Abstract
There are two major mechanisms reported to prevent the autoreactivity of islet-specific CD8+ T cells: ignorance and tolerance. When ignorance is operative, naïve autoreactive CD8+ T cells ignore islet antigens and recirculate without causing damage, unless activated by an external stimulus. In the case of tolerance, CD8+ T cells are deleted. Which factor(s) contributes to each particular outcome was previously unknown. Here, we demonstrate that the concentration of self antigen determines which mechanism operates. When ovalbumin (OVA) was expressed at a relatively low concentration in the pancreatic islets of transgenic mice, there was no detectable cross-presentation, and the CD8+ T cell compartment remained ignorant of OVA. In mice expressing higher doses of OVA, cross-presentation was detectable and led to peripheral deletion of OVA-specific CD8+ T cells. When cross-presentation was prevented by reconstituting the bone marrow compartment with cells incapable of presenting OVA, deletional tolerance was converted to ignorance. Thus, the immune system uses two strategies to avoid CD8+ T cell-mediated autoimmunity: for high dose antigens, it deletes autoreactive T cells, whereas for lower dose antigens, it relies on ignorance.
Keywords: antigen presentation, transgenic mice, ovalbumin, apoptosis, autoimmunity
The control of T cell-mediated autoimmunity has been extensively investigated in transgenic mice expressing model self antigens under the control of tissue-specific promoters. Particular effort has been invested into understanding the nature of T cell tolerance to pancreatic islet β cell antigens, because of the prominent role of T cells in the pathogenicity of diabetes mellitus type I. In some of the first of these studies, viral proteins were expressed in pancreatic islets (1, 2). Autoimmune diabetes did not occur in these mice, unless they were infected with the respective virus. These seminal studies demonstrated that CD8+ T cells with specificity for model self antigens could be induced to become autoagressive by cross-reactive pathogen-associated antigens, but that under normal circumstances they ignored their specific self antigens. Transgenic autoantigens are, however, not always ignored. In particular, when ovalbumin (OVA)-specific CD8+ T cells (OT-I) were injected into transgenic mice expressing membrane-bound OVA (mOVA) under the control of the rat insulin promoter (RIP-mOVA mice), which express mOVA in pancreatic islet β cells and kidney proximal tubular cells, they were activated by a mechanism termed cross-presentation and responded to the autoantigen OVA by proliferating in the renal and pancreatic lymph nodes (LNs) (3). This form of activation did not result in autoimmunity (unless unphysiologically high numbers of OT-I cells were transferred), but led to the deletion of OVA-specific T cells (4). Likewise, tolerance rather than ignorance was observed in transgenic mice expressing simian virus 40 T antigen (5) or influenza virus hemagglutinin (6). Hence, in the various transgenic models studied so far, both peripheral CD8+ T cell tolerance and ignorance to islet antigens have been reported. What determined whether ignorance or tolerance prevailed was unknown.
Recently, we demonstrated that antigen dose is a major factor influencing whether OT-I cells are activated by cross-presentation. Using two lines of transgenic mice expressing different amounts of secreted OVA under the control of the rat insulin promoter, it was revealed that only the higher dose was effectively cross-presented. This was despite the fact that both doses were sufficient to target islet cells for destruction by activated cytotoxic T lymphocytes (CTL). In RIP-OVAlo mice, which expressed the lower concentration of secreted OVA in pancreatic islets, OVA-specific CD8+ T cells were not activated by cross-presentation in the draining LNs (7). By contrast, RIP-OVAhi mice, which express a higher concentration, were able to stimulate T cells by cross-presentation. Because deletion has been shown to be associated with cross-presentation of OVA (4), these findings suggested that OVA-specific T cells might be deleted in RIP-OVAhi mice but not in RIP-OVAlo mice. In this study, we prove this hypothesis, demonstrating a link between autoantigen dose and the deletion of autoreactive CD8+ T cells.
Materials and Methods
Mice.
