusions
These results demonstrate a high prevalence of vitamin D deficiency in an uninsured, medically underserved female population. Uninsured women should be strongly encouraged to increase their vitamin D intake.
Key words: vitamin D deficiency, uninsured, women, prevalence
INTRODUCTION
Vitamin D deficiency continues to be present in epidemic proportions in this the “decade of bone health”.1 In a 2002 study of healthy young adults in Boston, investigators found that 36% of the 165 men and women screened in winter were vitamin D deficient as defined by 25(OH)D level less than 50 nmol/L.2 The prevalence is even higher in subjects of minority race. For example, the third National Health and Nutrition Examination Survey reported that 42% of African American women compared with only 4% of white women had 25(OH)D level less than or equal to 37.5 nmol/L.3 Vitamin D deficiency contributes to serious musculoskeletal conditions including osteoporosis, osteomalacia, muscle weakness, falls, and fracture.4–7 It has also been shown to influence the risks for diabetes, hypertension, and cardiovascular disease.8–11 Fortunately, if vitamin D deficiency is recognized early, supplementation is an inexpensive way to reduce the risk for these conditions and to slow their progression.12–14
An area of particular concern in public health is the medical care of racial minorities and underserved populations such as those without medical insurance. According to the latest U.S. Census Bureau estimates there are now 46.6 million uninsured Americans and the number is steadily increasing. The uninsured “working poor” seen in the free clinics across the country are theoretically at high risk for vitamin D deficiency as a result of limited sun exposure, low supplement use, and low milk consumption. This population is also more vulnerable to the effects of vitamin D deficiency because of its high prevalence of chronic disease.15,16 Nearly half (45%) of non-elderly uninsured adults report having 1 or more chronic health problems.17 After an in-depth review, we found a paucity of articles in refereed journals on the prevalence and current status of non-elderly, uninsured women with documented vitamin D deficiency.
The aim of this study is to evaluate the prevalence of vitamin D deficiency and identify the contributing factors in uninsured female patients at a County Free Medical Clinic in urban Michigan.
METHODS
Participants and Study Design
We conducted a survey of vitamin D status in uninsured female patients aged 18–65 who attended an Internal Medicine County Free Clinic in urban Michigan. The eligibility for care at the free clinic includes an income level no more than 200% of the federal poverty level.18 The participants were enrolled by the principal investigator or co-investigator during the study period (Jan 2005–Dec 2005) if they did not have the following exclusionary characteristics: known significant renal or hepatic dysfunction, diseases associated with significant malabsorption, use of prescription vitamin D, pregnancy, and treatment with medications that interfere with vitamin D metabolism. Whenever possible we verified information obtained from participants with their medical records to confirm that the exclusion criteria were met. Out of a convenience sample of 155 eligible patients, 145 women, provided written informed consent and were enrolled. The 10 eligible patients that refused enrollment were all aged less than 30 years and stated that they did not have interest in participating in the study. Assessment of vitamin D status required a separate laboratory visit, and 49 of the 145 enrolled patients were unwilling or unable to return for that visit. Thus, of the 145 participants enrolled, only the 96 with known vitamin D status could be included in the present analysis as shown in Table 1. The excluded participants were modestly younger than the included subjects, but the groups were similar in race distribution, BMI, vitamin D intake, and sun exposure. The Institutional Review Board of our institution reviewed and approved the study. None of the participants received any form of remuneration for participating in this study.
Table 1.
