Abstract
Background
The Veterans Health Administration (VHA) serves a population at high risk of influenza-related morbidity and mortality. The national public health response to the vaccine shortage of the 2004–2005 season resulted in prioritization of recipients and redistribution of available supply.
Objective
To characterize the impact of the 2004–2005 influenza vaccine shortage on vaccination among users of VHA facilities.
Design
Analysis using data from the cross-sectional VHA Survey of Healthcare Experiences of Patients.
Participants
Outpatients seen in VHA clinics during the months September 2004–March 2005.
Measurements
Sociodemographics, vaccination prevalence, setting of vaccination, and reasons cited for not getting vaccinated.
Results
Influenza vaccination prevalence among VHA outpatients aged 50–64 was 56% and for those aged ≥65 was 86%. Compared to the 2 previous seasons, this estimate was lower for patients age 50–64 but similar for patients ≥65. After adjustment for patient characteristics, unvaccinated patients aged 50–64 were 8.3 (95% CI 6.0, 11.4) times as likely to cite that they were told they were not eligible for vaccination because of the national shortage compared to patients ≥65. Regional VHA variation in vaccination receipt and shortage-related reasons for nonvaccination was small.
Conclusions
The national influenza vaccine shortage of 2004–2005 primarily affected VHA users aged 50–64, consistent with the tiered prioritization guidance issued by the Centers for Disease Control and Prevention and Advisory Committee on Immunization Practices. Despite the shortage, vaccination prevalence among VHA users ≥65 remained high.
KEY WORDS: influenza vaccine, veteran, vaccine shortage
INTRODUCTION
In 2004–2005, the United States (U.S.) suffered a shortage of influenza vaccine primarily because of the license suspension in October 2004 of Chiron Corporation by the United Kingdom’s Medicines and Healthcare Products Regulatory Agency. At the time, Chiron was anticipated to supply almost half of the estimated 100 million doses required in the U.S. for the 2004–2005 influenza season.1,2 The Centers for Disease Control and Prevention (CDC), in coordination with the Advisory Committee for Immunization Practices (ACIP), rapidly responded to news of the impending shortage by recommending priority groups of patients to receive vaccine that would be available from the only other FDA-approved manufacturer of inactivated influenza vaccine, Aventis Pasteur, Inc (now Sanofi Pasteur).1 In conjunction with state health departments, the CDC also began coordinating nationwide tracking of vaccine demand and supply to reallocate available doses where needed. In the U.S., this task is made difficult because many people get vaccinated in nongovernmental settings such as private health care offices/systems, work settings, and nonclinical community settings (grocery stores, senior centers, etc.).2
The Veterans Health Administration (VHA) is the nation’s largest integrated health care system, and as of June 2005 operated approximately 157 medical centers, 862 community-based outpatient clinics, 134 nursing homes, and 337 other types of facilities and programs.3 More than 5 million patients received care in VHA facilities in 2004 and VHA outpatient clinics registered nearly 54 million visits. Whereas many operations within VHA are delegated to the 21 regionally situated Veterans Integrated Service Networks (VISNs), some functions are retained centrally by VHA, including medication and vaccine procurement. Over the last several influenza seasons, VHA has increased the amount of inactivated influenza vaccine purchased for administration to beneficiaries and employees buying 1.58 million doses in 2002, 2.05 million in 2003, 2.10 million in 2004, and 2.49 million in 2005.
The patient population that VHA serves is generally older, sicker, and poorer than other clinic populations.4,5 Thus, there may be a limited ability to prioritize vaccine within a patient population where the majority of patients qualify as “high-risk” and that may not have resources to seek out other sources of vaccine. VHA was fortunate in that all of its 2004–2005 supply was ordered from Aventis Pasteur, Inc. Upon announcement of the shortage, discussions held with the manufacturer and CDC resulted in delivery of its entire 2004–2005 order with no redistribution outside VHA. Although VHA received its entire order, as a responsible federal partner, it prioritized patients for vaccination per CDC guidance and accepted its supply in batches distributed slowly over the course of the season.
