Abstract
Background
Screening elderly men for prostate cancer is not recommended because definitive treatments are unlikely to extend life expectancy.
Objective
Describe clinical outcomes after a negative prostate biopsy in a population-based cohort of men ages 65 and older.
Design
Retrospective cohort study.
Participants
9,410 Medicare-eligible men who underwent a prostate biopsy in Los Angeles or New Mexico in 1992.
Measurements
We used Medicare and SEER databases to identify a cohort with an initial negative biopsy (n = 7,119) and to ascertain survival, subsequent PSA testing, prostate biopsies, and prostate cancer detection and treatment through 1997.
Results
The overall 5-year survival was 79.4% (95% CI 78.4–80.3), but only 74.6% (72.4–76.7) for men ages 75–79 at the time of the initial negative biopsy and 55.0% (51.9–57.9) for men ages 80+. During a median 4.5 years follow-up, a cumulative 75.0% (73.9–76.1) of the cohort underwent PSA testing. Among men ages 75–79 and 80+, the cumulative proportions that underwent PSA testing were 75.4% (73.0–77.8) and 74.3% (71.1-77.5), respectively. Additionally, 29.1% (26.7–31.6) of men ages 75–79 and 20.1% (17.6–23.1) of men ages 80+ underwent repeat prostate biopsy, and 10.9% (9.4–12.7) and 8.3% (6.6–10.4), respectively, were diagnosed with cancer. Among men ages 75+ with localized cancers, approximately 34% received definitive treatment.
Conclusions
High proportions of men ages 75+ underwent PSA testing and repeat prostate biopsies after an initial negative prostate biopsy. Given the known harms and uncertain benefits for finding and treating localized cancer in elderly men, most continued PSA testing after a negative biopsy is potentially inappropriate.
KEY WORDS: prostate-specific antigen, prostate cancer screening, prostate cancer treatment, elderly
BACKGROUND
The benefits of prostate cancer screening and the optimal treatment for localized disease are uncertain. Prostate-specific antigen (PSA) testing is controversial because the efficacy of screening has yet to be confirmed in randomized controlled trials.1 Testing elderly men is considered problematic even by the professional societies whose guidelines support screening. The American Urologic Association, American Cancer Society, and the National Comprehensive Cancer Network all recommend against screening men with less than a 10-year life expectancy.2–4 Based on actuarial data, screening should stop at age 75 years for men in average health. Observational studies have shown that older men with localized cancers—the target of screening efforts—have relatively high rates of disease-specific survival even without treatment.5,6 Claims data have consistently shown that older men also have the highest rates of treatment morbidity and mortality, particularly with radical prostatectomy.7,8
Despite these concerns, survey, claims, and laboratory data show considerable PSA testing occurring in men ages 75 years and older.9–13 However, these data sources cannot always distinguish screening in asymptomatic men from diagnostic testing in men with findings suspicious for cancer. One cohort for whom further PSA testing should generally be considered inappropriate is comprised of men who have undergone a negative prostate biopsy at age 75 years or older. Although prostate needle biopsy is an imperfect gold standard.14,15 a negative biopsy substantially reduces the likelihood of subsequently detecting cancer.16 In addition, most cancers found on repeat biopsies are early stage.16 Given that the estimated mean lead time for PSA testing is at least 5 to 10 years,17,18 the majority of any subsequently detected early-stage cancers would not have been found in the absence of PSA testing. Furthermore, the single randomized trial that demonstrated efficacy for aggressively treating localized cancers found a survival advantage only for men age 65 years and younger at the time of diagnosis.19
We linked Medicare and Surveillance Epidemiology and End Results (SEER) data files to describe clinical outcomes after a negative prostate biopsy—subsequent PSA testing, repeat prostate biopsies, subsequent cancer diagnoses, and treatment for early-stage cancer—in a population-based cohort of older men.