Characteristics (Mean ± SD or %) of 145 Enrolled Subjects
| Variable | Included subjects (N = 96) | Subjects excluded due to missing 25(OH)D measurement (N = 49) | P |
|---|---|---|---|
| Age (years) | 48.3 ± 11.1 | 43.4 ± 11.5 | .014 |
| BMI (kg/m2) | 32.7 ± 8.4 | 32.3 ± 9.6 | .797 |
| Dietary Vitamin D intake (IU/d) | 125 ± 109 | 142 ± 126 | .431 |
| Total Vitamin D Intake (IU/d) | 264 ± 308 | 223 ± 207 | .403 |
| Use Vitamin D supplements | 24% | 21% | .712 |
| Sun exposure index | 18.9 ± 14.2 | 18.0 ± 13.6 | .715 |
| Serum 25(OH)D (nmol/L) | 18.5 ± 11.0 | – | – |
| Race | .944 | ||
| Caucasian | 66.7% | 59.2% | |
| African American | 21.9% | 32.7% | |
| Hispanic | 4.2% | 2.0% | |
| Native American | 2.1% | 2.0% | |
| Other | 5.2% | 4.1% |
Measurements
A brief health questionnaire was administered by a physician and included questions about demographics, medical history, vitamin supplement use, and sunlight exposure. Participants were asked to report the number of hours per week that they spent in direct sunlight and parts of their body that were exposed. A sun exposure index (SEI) was calculated by multiplying the number of hours per week by the percent of exposed body surface area (9% for the face, 9% for each arm, 18% for each leg and 1% for each hand). Sun exposure to the chest, back, and abdomen were not included in the calculation.19 A validated short food frequency screening instrument20 was used to assess vitamin D intake. It included 15 foods and beverages and have been shown to provide a reasonably accurate and quick assessment of vitamin D intake. Total vitamin D intake was estimated by adding vitamin D from diet and supplements. Height and weight were measured by a triage nurse. Body mass index (BMI) was calculated by dividing weight (kilograms) by height (meters squared).
Serum 25(OH)D concentrations were measured at Quest Diagnostics clinical laboratory in Auburn Hills, Michigan using a commercial radioimmunoassay kit from DiaSorin, Stillwater, MN. The manufacturer's normal reference range for this assay is 50–250 nmol/L (20–100 ng/mL). Our primary outcome measure, vitamin D deficiency, was defined as a 25(OH)D concentration less than 50 nmol/L. This was based on the use of a 50 nmol/L cutoff in previous studies2,4 and is also the level below which parathyroid hormone serum levels appear to increase.13,21,22 This is important because elevated parathyroid hormone is a risk factor for skeletal disease.23 Serum total calcium level was measured using standard automated calorimetric method. The laboratory reference range for total calcium is 2.1 to 2.6 mmol/L (8.5–10.4 mg/dl).
Data Analysis
Characteristics of participants with and without 25(OH)D measurements were compared with t tests for 2 independent samples and with Chi-squared tests. Pearson correlation coefficients were calculated to assess simple linear associations of 25(OH)D with other variables. Independent predictors of vitamin D deficiency were identified by logistic regression analysis. P values below .05 were considered to indicate statistical significance. Statistical analyses were performed using the SPSS 14.0 statistical package (SPSS Inc., Chicago, IL).
RESULTS
Associations of Vitamin D Intake and Other Variables with 25(OH)D
Mean and median 25(OH)D were 46.1 ± 27.5 nmol/L (Table 1) and 39.9, respectively. Dietary vitamin D intake was less than 200 IU/day in 77% of the participants and less than 400 IU/day in 98%. Twenty-four percent of participants reported taking a multivitamin and/or another supplement containing vitamin D. Both dietary (r = .26, P = .010) and total vitamin D intake (r = .55, P < .001) were positively correlated with 25(OH)D. Most of the study measurements (84%) were made from November through April when sun contribution to 25(OH)D level is minimal. As expected, mean 25(OH)D was lower during this period than from May through October (45 ± 25 nmol/L vs 54 ± 38 nmol/L), but this difference was modest and not statistically significant. Mean 25(OH)D was much higher in Caucasian women than others (55 ± 28 vs 28 ± 13, P < .001). Age, body mass index, and the sun exposure index were not significantly correlated with 25(OH)D.
Prevalence and Predictors of Vitamin D Deficiency
Sixty-seven percent of the participants in this study were vitamin D deficient as defined by a 25(OH)D concentration less than 50 nmol/L. Only 53% of Caucasian women compared with 94% of all non-Caucasian women were vitamin D deficient (P < .001), and 100% of the African-American women were vitamin D deficient. The prevalence of 25(OH)D concentrations less than 50 nmol/L and less than several other commonly used benchmarks for vitamin D status are illustrated in Figure 1.
Figure 1.