The impact of the various vaccine supply and demand forces resulting from the national shortage and subsequent prioritization efforts on VHA is not clear. A relative shortage of vaccine within VHA could have resulted from increased demand by enrolled beneficiaries who were not regular users of VHA services. Distribution delays resulting from CDC directed reallocation may also have affected VHA vaccination efforts, particularly early in the season. Finally, the national media and public health attention to the shortage may have resulted in increased demand by patients and overrationing of VHA vaccine supply by VHA staff. The purpose of this study was to evaluate the impact of the national influenza vaccine shortage and subsequent patient prioritization on vaccination receipt by users of VHA health care facilities aged ≥50 by examining vaccination prevalence, source of vaccination, reasons for not getting vaccinated, and regional variation within the VHA system during the 2004–2005 influenza season.
METHODS
Data Source and Study Population
This analysis examined responses to the mailed VHA system-wide Survey of Healthcare Experiences of Patients (SHEP) conducted by the VHA Office of Quality and Performance (OQP). Specific SHEP survey methods are detailed elsewhere.6 Briefly, the SHEP is a cross-sectional, mailed, monthly survey that uses a stratified random sample without replacement design and recruitment strategies consistent with Dillman’s tailored design method.7 The study population that is eligible for selection includes outpatients seen in VHA primary and specialty care clinics during the 60 days before the monthly administration of the SHEP. The survey uses a complex sampling design employing stratification based on the location of care (facility) and new or established primary or specialty care and accounts for an unequal probability of selection based on clinic size. A total of 218,775 patients were randomly selected to participate in the survey during the months of October 2004 through March 2005, the typical period for influenza vaccination. Of these, 188,739 were aged 50 or over. Of these, 124,744 (66%) responded to the survey (1,794 had bad addresses or were institutionalized and 62,201 refused or did not respond). Patients who did not respond as to whether or not they received the flu vaccine (item response missing = 3,992) were removed from the analysis for a final sample of 120,752 subjects or 64% of the eligible sample aged 50 and over. Data were analyzed in accordance with the governing Institutional Review Board approval from the Durham VA Medical Center and OQP data use agreement.
Measures
Prevalence of influenza vaccination was determined by an affirmative response to the question, “Did you get a flu shot in September 2004 or later?” Response options included “Yes”, “No”, or “I don’t know.” Patients who responded “No” to receiving influenza vaccine were then asked to endorse the main reason why they did not receive vaccine. “I was told I was not eligible to get the flu vaccine this year because of the shortage” (herein referred to as “told not eligible”) was 1 response. “Flu vaccine not available and I didn’t get it elsewhere” (herein referred to as “vaccine not available”) was another option. Nine other possible responses that have been found in prior studies to be barriers to influenza vaccination were also choices. Patients who reported receiving influenza vaccination were asked to indicate vaccination setting from the following options: at the VA (such as hospital, clinic, outreach mobile unit); Vet Center; non-VA hospital, clinic, doctor’s office, visiting nurse, or health department; community source (drug store, church, grocery store, etc.); other; don’t remember.
Demographic/socioeconomic information and other factors that might influence vaccine receipt were also collected, including whether the patient had a regular VA or non-VA medical provider, whether the patient had confidence or trust in his or her provider, whether the patient had health insurance other than VHA benefits, and patient’s self-reported health status.
Statistical Analyses
To calculate population estimates, sample weights (reflecting the probability of selection from a clinic within a facility) and nonresponse weights (reflecting propensity to respond based on age, gender, and clinic site) were applied for all analyses. All analyses accounted for intraclass correlation among patients introduced by shared organizational features of the clinics in which they were seen. Vaccination prevalences for the age groups 50–64 and ≥65 were estimated. These estimates and setting of vaccination were compared to historical data from similarly conducted SHEP surveys during influenza seasons 2002–2003 and 2003–2004 (years where there was minimal or no national vaccine shortage). Lastly, multivariate logistic regression models were used to examine regional variation in vaccination prevalence and to determine independent associations between citing “told not eligible” and “vaccine not available” as reasons for not getting vaccinated. All analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC) and variances were adjusted for the complex stratified sampling design using Taylor series approximations implemented in SAS-callable SUDAAN version 9.0 (RTI International, Research Triangle Park, NC).