All mice were bred and maintained at the Walter and Eliza Hall Institute for Medical Research (WEHI; Melbourne, Australia). Rag-1-deficient OT-I mice, RIP-mOVA mice, RIP-OVAhi mice, and RIP-OVAlo mice have been described previously (3, 7, 8).
Fluorescent Labeling of OT-I Cells.
5,6-Carboxyfluorescein diacetate, succinimidyl ester (CFSE) labeling was performed as previously described (4, 9). Briefly, Rag-1-deficient OT-I cells were resuspended in PBS containing 0.1% BSA (Sigma) at 107 cells per ml. For fluorescence labeling, 2 μl of a CFSE (Molecular Probes) stock solution (5 mM in DMSO) was incubated with 107 cells for 10 min at 37°C.
Adoptive Transfer and FACS Analysis.
Preparation and adoptive transfer of Rag-1-deficient OT-I cells and analysis on a FACScan (Becton Dickinson) were carried out as described (3). In adoptive-transfer experiments, OT-I cells were identified in recipient mice by staining with anti-Vα2-FITC (B20.1), anti-CD8-phycoerythrin (Caltag, South San Francisco, CA), and anti-Vβ5-biotin (MR9-40) revealed with Streptavidin-Tricolor (Caltag). An average of 1.4% of CD8+ cells were Vα2+Vβ5+ in uninjected mice. The total number of OT-I cells was derived using the formula [(% Vα2+Vβ5+ cells in the CD8+ cells − 1.4%) × (% CD8+ T cells in live cells) × (number of live cells)]. For analysis of fluorescent-labeled cells, 50,000 CD8+ cells were collected and analyzed using weasel software (F. Battye, WEHI).
Bone Marrow Chimeras.
RIP-mOVA mice expressing the MHC class I molecule H-2Kb on non-bone marrow-derived tissue cells, and either H-2Kbm1 or H-2Kb on their bone marrow-derived cells, were generated by irradiating H-2b RIP-mOVA mice with 900 cGy and injecting them with 5 × 106 bm1 or C57BL/6 (B6) T-depleted bone-marrow cells as described (3). The next day, radioresistant T cells were depleted by i.p. injection of 100 μl of T24 ascites fluid (anti-Thy-1). These mice were termed bm1→RIP-mOVA mice and B6→RIP-mOVA mice, respectively.
Proliferative Response.
LN cells from mice injected with Rag-1-deficient OT-I cells were used as responders. The number of OT-I cells was determined by FACS analysis on the day of culture, as described above. Stimulator cells consisted of OVA257–264 peptide-pulsed or unpulsed syngeneic 1500-cGy-irradiated B6 spleen cells. Cells were cultured in 96-well plates in RPMI 1640 supplemented with 10% FCS and 50 μM 2-mercaptoethanol for 3 days. After pulsing the wells with 1 μCi of [3H]thymidine for 6 h, cells were harvested onto glass-fiber filters for scintillation counting. The OVA257–264 peptide SIINFEKL was synthesized by using an Applied Biosystems model 431A synthesizer and provided by J. Fecondo (Swinburne University of Technology, Hawthorn, Victoria, Australia).
OVA-Specific CTL Generation.
OVA-specific CTL were generated as previously described (8). Briefly, mice were primed with 10 μg of OVA257–264 peptide in complete Freund’s adjuvant. After 7 days, spleens were removed and cultured with 108 1500-cGy-irradiated OVA-loaded B6 spleen cells for 6 days. Cytotoxicity was assessed in a conventional 51Cr-release assay using the H-2b cell line EL4 and the OVA-expressing transfectant E.G7 as targets.
Quantitation of OVA Expression by Western Blot Analysis.