METHODS
Cohort Assembly We retrospectively assembled a study cohort using the 1992 Medicare Denominator and Physician Part B claims files to identify subjects residing in either the state of New Mexico or Los Angeles County who underwent prostate biopsy from January 1, 1992 through December 31, 1992. We identified prostate biopsies or ultrasound-guided biopsies by Physicians Current Procedure Terminology (CPT) Fourth Edition codes 55700, 55705, 76942, and 76943.We linked the Medicare claims files for men who underwent prostate biopsy in 1992 with the New Mexico and Los Angeles County SEER tumor registry databases. Linkages were based on either (1) an exact match of the social security number and at least 6 of 8 digits for date of birth or (2) an exact match on date of birth and at least 8 digits of the social security number. We used the linkage to exclude men with prevalent prostate cancers before the initial 1992 (index) prostate biopsy and to identify cases of prostate cancer detected by the index biopsy as well as those detected by a subsequent biopsy. A subject was considered to have undergone a negative index biopsy if we found no cancer match in the 8 weeks after the index biopsy. For cancers identified through the linkage, we obtained additional information on tumor stage, grade, and treatment. Stage was classified as localized (confined to the prostate), regional (extension beyond the prostate capsule including regional lymph nodes), and distant (metastasis). Stage was based on clinical findings unless the patient underwent radical prostatectomy, which provides pathologic staging. The SEER registries classify tumor grade based on level of cellular differentiation. Grade I is well differentiated (corresponding to Gleason scores 2–4), Grade II is moderately well differentiated (Gleason 5–7), and Grade III is poorly differentiated (Gleason 8–10). The SEER registries report initial treatment (particularly radical prostatectomy or radiation therapy) received or planned within 4 months of diagnosis. Reliable data on androgen deprivation treatment are not routinely available.
Follow-up We used Medicare claims data to follow the negative biopsy cohort through December 31, 1997, using CPT and ICD-9 codes to identify subsequent prostate-specific antigen testing (CPT code 84153) and prostate biopsies (CPT codes 55700, 55705, 76942, and 76943; ICD-9 code 60.1). We linked the cohort to the New Mexico and Los Angeles County tumor registry databases to identify prostate cancers found subsequent to the negative index biopsy and to obtain information on cancer stage, grade, and treatment. If a cancer was diagnosed without a preceding CPT code for a prostate biopsy, we counted the patient as having had a biopsy unless we found a preceding code for transurethral resection of the prostate (52601, 52612, 52614, 52620, 52630) or if prostate cancer was diagnosed only at the time of death. We used Medicare vital status files to determine date of death.
Data Analysis We used Kaplan–Meier analyses to estimate the cumulative proportions of patients undergoing PSA testing, undergoing repeat prostate biopsy, and receiving a prostate cancer diagnosis through the end of the study period. We used the log-rank test to test for differences in cumulative proportions of the clinical outcomes across age groups (65–69, 70–74, 75–79, 80+) at the time of the index biopsy. We also estimated rates of PSA testing, prostate biopsy, and cancer detection based on the number of events over person years at risk. Subjects were censored at the time of death, cancer diagnosis, or end of study (December 31, 1997) for analyses of PSA testing and repeat prostate biopsy. They were also censored for cancer detection analyses when they moved away from their initial geographic area (based on Medicare denominator files) because we could no longer obtain tumor registry data. We used chi-square tests to compare demographics between patients with cancer diagnosed on the initial biopsy and those who were diagnosed on a subsequent biopsy and to compare cancer treatments across age groups. We classified treatment as definitive if the subject underwent radical prostatectomy or radiation therapy.
RESULTS
We identified 10,059 men from the Medicare file who underwent an index prostate biopsy in 1992 either in Los Angeles County (8,197) or New Mexico (1,862). We excluded 649 men with a prevalent prostate cancer before the index biopsy. Overall, 2,291 of 9,410 (24.3%) men were diagnosed with prostate cancer with the index biopsy; cancer detection was significantly associated with older age and location (Table 1).
Table 1.