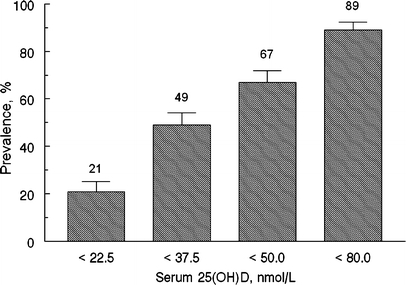
The prevalence of 25(OH)D concentrations below established benchmarks
Independent predictors of vitamin D deficiency were examined with logistic regression models that included age, race, BMI, season, and vitamin D intake (Table 2). Non-Caucasians were 3 times as likely as Caucasians to be deficient when other factors were held constant, and women with vitamin D intakes less than 400 IU/day were more than 10 times more likely than others to be deficient.
Table 2.
Independent Predictors* of Vitamin D Deficiency in 96 Women
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Age (Yrs) | |||
| <50 | Reference | ||
| ≥50 | 2.24 | 0.78–6.42 | .134 |
| Race | |||
| Caucasian | Reference | ||
| Non-Caucasian | 3.22 | 1.00–10.36 | .049 |
| BMI (kg/m2) | |||
| <29.9 | Reference | ||
| ≥30.0 | 2.34 | 0.86–6.40 | .098 |
| Season | |||
| May–October | Reference | ||
| November–April | 1.27 | 0.34–4.79 | .725 |
| Vitamin D intake (IU/d)† | |||
| ≥400 IU/d | Reference | ||
| <400 IU/d | 10.20 | 2.84–36.58 | <0.001 |
*Each predictor adjusted for the others
†From diet and supplements
DISCUSSION
More than two-thirds of women in this uninsured, medically underserved northern US population were vitamin D deficient as defined by a 25(OH)D concentration less than 50 nmol/L. This is a staggering statistic, particularly when you consider that many experts feel that optimal 25(OH)D concentrations are closer to 80 nmol/L, a level that 89% of our participants did not meet.24 Furthermore, all African-American women in the study were vitamin D deficient, adding to extensive previous evidence that this group is more vulnerable to vitamin D deficiency than others because skin pigmentation inhibits skin synthesis of vitamin D.4,25,26 The extent of vitamin D deficiency in our study was greater than that reported in women aged 30–59 in NHANES III (31% for non-Hispanic whites and 76% for non-Hispanic blacks.27 The same assay and cut points were used for NHANES III and for our study. One explanation may be that, for logistical reasons, NHANES III made wintertime 25(OH)D measurements only in Southern states where sun exposure stimulates greater vitamin D production than in Northern states during the same period. Alternatively, the high prevalence of vitamin D deficiency in our study may be linked to lower vitamin D intake. Although self-reported total vitamin D intake in our study was similar to that of adult women in NHANES,26 actual intake in our study may be lower. This may be caused by sporadic use of vitamin D supplements resulting from income or time constraints. We did not collect information about regularity of supplement use.
Our study is limited by its small size and the fact that 32% of the participants we enrolled did not have measurements of 25(OH)D. However, those in whom 25(OH)D measurements were missing were similar to the others in the most important determinants of vitamin D status: vitamin D intake and race, suggesting that results in those we measured likely reflected those of the clinic population as a whole. Although we cannot know for sure whether the insured women have vitamin D levels that are similar to uninsured women, having a control group of insured women would have been helpful in answering this question. Uninsured individuals are typically a difficult population to recruit and study, and thus our demonstration of poor vitamin D status in this group is a strength of this study. However, because the clinic only served uninsured patients, we were unable to compare vitamin D status of uninsured and insured women. Thus, this study does not provide evidence that vitamin D status of the uninsured is worse than that of the insured.
Our findings point out the pressing need for health education regarding the prevention and treatment of vitamin D deficiency in uninsured women. In particular, our results demonstrate the importance of increasing vitamin D intake to 400 IU/day or higher, an intake that cannot generally be achieved from diet alone. Many experts now feel that the current recommended dietary allowances (RDAs), which range from 200 to 400 IU/day in the age group we studied, are too low and that many individuals need intakes as high as 1,000 IU/day to achieve optimal vitamin D status.24 Only 24% of our study population was taking supplemental vitamin D, suggesting that clinical and public health efforts to increase the use of vitamin D supplementation among uninsured individuals could have an important impact on improving vitamin D status in this group. The interventions to improve Vitamin D intake is a low-cost public health intervention. Approximately 47 million people in this country currently lack medical insurance and the number is steadily increasing. The main findings of the Care Without Coverage: Too Little, Too Late Institute of Medicine 2002 report are that working-age Americans without health insurance are more likely to receive too little medical care and receive it too late; be sicker and die sooner; and receive poorer care when they are in the hospital, even for acute situations like a motor vehicle crash. The prevention and treatment of vitamin D deficiency may be 1 way to reduce the risk for and consequences of serious medical conditions in this vulnerable population.