Pancreata were collagenase-digested, and islets were separated by density gradient centrifugation (10). Islets were handpicked under an inverted microscope and lysed in buffer containing 1% Triton X-100 and proteinase inhibitors (11). The soluble fraction was recovered after centrifugation at 15,000 × g. The total protein content was estimated by Lowry assay using BSA as the standard. Proteins were fractionated on SDS/10% polyacrylamide gels and electroblotted onto nitrocellulose membranes. The membranes were incubated with rabbit anti-OVA serum at 1:100 (prepared in the Autoimmunity and Transplantation Division, WEHI) followed by swine anti-rabbit serum at 1:2000 (Dako), and immunoreactive proteins were detected with 125I-labeled protein A. Purified OVA (Sigma) was included as a standard on all blots. Stained proteins were detected by using a PhosphorImager (Molecular Dynamics), and quantified by using imagequant software (Molecular Dynamics).
Results and Discussion
The Deletion of Autoreactive CD8+ T Cells Induced by Cross-Presentation Is Restricted to High-Dose Self Antigens.
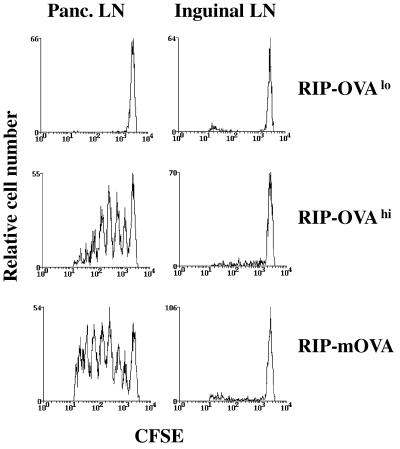
We have previously shown that OVA-specific CD8+ T cells (OT-I cells) adoptively transferred into RIP-mOVA mice, which express mOVA in pancreatic islet β cells and kidney proximal tubular cells, were activated in LNs draining OVA-expressing tissues by a mechanism termed cross-presentation (3). This led to the deletion of OT-I cells, suggesting a role for cross-presentation in peripheral CD8+ T cell tolerance (4). To investigate the role of antigen dose in this process, we generated several lines of transgenic mice expressing secreted OVA under the control of the rat insulin promoter. Two of these lines, RIP-OVAhi and RIP-OVAlo, were chosen for further analysis because of their clear differences in the level of OVA expression (7). In earlier work, we provided evidence for different levels of antigen expression based on the fact that RIP-OVAhi but not RIP-OVAlo mice expressed sufficient antigen in pancreatic islets to be detected by immunohistology (7). We have now quantitated OVA expression by Western blotting of protein extracted from the islets. This revealed that RIP-OVAhi mice expressed 1.0 ± 0.4 ng of OVA per μg of protein in the pancreatic islets. In contrast, RIP-OVAlo mice expressed an undetectably low level of antigen that was <0.03 ng of OVA per μg of protein, which is at least 33-fold less antigen than the high expressor. Both lines expressed physiologically relevant doses of OVA, as shown by the ability of in vitro-activated OT-I cells to recognize and destroy the islet β cells of each line (7). They differed, however, in their ability to cross-present OVA in the pancreatic draining LNs (Fig. 1, and see ref. 7). Like RIP-mOVA mice, RIP-OVAhi mice were able to cross-present OVA in the draining LNs of the pancreas, whereas RIP-OVAlo mice were unable to cause such CD8+ T cell activation. Our analysis of the level of antigen expressed by islet cells in RIP-mOVA mice has been somewhat problematic because of mOVA running at a higher molecular weight (possibly due to multimerization), leading to its suboptimal transfer onto nitrocellulose. However, two separate preparations yielded expression averaging 2.2 ng per μg of islet cell protein, which is very similar to the level of secreted OVA expressed in RIP-OVAhi mice.
Figure 1.
Cross-presentation of OVA depends on antigen dose. A total of 5 × 106 Rag-1-deficient OT-I cells were labeled with CFSE and adoptively transferred i.v. into either RIP-OVAlo mice (Top), RIP-OVAhi mice (Middle), or RIP-mOVA mice (Bottom). Three days later, the pancreatic (Left) and inguinal (Right) LN cells were analyzed by flow cytometry. As previously reported (7), negative littermates did not induce proliferation of Rag-1-deficient OT-I cells (data not shown). Histograms were gated on CD8+CFSE+ cells.