Baseline Characteristics of Men at the Time of 1992 Index Prostate Biopsy
| Positive biopsy | Negative biopsy | Cancer detection (%) | |||
|---|---|---|---|---|---|
| N | Column % | N | Column % | ||
| Total | 2,291 | 100.0 | 7,119 | 100.0 | 24.3 |
| Age at biopsy* | |||||
| 65–69 | 549 | 24.0 | 2,151 | 30.2 | 20.3 |
| 70–74 | 748 | 32.6 | 2,339 | 32.9 | 24.2 |
| 75–79 | 585 | 25.5 | 1,573 | 22.1 | 27.1 |
| 80+ | 409 | 17.9 | 1,056 | 14.9 | 27.9 |
| Location† | |||||
| Los Angeles | 1,757 | 76.7 | 5,954 | 83.6 | 22.8 |
| New Mexico | 534 | 23.3 | 1,165 | 16.4 | 31.4 |
*P value < .001
†P value < .0001
The follow-up study cohort was comprised of the 7,119 men with an initial negative biopsy. By December 31, 1997, the cohort had accumulated 31,938 person years of follow-up, an average of 4.5 years per person. The overall 5-year survival was 79.4% (95% CI 78.4–80.3%). However, 5-year survival was only 74.6% (95% CI 72.4–76.7%) for men ages 75 to 79 years at the time of initial biopsy and 55.0% (95% CI 51.9–57.9%) for those age 80 years and older. Meanwhile, 5-year survival for men ages 65 to 69 years was 89.9% (95% CI 88.6–91.1) and 84.0% (95% CI 82.4–85.4%) for men ages 70 to 74 years. The PSA testing rate was 74.1/100 person years (95% CI 73.2–75.1/100 person years). During follow-up, the cohort underwent 23,671 PSA tests with a cumulative testing proportion of 75.0% (95% CI 73.9–76.1%) (Table 2, Fig. 1). Table 2 also shows the cumulative proportion of men undergoing PSA testing by age group at the time of the initial biopsy. We found no significant age-specific differences in PSA testing. The majority of PSA tests were not linked to a provider type.
Table 2.
Cumulative Proportions of Men Undergoing PSA Testing, Repeat Biopsy, and Cancer Detection within 5 Years After Negative Index Prostate Biopsy, Overall and Stratified by Age at Index Biopsy
| Age at index biopsy | PSA testing % (95% CI) | Repeat biopsy % (95% CI) | Cancer detection % (95% CI) |
|---|---|---|---|
| All ages | 75.0 (73.9, 76.1) | 32.2 (31.1, 33.4) | 10.7 (9.9, 11.5) |
| 65–69 (reference) | 74.6 (72.6, 76.5) | 36.0 (34.0, 38.2) | 10.2 (9.0, 11.6) |
| 70–74 | 75.4 (73.4, 77.2) | 35.1 (33.1, 37.1) | 11.8 (10.5, 13.2) |
| 75–79 | 75.4 (73.0, 77.8) | 29.1 (26.7, 31.6) * | 10.9 (9.4, 12.7) |
| 80+ | 74.3 (71.1, 77.5) | 20.1 (17.6, 23.1) * | 8.3 (6.6, 10.4)† |
*P < .0001 vs reference
†P = .05 vs reference
Fig. 1.
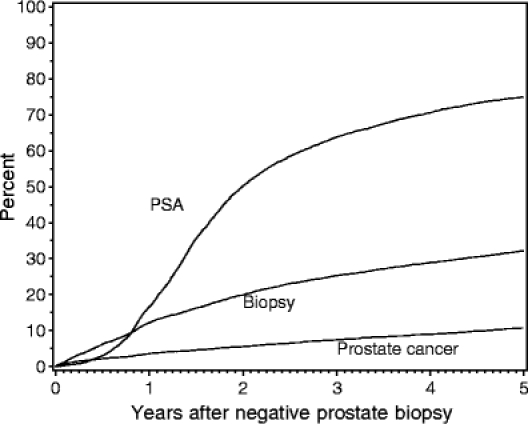
Cumulative proportions of PSA testing, repeat biopsy, and cancer diagnosis following initial negative biopsy.