Acknowledgment
We would like to thank Dr. Susan Blalock for granting us permission to use the food questionnaire, Dr. Mindy Smith and Dr. Carole Keefe for their mentorship, Dr. Brenda Gillespie for her expert statistical review and the staff at Genesee County free medical clinic for all their support during this study. This article was presented at the Flint Area Medical Education (FAME) research meeting, May 2005, ACP Michigan Associates Meeting, May 2005 and ACP Michigan Chapter meeting, Sept. 2005)
Conflict of interest None disclosed.
References
- 1.Bone Health and Osteoporosis: A Report of the Surgeon General. Available at: http://www.surgeongeneral.gov/library/bonehealth/docs/exec_summ.pdf. [PubMed]
- 2.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112(8):659–62. [DOI] [PMC free article] [PubMed]
- 3.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–92. [DOI] [PubMed]
- 4.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85(11):4125–30. [DOI] [PubMed]
- 5.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78(12):1463–70. [DOI] [PubMed]
- 6.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501. Review. [DOI] [PubMed]
- 7.Holick MF. Vitamin D. The underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes 2002;9:87–98. [DOI]
- 8.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–5. [DOI] [PubMed]
- 9.Lind L, Hanni A, Lithell H, Hvarfner A, Sorensen OH, Ljunghall S. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens. 1995; 8(9):894–901. [DOI] [PubMed]
- 10.Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia. 1995;38(10):1239–45. [DOI] [PubMed]
- 11.Gedik O, Akalin S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia. 1986;29(3):142–5. [DOI] [PubMed]
- 12.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003; 326(7387):469–72. [DOI] [PMC free article] [PubMed]
- 13.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351(9105):805–6. [DOI] [PubMed]
- 14.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA. 2004;291(16):1999–2006. Review. [DOI] [PubMed]
- 15.Fiscella K. Is lower income associated with greater biopsychosocial morbidity? Implications for physicians working with underserved patients. J Fam Pract. 1999;48(5):372–7. [PubMed]
- 16.Reed MC, Tu HT. Triple jeopardy: low income, chronically ill and uninsured in America. Issue Brief Cent Stud Health Syst Change. 2002;(49):1–4. [PubMed]
- 17.Uninsured Americans with Chronic Health Conditions: Key Findings from the National Health Interview Survey. Available at: http://www.urban.org/UploadedPDF/411161_uninsured_americans.pdf.
- 18.The 2007 HHS Poverty Guidelines. Available at: http://aspe.hhs.gov/poverty/07poverty.shtml.
- 19.Holick MF, Siris ES, Binkley N, et al. Prevalence of vitamin D inadequacy among postmenopausal north American woman receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–24. [DOI] [PubMed]
- 20.Blalock SJ, Norton LL, Patel RA, Cabral K, Thomas CL. Development and assessment of a short instrument for assessing dietary intakes of calcium and vitamin D. J Am Pharm Assoc (Wash DC). 2003;43(6):685–93. [DOI] [PubMed]
- 21.Kauppinen-Makelin R, Tahtela R, Loyttyniemi E, Karkkainen J, Valimaki MJ. A high prevalence of hypovitaminosis D in Finnish medical in- and outpatients. J Intern Med. 2001; 249(6):559–63. [DOI] [PubMed]
- 22.Lips P, Duong T, Oleksik A, Black, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86(3):1212–21. [DOI] [PubMed]
- 23.Harris SS, Soteriades E, Dawson-Hughes B. Secondary hyperparathyroidism and bone turnover in elderly blacks and whites. J Clin Endocrinol Metab. 2001;86:3801–3804 [DOI] [PubMed]
- 24.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis Int. 2005;16:713–6. [DOI] [PubMed]
- 25.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67(6):1232–6. [DOI] [PubMed]
- 26.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–7. [DOI] [PubMed]
- 27.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr 2005;135:2478–85 [DOI] [PubMed]