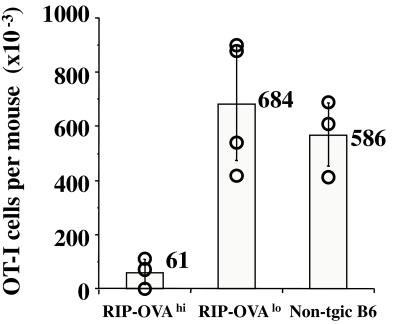
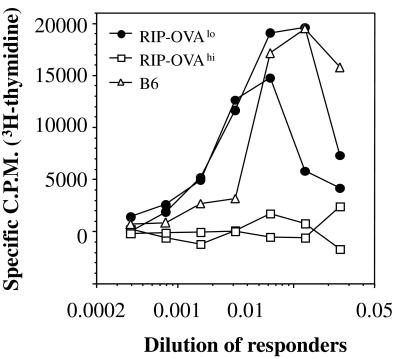
To investigate whether the absence of cross-presentation in RIP-OVAlo mice resulted in a lack of peripheral deletion, we followed the fate of OT-I cells after adoptive transfer into RIP-OVAlo vs. RIP-OVAhi mice (Fig. 2). Four weeks after adoptive transfer, very few OT-I cells were left in RIP-OVAhi mice, whereas the number of OT-I cells remaining in RIP-OVAlo mice was similar to that in nontransgenic littermates. This indicated that OT-I cells were deleted in RIP-OVAhi mice but not in RIP-OVAlo mice. To determine the functional status of the OT-I cells remaining in the RIP-OVAlo mice after 4 wk in vivo, LN cells from these mice were rechallenged with antigen in vitro (Fig. 3). In this experiment, OT-I cells from RIP-OVAlo mice were able to proliferate in response to antigen, indicating that they had not been anergized. In contrast, LN cells from RIP-OVAhi mice, in which most OT-I cells had been deleted (Fig. 2), showed a very weak OVA-specific proliferative response (Fig. 3). To further support the idea that OT-I cells ignored OVA in RIP-OVAlo mice, we examined the expression of T cell activation markers after adoptive transfer of CFSE-labeled OT-I cells into both RIP-OVAlo mice and nontransgenic B6 mice. At 2 wk after transfer, there was no difference in the expression of CD44, CD62 ligand, and CD69 on OT-I cells in either recipient (data not shown).
Figure 2.
OT-I cells are deleted in RIP-OVAhi but not RIP-OVAlo mice. A total of 5 × 106 Rag-1-deficient OT-I cells were adoptively transferred into RIP-OVAhi mice, RIP-OVAlo mice, or nontransgenic B6 mice. Four weeks later, the total number of OT-I cells remaining in the spleen and the LNs of the recipient mice was determined by flow cytometry, as previously described (4). The bars indicate the average number of OT-I cells found in the respective groups. These results are representative of three such experiments.
Figure 3.
OT-I cells are not anergized in RIP-OVAlo mice. A total of 5 × 106 Rag-1-deficient OT-I cells were adoptively transferred into RIP-OVAhi mice (□, two recipients shown), RIP-OVAlo mice (●, two recipients shown), and a nontransgenic B6 mouse (▵). After 4 wk, various numbers of LN cells were restimulated with 1500-cGy-irradiated spleen cells pulsed with the OVA257–264 peptide at a concentration of 5 μg/ml. After another 3 days, their proliferative response to OVA was determined by [3H]thymidine incorporation. These results are representative of two such experiments.