Overall, the cohort underwent 3,007 repeat biopsies, with a biopsy rate of 9.4/100 person years (95% CI 8.9–10.0/100 person years). The number of repeat biopsies per person ranged from 1 to 7, with a median of 1 (data not shown). The cumulative proportion of men undergoing repeat biopsy was 32.2 % (95% CI 31.1–33.4%) (Table 2, Fig. 1). The cumulative proportion undergoing biopsy was significantly lower for older men compared to younger men, although 29.1% of men ages 75 to 79 years and 20.1% of men ages 80 years and older underwent a repeat biopsy (Table 2). Additionally, 16% of the repeat biopsies in men ages 70 to 74 years at the time of the index biopsy occurred when the subject was age 75 years or older. The overall proportion of biopsies to PSA tests was lower for men ages 75 years and older (10.0%) than for younger men (14.6%), P < .0001.
Prostate cancer was detected in 741 subjects by the end of the study period with a cancer detection rate of 2.3/100 person years (95% CI 2.2-2.5/100 person years). The cumulative proportion of men diagnosed with cancer was 10.7% (95% CI 9.9–11.5%) (Table 2, Fig. 1). Table 2 also shows the cumulative proportion of cancer detection by age group at the time of the initial biopsy. The cumulative proportions of cancer detection were similar for the 3 youngest age groups, but slightly lower for men ages 80 years and older.
The proportion of men receiving definitive treatment for localized prostate cancer was significantly lower for men ages 75 years and older at the time of the negative index biopsy, although 42.7% of men ages 75 to 79 years received radical prostatectomy or radiation therapy (Table 3). We also re-analyzed the data after reclassifying the 292 men with regional stage cancers who underwent radical prostatectomy as having a clinically localized cancer at diagnosis. We did this because the summary SEER stage reflects pathologic staging in men who underwent radical prostatectomy—which could lead to underestimating the number of subjects with clinically localized cancer. Even with the stage reclassification, the associations between age group at diagnosis and treatment received were unchanged (data not shown).
Table 3.
Treatment Received for Localized Prostate Cancer Detected by Subsequent Biopsy Stratified by Age at Negative Index Biopsy
| Age at negative index biopsy | N | Definitive treatment* % (95% CI) | Radical prostatectomy % (95% CI) |
|---|---|---|---|
| 65–69 (reference) | 177 | 75.7 (69.2, 82.1) | 55.9 (48.5, 63.4) |
| 70–74 | 208 | 66.4 (58.9, 72.6)† | 31.7 (11.2, 21.9)‡ |
| 75–79 | 117 | 42.7 (34.1, 52.7)‡ | 17.1(10.9, 25.5)‡ |
| 80+ | 45 | 11.1 (3.7, 24.1)‡ | 2.2(0.1, 11.8)‡ |
*Radical prostatectomy or radiation therapy
†P < 0.03 vs reference
‡P < 0.0001 vs reference
DISCUSSION
The 5-year overall cumulative proportion of PSA testing in elderly men ages 75 years and older after a negative prostate biopsy was approximately 75%, very similar to the testing rate in younger men. However, the 5-year survival was much lower for the elderly men. Nonetheless, as a result of this PSA testing, 29.1% of men ages 75 to 79 years and 20.1% of men ages 80 years and older underwent at least 1 subsequent prostate biopsy. Cancer was detected in 10.9% of men ages 75 to 79 years and in 8.3% of those ages 80 years and older; nearly 34% of the elderly men diagnosed with early-stage cancer received definitive treatment.