RESULTS
Frequencies and weighted population percentage estimates for various demographic and other characteristics of VHA users aged ≥50 are provided in Table 1. These estimates indicate that most VHA users aged ≥50 were white (82.0%), male (97.3%), and almost half had at least some college education. Almost two-thirds of this population aged 50 and over were also over age 65. Most (78.3%) had additional health insurance other than VHA health benefits.
Table 1.
Characteristics of Survey of Healthcare Experiences of Patients (SHEP) Survey Respondents Aged ≥50 during Influenza Season 2004–2005 (N = 120,752)
| Characteristic | Frequency* | (Weighted %) | ||
|---|---|---|---|---|
| Age≥65 | 79,976 | (63.4) | ||
| Age 50–64 | 40,776 | (36.6) | ||
| Male | 117,714 | (97.3) | ||
| Marital Status | ||||
| Married | 82,340 | (65.7) | ||
| Never married | 4,890 | (5.1) | ||
| Divorced | 17,047 | (16.9) | ||
| Widowed | 11,608 | (9.8) | ||
| Separated | 2,116 | (2.5) | ||
| Ethnicity/Race | ||||
| White, non-Hispanic | 104,204 | (82.0) | ||
| Black, non-Hispanic | 6,002 | (9.4) | ||
| Asian | 628 | (0.6) | ||
| Native Hawaiian or Pacific Islander | 282 | (0.2) | ||
| American Indian or Alaska Native | 2,479 | (2.3) | ||
| Hispanic | 4,743 | (5.5) | ||
| Education | ||||
| Did not complete high school | 23,067 | (19.4) | ||
| High school graduate or GED | 45,097 | (36.3) | ||
| Some college | 29,256 | (26.4) | ||
| College graduate or beyond | 20,708 | (17.9) | ||
| Total Household Income | ||||
| $15,000 or less | 33,184 | (33.3) | ||
| $15,001 to $30,000 | 48,687 | (40.5) | ||
| ≥$30,000 | 23,397 | (26.2) | ||
| Non-VHA health insurance benefits | 92,964 | (78.3) | ||
| Employment | ||||
| Employed or student | 23,331 | (17.7) | ||
| Retired or homemaker | 70,491 | (55.4) | ||
| Unable to work | 21,710 | (24.4) | ||
| Unemployed | 2,943 | (2.5) | ||
| Primary Care Provider (PCP) | ||||
| PCP is a VA provider | 53,435 | (62.3) | ||
| PCP is a non-VA provider | 44,440 | (26.5) | ||
| No PCP | 14,267 | (11.2) | ||
| Confidence/Trust in Provider | ||||
| Complete confidence/trust in provider | 93,314 | (77.3) | ||
| Some confidence/trust in provider | 22,557 | (18.7) | ||
| No confidence/trust in provider | 2,527 | (2.1) | ||
| Overall Health Status | ||||
| Poor | 10,419 | (10.9) | ||
| Fair | 35,390 | (33.3) | ||
| Good | 47,245 | (37.5) | ||
| Very good | 21,597 | (15.5) | ||
| Excellent | 3,679 | (2.8) | ||
*Frequencies within each characteristic may not sum to overall survey N because of individual item nonresponse.
Based on this sample, the population prevalence estimates for influenza vaccination were 56.0% for the age group 50–64 and 86.2% for those ≥65 in the 2004–2005 influenza season. Figure 1 compares historical SHEP data on vaccination prevalence. The vaccination prevalence remained stable for those aged ≥65 over the 3 influenza seasons compared. For those aged 50–64, vaccination in 2004–2005 declined by 35% when compared to 2002–2003 and by 20% when compared to 2003–2004. In both age groups, the proportion of those vaccinated who received vaccine from a VHA source increased over this period.