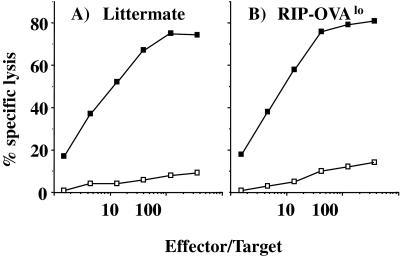
Consistent with the lack of tolerance of transgenic OT-I cells transferred into RIP-OVAlo mice, the normal CD8+ T cell repertoire of these mice was not tolerant to OVA, as indicated by their ability to generate an OVA-specific cytotoxic T cell response (Fig. 4). Because only high-affinity CD8 T cells can lyse transfectants (12), the ability of CTL from RIP-OVAlo mice to kill the E.G7 targets (EL4 transfectants expressing OVA) indicated that the CTL generated in these mice were not simply low-affinity T cells that remained after deletion of high-affinity clones. Thus, in the absence of cross-presentation, OVA-specific CD8 T cells ignored islet antigens and were fully responsive in vitro. This contrasts with what we observed for the normal CD8 T cell repertoire of RIP-mOVA mice. These mice express small amounts of OVA in the thymus, but when central deletion is prevented by thymectomy followed by bone marrow reconstitution and grafting of a normal B6 thymus graft, RIP-mOVA chimeras were still unable to generate OVA-specific CTL (Fig. 5). This indicated that cross-presentation can tolerize a normal repertoire. Like RIP-mOVA mice, RIP-OVAhi mice also express OVA in the thymus, and we have yet to determine whether cross-presentation in this line leads to tolerance of their normal T cell repertoire. However, this outcome seems highly likely considering our findings with the RIP-mOVA chimeras.
Figure 4.
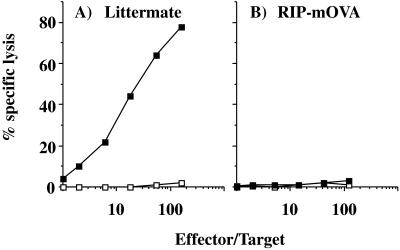
Priming of RIP-OVAlo mice leads to the generation of OVA-specific CTL. A RIP-OVAlo mouse or a nontransgenic littermate (A) was injected with OVA peptide in complete Freund’s adjuvant. Then, 1 wk later, their spleens were removed and cultured in vitro with OVA-loaded spleen as previously described (8). After 6 days, cultures were assayed for OVA-specific lytic activity by assessing their ability to lyse the OVA-transfectant E.G7 (■) or EL4 (□) cells.
Figure 5.
Priming of thymus-grafted, bone marrow-reconstituted, RIP-mOVA mice does not lead to the generation of OVA-specific CTL. Mice were thymectomized at 4–5 wk, reconstituted with B6 bone marrow at 7 wk, and then grafted with a B6 neonatal thymus at 11 wk. After another 19 wk, to allow T cell reconstitution, a RIP-mOVA mouse (B) or a nontransgenic littermate (A) was injected with OVA peptide in complete Freund’s adjuvant. Then, 1 wk later, their spleens were removed and cultured in vitro with OVA-loaded spleen as previously described (8). After another 6 days, cultures were assayed for OVA-specific lytic activity by assessing their ability to lyse the OVA257–264 peptide-coated EL4 cells (■) or uncoated EL4 cells (□).
Incapacitating Cross-Presentation Prevents Deletion.
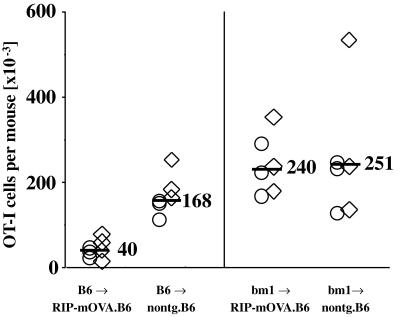
The above data suggested that cross-presentation was essential for deletion of OVA-specific CD8 T cells. If this was true, then deletion should be abolished in mice expressing high doses of OVA if cross-presentation is prevented. Such mice could be generated by introducing bone marrow of the bm1 haplotype (which cannot present OVA to OT-I cells) into RIP-mOVA mice of the B6 background (bm1→RIP-mOVA mice). This type of chimera is unable to cross-present OVA on its bone marrow-derived cells (3) but has the potential capacity to directly present OVA on parenchymal cells, such as the islet β cells. In such bm1→RIP-mOVA mice, deletion of OT-I cells was not evident, although it was detectable in control B6→RIP-mOVA mice, which could cross-present OVA (Fig. 6). OT-I cells recovered from bm1→RIP-mOVA mice proliferated in vitro when rechallenged with antigen, indicating that they had not been anergized (data not shown). Consequently, incapacitation of cross-presentation converted deletional tolerance into ignorance.