The high proportion of men older than age 75 years who underwent PSA testing is consistent with testing rates reported by the 2000 National Health Interview Survey9,11 and the 1999 to 2002 National Ambulatory Medical Care Survey.10 Among men ages 75 years and older, over 30% reported PSA testing within the past year, including a substantial proportion who self-described themselves as being in fair or poor health.10 Walter and colleagues determined that 51% of veterans over age 75 years underwent PSA screening tests in 2003, with relatively similar within age-group testing rates regardless of health status as measured by the Charlson–Deyo Comorbidity Index.13 These PSA testing practices are concerning. Efforts to find cancers in men with less than a 10-year life expectancy by screening are not supported by any guidelines because elderly men are not expected to live long enough to gain a survival benefit from aggressively treating an early-stage cancer.1 However, PSA testing in elderly patients could appropriately be driven by lower urinary tract or systemic symptoms because diagnosing clinically–advanced prostate cancer could alter treatment strategies.
Our Medicare cohort of men ages 75 years and older all had undergone a negative biopsy. Guidelines do suggest repeating a prostate biopsy when the pathology is suspicious for cancer without necessarily first repeating a PSA.2 Accordingly, we found that a higher cumulative proportion of men underwent biopsy than underwent PSA tests in the 6 months after the index negative biopsy (Fig. 1). Continued surveillance PSA testing could reflect clinical suspicion (based on PSA level and/or digital rectal examination findings) that a cancer was missed, and a rising or persistently elevated PSA might be an indication for a repeat biopsy. However, the chances that the index biopsy missed an advanced-stage tumor are small.16 The relatively indolent course of early-stage prostate cancer in the elderly5,6—and the lack of evidence supporting aggressive treatment for the elderly19—suggests that continued PSA testing would not be expected to increase survival. In addition, it is unlikely that new urinary tract or systemic symptoms—which could prompt diagnostic PSA testing—occurring within 5 years of a negative biopsy could be attributed to prostate cancer.
A limitation of the study is that we do not know the indication for PSA testing. However, based on previous reports characterizing PSA testing in the elderly,9–13 cancer detection after a negative biopsy,16 and the natural history of prostate cancer, 5,6 we believe that most subsequent PSA testing in our cohort was potentially inappropriate, whether it was intended for screening, surveillance, or diagnosis.
The majority of PSA testing after the initial negative biopsy was not linked to a provider type. However, based on previous national survey data, we suspect that primary care providers likely drive the high rate of PSA testing in older men.20–22 According to these studies, primary care providers are much more aggressive in ordering PSA testing than urologists and have greater expectations for the benefits of definitive treatment than is warranted by the evidence. Although the cumulative proportion of PSA testing approached 75% in the older age groups, a relatively smaller proportion underwent re-biopsy. Given that the proportion of elevated PSA levels was probably higher in the older age groups than in the younger age groups,23 these findings are consistent with a survey report that urologists are less inclined than primary care providers to routinely recommend biopsies for older men with an elevated PSA.20
When cancers were detected in elderly men, about one-third of them were definitively treated, with just 13% undergoing radical prostatectomy. This conservative management is reasonable given the higher complication risk for older men after radical prostatectomy7,8,24 and the limited treatment benefit for older men.19,25 However, this raises a question about the purpose of testing men ages 75 and older for prostate cancer. The goal of screening is to identify early-stage cancers because they are potentially curable with definitive treatment. Finding, but not definitively treating—or needing to definitively treat—early-stage cancers in elderly men undermines the purpose of screening. Such men face the psychological burden of a cancer diagnosis without any expectation or need for a cure. Although elderly men may receive androgen deprivation therapy, these treatments can impair quality of life26 and potentially cause diseases27 with no evidence that they prolong life for men with early-stage disease. The quandary surrounding treatment decisions for elderly men with early-stage cancers can be avoided by not screening them in the first place.