Fig. 1.
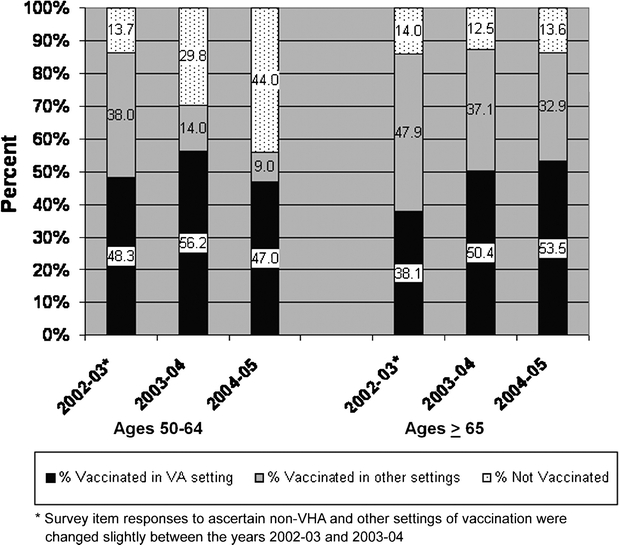
Prevalence and setting of influenza vaccination among users of VHA facilities aged ≥50. Data from the Survey of Healthcare Experiences of Patients (SHEP) during influenza season 2004–2005. (N = 120,752)
The main reason endorsed for not getting vaccinated among the unvaccinated is presented in Figure 2. “Did not want a flu shot” was the reason cited most frequently for both age groups. “Told not eligible” was the next most common reason endorsed among those aged 50–64 but was endorsed infrequently among the older age group. “Vaccine not available” was the 4th most common reason endorsed by those aged 50–64 and the 2nd most common reason for those aged ≥65.
Fig. 2.
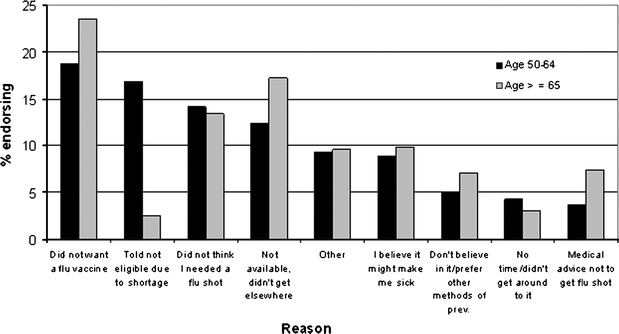
Reasons cited by respondents for not getting influenza vaccination. Data from the Survey of Healthcare Experiences of Patients (SHEP) during influenza season 2004–2005 (N = 30,937 unvaccinated respondents)
Lastly, we performed multivariate logistic regression analyses to further evaluate variation in vaccination prevalence. When adjusted for other patient characteristics including VISN, patients aged ≥65 were significantly more likely (odds ratio [OR] 3.7, 95% CI 3.4, 4.0) to have received vaccination compared to those aged 50–64. VISN was a small but significant factor associated with vaccine receipt by patients. For the age group 50–64, patients in 1 VISN had a vaccination prevalence significantly lower (OR 0.58, 95% CI 0.4, 0.9) than patients in the median vaccination prevalence VISN. For the age group ≥65, patients in 2 VISNs had significantly lower vaccination prevalences than patients in the median vaccination prevalence VISN (OR 0.8, 95% CI 0.6, 0.99 and OR 0.7, 95% CI 0.5, 0.95), and patients in 1 VISN had a significantly higher vaccination prevalence (OR 1.4, 95% CI 1.02, 1.8) than the median.