Figure 6.
Cross-presentation of high-dose OVA is essential for the deletion of OT-I cells. RIP-mOVA mice and nontransgenic littermates on a B6 (H-2b) background were lethally irradiated with 900 cGy and then reconstituted with either B6 or bm1 (H-2bm1) bone marrow (3). Eight weeks later, 9 × 106 Rag-1-deficient OT-I cells were transferred into these mice. After another 6 wk, the total number of OT-I cells remaining in the spleen and the LNs of the recipient mice was determined by flow cytometry (4). The bars indicate the average number of OT-I cells found in the respective groups. These data were obtained in two separate experiments identified by ○ or ⋄. It should be noted that in earlier publications we examined deletion using B6→bm1 RIP-mOVA chimeras and justified the necessity to use such chimeras because normal B6 RIP-mOVA mice became diabetic when transferred with the large doses of cells (i.e., 5 × 106 cells). For an as yet undetermined reason, B6→B6 RIP-mOVA chimeras are less sensitive to induction of diabetes than normal B6 RIP-mOVA mice and can often be transferred with 5 × 106 OT-I cells without leading to diabetes. This was the case for mice used in this experiment.
Two apparently incompatible mechanisms have been reported to control CD8+ T cell-mediated autoimmunity to islet antigens, i.e., tolerance (4–6) and ignorance (1, 2, 13). This discrepancy is reflected by our transgenic mice expressing different concentrations of OVA. RIP-OVAhi mice showed deletional tolerance, whereas RIP-OVAlo mice, which express at least 33-fold less OVA, showed ignorance. We have recently demonstrated that only mice expressing relatively high doses of OVA are able to cross-present this protein (14), and here we show that deletional tolerance was induced only in those mice that could cross-present OVA. This strongly implies that cross-tolerance depends on antigen dose. Consistent with this relationship between the presence of tolerance and the ability to cross-present a self antigen, we found that tolerance to hemagglutinin transgenically expressed in the pancreatic islets (6) also correlated with the ability to cross-present this molecule in the pancreatic draining LNs (15). Furthermore, in mice expressing the MHC molecule H-2Kb as a transgene in their islet cells, ignorance was seen (13). Based on our findings, this is predictable because Kb must be recognized as an entire MHC molecule and cannot be cross-presented to Kb-specific CD8+ T cells in the draining nodes. This latter point was reiterated in bm1→RIP-mOVA mice, which could not cross-present OVA because their bone marrow compartment expressed an inappropriate class I MHC haplotype. Without cross-presentation, CD8+ T cell deletion was abrogated, even when the antigen was expressed at a concentration known to be capable of cross-presentation.
Thus, our data provide direct evidence that different levels of expression of the same self antigen (in this case secreted OVA) can lead to completely different outcomes—deletional tolerance for high doses, and ignorance for low doses. Second, we provide direct correlation between cross-presentation and deletion, as we convert a deletional response in the RIP-mOVA mice to ignorance simply by preventing cross-presentation. It will be interesting to examine the relationship between antigen expression levels, cross-presentation, and peripheral CD8+ T cell tolerance in the other transgenic models used in this field.