Our study had some potential limitations. Although we found that older men with clinically localized cancers were less likely than younger men to receive aggressive treatment, we were unable to adjust the comparisons for comorbidities or know whether patients received androgen deprivation therapy. However, if comorbid conditions prevented men from receiving definitive treatments, these conditions should potentially also have limited efforts to detect cancer. Possibly, our results are now less relevant given temporal changes in prostate cancer diagnosis and treatment. Surveillance Epidemiology and End Results (SEER) data have shown declining prostate cancer incidence rates for older men. For example, the age-specific incidence rate was 1118.5 per 100,000 for men ages 75 to 79 years during the period 1993 to 1997.28 The most recent SEER data (2000 to 2003) reported a rate of only 1,032.3 per 100,000.29 Even larger declines in incidence rates were reported for men ages 80 to 84 years, from 1018.6 to 842.4 per 100,000. However, multiple data sources still document high testing rates in elderly men.9–13 Although recent data suggest that fewer men over age 70 years receive radical prostatectomy, the proportion being treated with brachytherapy has increased and the overall treatment rates are relatively stable.7
We may have underestimated cancer detection if we missed matching Medicare and tumor registry data owing to errors in personal identifiers. We may have incorrectly included men with a prevalent cancer in our cohort if they had not been diagnosed in Los Angeles or New Mexico. This would primarily result in misclassifying surveillance PSA testing as diagnostic testing, although it should not substantially alter our estimates for biopsies. Finally, our results may not be generalizable to geographic locations other than Los Angeles or New Mexico.
We found a high rate of PSA testing in a Medicare cohort of elderly men after a negative prostate biopsy. Other studies have also reported that PSA testing is common among older men.9–13 Unlike these studies, we were also able to provide data on the clinical outcomes associated with PSA testing. We found that the problem of potentially inappropriate PSA testing in men ages 75 years and older is compounded by relatively high rates of repeat biopsies and aggressive therapy for men with early-stage cancer despite their decreased life expectancy, particularly for those older than age 80 years. Given that the benefit of finding and treating prostate cancer in elderly men is uncertain, primary care providers have an important responsibility to avoid recommending and/or ordering unnecessary PSA tests. Older patients need to understand that continued efforts to find prostate cancer are unlikely to improve survival while potentially subjecting them to the psychological harms of diagnosis and the physical and financial burdens of treatment.
Acknowledgements
This project was supported by the National Cancer Institute: N01-PC-85063-20. This project was also supported in part by National Cancer Institute Contract NO1-PC-35138 for the New Mexico Tumor Registry and Contract N01-PC-67010 for the Los Angeles County Tumor Registry.
This project was also supported by the New Mexico VA Health Care System and the University of New Mexico Cancer Research and Treatment Center.
The collection of the Los Angeles cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institutes Surveillance, Epidemiology and End Results Program under contract N01-PC-35139 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Preventions National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Health Services, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
The authors thank Arnold Potosky, PhD, for his helpful comments on an earlier manuscript draft, and Meg Adams-Cameron, MPH, for her help with grant preparation and data acquisition.
Conflicts of Interest None disclosed.
Footnotes
This work was presented in part at the Annual National Meeting of the Society for General Internal Medicine. Los Angeles, California, May 2006.
References
- 1.Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(11):917–29. [DOI] [PubMed]
- 2.National Comprehensive Cancer Network. Prostate Cancer Early Detection. Clinical Practice Guidelines in Oncology. Version 1.2005 ed., vol. 2005; 2005. [DOI] [PubMed]