We further determined factors independently associated with citing either “told not eligible” or “vaccine not available” as reasons for not getting vaccinated (Table 2). The unvaccinated aged 50–64 were significantly more likely (OR 8.3, 95% CI 6.0, 11.4) to cite “told not eligible” as their main reason for not getting vaccinated as compared to those aged ≥65, but were just as likely to cite “vaccine not available”. Compared to patients who reported no primary care provider, patients with a non-VHA primary care provider were somewhat more likely to cite “vaccine not available” (OR 1.5, 95% CI 1.2, 1.9) as a reason for not getting vaccinated. Patients with health insurance benefits in addition to VHA benefits were significantly more likely to cite both shortage-related reasons (“told not eligible” OR 1.2, OR 95% CI 1.01, 1.5; “vaccine not available” OR 1.3, 95% CI 1.1, 1.6). Race/ethnicity, education, and income were also significantly related to citing 1 of the shortage-related options as a reason for not getting vaccinated.
Table 2.
Adjusted* OR and 95% CI for Citing “I was told I was not eligible due to a shortage” and “Vaccine not available, didn’t get it elsewhere” as Reasons for not Getting Vaccinated
| Adjusted OR* (95% CI) | ||
|---|---|---|
| Characteristic | Model 1: “Told I was not eligible due to shortage” | Model 2: “Not available, did not get it elsewhere” |
| Age 50–64 | 8.3†(6.0, 11.4) | 0.8 (0.7, 1.0) |
| Age ≥65 | Referent | Referent |
| Male | 1.3 (0.8, 1.9) | 1.2 (0.8, 1.9) |
| Female | Referent | Referent |
| Race/ethnicity | ||
| Black, non-Hispanic | 0.9 (0.7, 1.2) | 0. 6 (0.5, 0.9) |
| Hispanic | 1.1 (0.8, 1.6) | 1.0 (0.7, 1.4) |
| American Indian or Alaskan Native | 0.7 (0.4, 1.1) | 0.9 (0.6, 1.4) |
| Asian | 1.2 (0.5, 3.3) | 0.9 (0.3, 2.8) |
| Native Hawaiian or Pacific Islander | 0.7 (0.2, 2.4) | 1.6 (0.3, 7.2) |
| White, non-Hispanic | Referent | Referent |
| Education level | ||
| College graduate or beyond | 1.8 (1.3, 2.6) | 0.9 (0.7, 1.2) |
| Some college | 1.7 (1.2, 2.4) | 0.9 (0.7, 1.2) |
| High school graduate or GED | 1.7 (1.3, 2.4) | 0.9 (0.7, 1.1) |
| Did not complete high school | Referent | Referent |
| Total household income $15,000 or more | 1.3 (1.1, 1.6) | 1.0 (0.9, 1.2) |
| Total household income less than $15,000 | Referent | Referent |
| Employment status | ||
| Retired or homemaker | 0.8 (0.7, 1.0) | 1.0 (0.8, 1.2) |
| Unable to work | 0.8 (0.6, 0.9) | 1.0 (0.8, 1.3) |
| Unemployed | 1.0 (0.7, 1.4) | 0.9 (0.6, 1.3) |
| Employed or student | Referent | Referent |
| Non-VA health insurance benefits | 1.2 (1.01, 1.5) | 1.3 (1.1, 1.6) |
| No other health insurance benefits | Referent | Referent |
| PCP is a VA provider | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.2) |
| PCP is not a VA provider | 1.1 (0.8, 1.4) | 1.5 (1.2, 1.9) |
| No PCP | Referent | Referent |
| Self-reported health status fair or poor | 1.2 (1.0, 1.5) | 0.9 (0.7, 1.1) |
| Self-reported health status excellent, very good, or good | Referent | Referent |
| Marital status | ||
| Never married | 1.2 (0.9, 1.7) | 0.9 (0.7, 1.4) |
| Divorced | 1.1 (0.9, 1.3) | 1.2 (0.9, 1.4) |
| Separated | 1.1 (0.6, 1.8) | 1.4 (0.9, 2.2) |
| Widowed | 0.9 (0.6, 1.4) | 0.9 (0.7, 1.2) |
| Married | Referent | Referent |
| Confidence and trust in provider | ||
| Complete confidence/trust in provider | 1.0 (0.6, 1.7) | 1.2 (0.8, 1.8) |
| Some confidence/trust in provider | 1.3 (0.8, 2.2) | 1.3 (0.8, 2.0) |
| No confidence/trust in provider | Referent | Referent |
Data are from the Survey of Healthcare Experiences of Patients (SHEP) survey during influenza season 2004–2005 (N = 24,939 unvaccinated respondents with complete covariate information)
*Adjusted for VISN, age, gender, race/ethnicity, education level, income, employment, health insurance status, having a primary care provider, overall health status, marital status, and level of confidence/trust in provider
†Example interpretation: unvaccinated VHA users age 50–64 were 8.3 times as likely as unvaccinated VHA users aged ≥65 to cite that they were told they were not eligible for vaccination because of the shortage