Although OT-I cells were not activated in the pancreatic LNs of RIP-OVAlo mice, it was possible that this antigen was cross-presented, but at a dose too low to activate OT-I cells. The lack of CD8+ T cell tolerance in the normal repertoire of these mice suggested that there was no cross-presentation. It is possible, however, that the nature of tolerance in a normal T cell repertoire is more complex, with high-affinity/avidity clones being deleted by cross-presented low-dose antigens, and lower-affinity/avidity clones remaining ignorant. Consequently, for each antigen dose there would be a specific threshold affinity cut-off for deletion. This would also suggest that tolerance or ignorance observed in the transgenic systems studied so far would depend not only on antigen dose, but also on the affinity of the TCR transgene used.
In conclusion, our data provide an explanation for why some self antigens induce CD8+ T cell tolerance, whereas others are ignored. That is, the dose of self antigen determines whether it will be cross-presented, and this in turn determines whether deletional tolerance will ensue. High-dose antigens are crosspresented and induce tolerance, whereas low-dose antigens are ignored. These findings reconcile a major discrepancy in the field of peripheral T cell tolerance.
Acknowledgments
We thank Tatiana Banjanin, Freda Karamalis, Loretta Clovedale, and Paula Nathan for their technical assistance. This work was funded by National Institutes of Health Grant AI43347-01 and grants from the National Health and Medical Research Council of Australia, the Australian Research Council, and an Angelo and Susan Alberti Program grant from the Juvenile Diabetes Foundation International-National Institutes of Health. C.K. was supported by a fellowship from the Deutsche Forschungsgemeinschaft (Grant Ku1063/1-2).
Abbreviations
- OVA
ovalbumin
- mOVA
membrane-bound OVA
- LNs
lymph nodes
- RIP
rat insulin promoter
- B6
C57BL/6
- OT-I
OVA-specific class I-restricted T cell receptor transgenic
- CFSE
5,6-carboxyfluorescein diacetate, succinimidyl ester
- CTL
cytotoxic T lymphocytes
References
- 1.Ohashi P S, Oehen S, Buerki K, Pircher H, Ohashi C T, Odermatt B, Malissen B, Zinkernagel R M, Hengartner H. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 2.Oldstone M B, Nerenberg M, Southern P, Price J, Lewicki H. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 3.Kurts C, Heath W R, Carbone F R, Allison J, Miller J F A P, Kosaka H. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurts C, Kosaka H, Carbone F R, Miller J F A P, Heath W R. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams T E, Alpert S, Hanahan D. Nature (London) 1987;325:223–228. doi: 10.1038/325223a0. [DOI] [PubMed] [Google Scholar]
- 6.Lo D, Freedman J, Hesse S, Palmiter R D, Brinster R L, Sherman L A. Eur J Immunol. 1992;22:1013–1022. doi: 10.1002/eji.1830220421. [DOI] [PubMed] [Google Scholar]
- 7.Kurts C, Miller J F A P, Subramanium R, Carbone F R, Heath W R. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanas E, Carbone F R, Allison J, Miller J F A P, Heath W R. Science. 1996;274:1707–1709. doi: 10.1126/science.274.5293.1707. [DOI] [PubMed] [Google Scholar]
- 9.Lyons A B, Parish C R. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 10.Liu M, Shapiro M E. Transplant Proc. 1995;27:3208–3210. [PubMed] [Google Scholar]
- 11.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 12.Carbone F R, Moore M W, Sheil J M, Bevan M J. J Exp Med. 1988;167:1767–1779. doi: 10.1084/jem.167.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heath W R, Karamalis F, Donoghue J, Miller J F A P. J Immunol. 1995;155:2339–2349. [PubMed] [Google Scholar]
- 14.Kurts C, Heath W R, Kosaka H, Miller J F A P, Carbone F R. J Exp Med. 1998;188:415–420. doi: 10.1084/jem.188.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan D J, Kurts C, Kreuwel H T C, Holst K, Heath W R, Sherman L A. Proc Natl Acad Sci USA. 1999;96:3854–3858. doi: 10.1073/pnas.96.7.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]