- 3.Smith RA, Cokkinides V, von Eschenbach AC, et al. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2002;52(1):8–22. [DOI] [PubMed]
- 4.Carroll P, Coley C, McLeod D, et al. Prostate-specific antigen best practice policy. Part II. prostate cancer staging and post-treatment follow-up. Urology. 2001;57(2):225–9. [DOI] [PubMed]
- 5.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095–101. [DOI] [PubMed]
- 6.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291(22):2713–9. [DOI] [PubMed]
- 7.Bubolz T, Wasson JH, Lu-Yao G, Barry MJ. Treatments for prostate cancer in older men: 1984–1997. Urology. 2001;58(6):977–82. [DOI] [PubMed]
- 8.Lu-Yao GL, Albertsen P, Warren J, Yao SL. Effect of age and surgical approach on complications and short-term mortality after radical prostatectomy—a population-based study. Urology. 1999;54(2):301–7. [DOI] [PubMed]
- 9.Ross LE, Coates RJ, Breen N, Uhler RJ, Potosky AL, Blackman D. Prostate-specific antigen test use reported in the 2000 National Health Interview Survey. Prev Med. 2004;38(6):732–44. [DOI] [PubMed]
- 10.Scales CD, Jr., Curtis LH, Norris RD, Schulman KA, Albala DM, Moul JW. Prostate specific antigen testing in men older than 75 years in the United States. J Urol. 2006;176(2):511–4. [DOI] [PubMed]
- 11.Lu-Yao G, Stukel TA, Yao SL. Prostate-specific antigen screening in elderly men. J Natl Cancer Inst. 2003;95(23):1792–7. [DOI] [PubMed]
- 12.Richter F, Dudley AW, Jr., Irwin RJ, Jr., Sadeghi-Nejad H. Are we ordering too many PSA tests? Prostate cancer diagnosis and PSA screening patterns for a single Veterans Affairs Medical Center. J Cancer Educ. 2001;16(1):38–41. [DOI] [PubMed]
- 13.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296(19):2336–42. [DOI] [PubMed]
- 14.Stroumbakis N, Cookson MS, Reuter VE, Fair WR. Clinical significance of repeat sextant biopsies in prostate cancer patients. Urology. 1997;49(3A Suppl):113–8. [DOI] [PubMed]
- 15.Djavan B, Zlotta A, Remzi M, et al. Optimal predictors of prostate cancer on repeat prostate biopsy: a prospective study of 1,051 men. J Urol. 2000;163(4):1144–8; discussion 1148–9. [DOI] [PubMed]
- 16.Roobol MJ, Van der Cruijsen IW, Schroder FH. No reason for immediate repeat sextant biopsy after negative initial sextant biopsy in men with PSA level of 4.0 ng/mL or greater (ERSPC, Rotterdam). Urology. 2004;63(5):892–7; discussion 897–9. [DOI] [PubMed]
- 17.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95(12):868–78. [DOI] [PubMed]
- 18.Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273(4):289–94. [DOI] [PubMed]
- 19.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977–84. [DOI] [PubMed]
- 20.Fowler FJ, Jr., Bin L, Collins MM, et al. Prostate cancer screening and beliefs about treatment efficacy: a national survey of primary care physicians and urologists. Am J Med. 1998;104(6):526–32. [DOI] [PubMed]
- 21.McNaughton Collins M, Stafford RS, Barry MJ. Age-specific patterns of prostate-specific antigen testing among primary care physician visits. J Fam Pract. 2000;49(2):169–72. [PubMed]
- 22.Han PK, Coates RJ, Uhler RJ, Breen N. Decision making in prostate-specific antigen screening national health interview survey, 2000. Am J Prev Med. 2006;30(5):394–404. [DOI] [PubMed]
- 23.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270(7):860–4. [DOI] [PubMed]
- 24.Wilt TJ, Cowper DC, Gammack JK, Going DR, Nugent S, Borowsky SJ. An evaluation of radical prostatectomy at Veterans Affairs Medical Centers: time trends and geographic variation in utilization and outcomes. Med Care. 1999;37(10):1046–56. [DOI] [PubMed]
- 25.Ross KS, Guess HA, Carter HB. Estimation of treatment benefits when PSA screening for prostate cancer is discontinued at different ages. Urology. 2005;66(5):1038–42. [DOI] [PubMed]
- 26.Potosky AL, Reeve BB, Clegg LX, et al. Quality of life following localized prostate cancer treated initially with androgen deprivation therapy or no therapy. J Natl Cancer Inst. 2002;94(6):430–7. [DOI] [PubMed]
- 27.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–56. [DOI] [PubMed]
- 28.Ries LAG, Eisner MP, Kosary CL, et al. (National Cancer Institute). SEER Cancer Statistics Review, 1973–1997; 2000.
- 29.Ries LAG, Harkins D, Krapcho M, et al. (National Cancer Institute). SEER Cancer Statistics Review, 1975-2003; 2006.