VISN was a small but significant factor associated with citing a shortage-related reason for nonvaccination. When compared to the VISN with the median vaccination prevalence, patients in 3 VISNs were significantly less likely to cite “told not eligible” as a reason for not getting vaccinated. Patients in 2 different VISNs were significantly less likely to cite “vaccine not available” as a reason for not getting vaccinated. These VISNs were geographically diverse including the portions of the east coast, south, midwest, and west coast. No VISNs were significantly more likely to have patients citing either shortage-related barriers as a reason for not getting vaccinated.
DISCUSSION
Despite the national shortage, vaccination prevalence remained high among users of outpatient VHA facilities aged ≥65. This finding is consistent with prior studies of influenza vaccination among veterans and VA users.8 VHA has many organizational features that influence influenza vaccination delivery including the system-wide use of electronic clinical reminders, an influenza vaccination performance measure with facility-level feedback, and national flu and infection control campaigns that include facility-level toolkits to increase staff and patient awareness of influenza prevention. Additional actions during the 2004–2005 season may have helped sustain high vaccination rates, which included a requirement that systems be in place to call back VHA patients deferred from vaccination because of initial shortages and clearly defining “direct patient care” health care providers to help preserve vaccine supply for patients.
Our findings suggest that VHA users between ages 50 and 64 were most affected by the national shortage as indicated by decreased influenza vaccination prevalence in this age group compared to previous years. Furthermore, among the unvaccinated we found a strong association with citing the “told not eligible” reason for the age group 50–64 as compared to the age group ≥65. VHA redistributed vaccine within its own system, a measure we viewed as largely successful because VISN (the administrative unit by which redistribution occurred) was not strongly associated with either vaccine receipt or with citing a shortage-related reason for not getting vaccinated.
Similar to our results, the decline in vaccination nationally was smallest for adults aged ≥65 and larger for younger age groups. Data from the CDC’s Behavioral Risk Factor Surveillance System found that vaccination prevalence for respondents ≥65 years decreased from 67.6% to 63.3% between 2004 and 2005.9 Vaccination for adults in nonpriority groups declined by about half and experts attribute this to approximately 17.5 million adults who cited wanting to save vaccine for those who needed it more as the reason for not being vaccinated.10 The National Committee for Quality Assurance analyzed data from commercial managed care plans and found vaccination prevalence among those aged 50–64 declined by nearly half from 52.4% in 2003–2004 to 28.1% in 2004–2005.11 In this study, older patients and those who reported poorer health status experienced smaller relative decreases between seasons compared to younger patients or those reporting excellent or good health.
In our study, shortage-related reasons were among the most frequently cited reasons reported for not getting vaccinated in patients of both age groups. Unfortunately, these reasons were not available as item responses on the SHEP survey before 2004–2005 limiting direct comparison to earlier years. Vaccine unavailability first appeared in 2000–2001 as 1 of the leading reasons for nonvaccination in the Medicare Current Beneficiary Survey with nearly 12.7% of respondents reporting vaccine unavailability.12 Santibanez et al.13 reported no change in the overall vaccination rate in 1 large metropolitan area after a vaccine shortage in 2000; however, these investigators did find that subjects who were patients of practices who received their vaccine late in the season were more likely to report a source of vaccination other than their regular doctor. These findings all suggest that even further gains in vaccination both within and outside of VHA could be achieved by simply ensuring that a timely and adequate vaccine supply is available.
VHA was able to use its position as a national health care system to advantage in managing the shortage. On announcement of the shortage, VHA leadership immediately entered discussions with Aventis Pasteur, Inc. to confirm that its contracted vaccine supply would be delivered and with CDC to ensure recognition of VHA as the source of vaccine for a sizable number of persons in high-priority groups. VHA communicated information on vaccine supply, distribution, nonvaccine preventive measures and policies on prioritization frequently throughout the season to its VISN leaders and facility staff via a series of 7 nationally electronically mailed advisories from the VHA executive, the Under Secretary for Health.14 VHA assessed its vaccine supplies within VISNs in mid-December 2004, mid-February 2005, and early April 2005 to redistribute between VISNs and to prepare for possible redistribution of any unused VHA-owned vaccine outside the VHA system. When it became clear by late January that redistribution outside VHA would not be needed, VHA encouraged “late season” vaccination within its health care system, an action that resulted in over 17% of its supply being administered after the middle of December, the traditional end of influenza vaccination in most years. By April 6th, only 9% of the 2.1 million doses received remained unused.
One of the unanticipated benefits of the national shortage for VHA was the establishment of a mechanism for providing timely updates to VHA staff in the field via the use of “Flu Advisory” emails and web postings. Non-vaccine preventive measures were emphasized with the initiation of a hand-washing and respiratory-hygiene promotional campaign within VHA called Infection: Don’t Pass It On, which has evolved into an ongoing effort.15 VHA can build upon this infrastructure in emergency preparedness planning for future public health emergencies such as pandemic influenza, bioterrorist emergencies, and other urgent national public health threats.
This study has several strengths. First, we used SHEP data, a survey with a good response rate collected from a large, geographically representative sample of VHA outpatients. The survey items used to assess reasons for not getting vaccinated included items specifically related to a vaccine shortage. Lastly, historical vaccination prevalence from similarly designed and conducted SHEP surveys was available for comparison.
Our study has several limitations. We lacked information based on VHA administrative data to determine the degree of bias owing to SHEP survey nonresponse. However, nonresponse weights based on the available demographics were applied to minimize this bias. The use of self-reported vaccination status is also a limitation; however, previous studies have confirmed reliability of self-reported influenza vaccination.16–18 We did not have information on medical comorbidities of respondents. This would have been helpful for patients aged 50–64 because this information would better characterize eligibility for vaccination. Finally, the large sample size in our study may have yielded statistically significant results with little or no clinical significance.
We are limited in drawing definitive conclusions about specific supply and demand forces at play during the 2004–2005 influenza season. Our estimates were derived from a survey sample; we do not know the absolute numbers of patients that were vaccinated. Because some of the VHA vaccine supply is used to vaccinate employees, we are unable to estimate the absolute numbers of vaccinated patients based on vaccine supply that was used. Furthermore, we have no information on the absolute numbers of patients who sought vaccination but were turned down because of the shortage. Because of changes in the item used to determine vaccination setting between 2002–2003 and 2003–2004, we are not able to draw conclusions based on changes in vaccination source. These data would have better characterized the supply and demand forces.
CONCLUSION
Our data suggest that VHA users aged 50–64 were most affected by the national influenza vaccine shortage, but the impact on VHA patients appears smaller than what was experienced outside VHA. Unless the vaccine manufacturing and distribution mechanisms change radically, shortages will likely continue to threaten attainment of Healthy People 2010 goals for influenza vaccination nationally. Because of this, future studies on vaccination uptake should examine changes in vaccine supply, setting and timing of vaccination within the flu season, and the impact of health system policy on vaccination prevalence. Demonstrating the impact of vaccine shortages on influenza-related health outcomes and health care utilization may help drive changes in policy to ensure a consistent supply of vaccine in the future. VHA is poised to contribute to future research in this area.
Acknowledgments
The authors would like to acknowledge Steven Wright, PhD, VHA Office of Quality and Performance for his review and comments on this manuscript.
Conflict of interest All authors are employees of the Veterans Health Administration, Department of Veterans Affairs.
Disclaimer The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- 1.CDC. Interim influenza vaccination recommendations—2004–2005 influenza season. Morb Mort Wkly Rep. 2004;53(39):923–4. [PubMed]
- 2.Lister SA. Influenza Vaccine Shortages and Implications. Congressional Research Service. Library of Congress. Order Code RL 32655. Available at: http://fas.org/sgp/crs. Accessed February 28, 2006.
- 3.Department of Veterans Affairs. Fact Sheet June 2005—Facts about the Department of Veterans Affairs. Updated version available from: http://www1.va.gov/opa/fact/vafacts.asp. Accessed January 31, 2006.
- 4.Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626–32. [DOI] [PubMed]
- 5.Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252–7. [DOI] [PubMed]
- 6.Wright SM, Craig T, Campbell S, Schaefer J, Humble C. Patient satisfaction of female and male users of Veterans Health Administration Services. J Gen Intern Med. 2006;21 Suppl 3:S26–32. [DOI] [PMC free article] [PubMed]
- 7.Dillman DA. Mail and Internet Surveys: The Tailored Design Method—2nd ed. New York: John Wiley and Sons, Inc.; 2000.
- 8.Ru-Chien C, Reiber GE, Neuzil KM. Influenza and pneumococcal vaccination in older veterans: results from the behavioral risk factor surveillance system. J Am Geriatr Soc 2006;54:217–23. [DOI] [PubMed]
- 9.CDC. Influenza and pneumococcal vaccination coverage among persons aged ≥65 years—United States, 2004–2005. Morb Mort Wkly Rep 2006;55:1065–8. [PubMed]
- 10.CDC. Estimated influenza vaccination coverage among adults and children—United States, September 1, 2004—January 31, 2005. Morb Mort Wkly Rep 2005;54:304–8. [PubMed]
- 11.CDC. Influenza vaccination coverage among persons aged 50–64 years enrolled in commercial managed health-care plans—United States, 2003–04 and 2004–05 influenza seasons. Morb Mort Wkly Rep 2005;54:921–3. [PubMed]
- 12.CDC. Influenza vaccination and self-reported reasons for not receiving influenza vaccination among medicare beneficiaries aged ≥65 years—United States, 1991–2002. November 5, 2004. Morb Mort Wkly Rep 2004;53:1012–15. [PubMed]
- 13.Santibanez TA, Nowalk MP, Zimmerman RK, Bruehlman RD. Effects of the year 2000 influenza vaccine delay on elderly patients’ attitudes and behaviors. Prev Med. 2003;37(5):417–23. [DOI] [PubMed]
- 14.Department of Veterans Affairs [homepage on the internet]. Washington: Veterans Health Administration, Public Health Strategic Healthcare Group. Vaccine Advisories. 2004–2005 Influenza Season. Available at: http://www.publichealth.va.gov/flu/advisory.htm. Accessed September 3, 2006.
- 15.Department of Veterans Affairs [homepage on the internet]. Washington: Veterans Health Administration, Public Health Strategic Healthcare group. Infection: Don’t Pass It On. Available at: http://www.publichealth.va.gov/InfectionDontPassItOn/. Accessed September 3, 2006.
- 16.Mac Donald R, Baken L, Nelson A, Nichol KL. Validation of self-report of influenza and pneumococcal vaccination status in elderly outpatients. Am J Prev Med 1999;16:173–7. [DOI] [PubMed]
- 17.Hutchison BG. Measurement of influenza vaccination status of the elderly by mailed questionnaire: response rate, validity and cost. Can J Public Health 1989;80:271–5. [PubMed]
- 18.Zimmerman RK, Raymund M, Janosky JE, Nowalk MP, Fine MJ. Sensitivity and specificity of patient self-report of influenza and pneumococcal polysaccharide vaccinations among elderly outpatients in diverse patient care strata. Vaccine 2003;21(13–14):1486–91. [DOI] [PubMed]


