Abstract
BACKGROUND
Understanding disease seasonality can provide guidance for future biomedical research.
OBJECTIVE
To examine whether meteorological factors and calendar months impact duodenal ulcer (DU) exacerbations.
DESIGN
We conducted a retrospective time series analysis of population-based claims data.
PARTICIPANTS
DU inpatients (1997–2003; all endoscopy confirmed) from Taiwan, a small island nation, n = 160,510. Inpatient admission was used as a proxy for exacerbation because 98.5% of cases had hemorrhage or perforation or both.
MEASUREMENTS
We used multivariate autoregressive integrated moving average (ARIMA) modeling to examine if DU admissions/100,000 was associated with calendar month, ambient temperature, relative humidity, rainfall, atmospheric pressure, and sunshine hours, controlling for available DU-relevant comorbidities.
RESULTS
DU admissions increased with age. ARIMA modeling showed a February (Chinese New Year-related) trough in all age groups (all p < 0.001; adjusted for meteorological variables and comorbidities), consistent with a February dip in all-cause admissions. Among 35–49 and 50+ age groups, DU admissions were negatively associated with temperature (both p < 0.05; model R2 = 0.875 and 0.920, respectively), representing a winter peak and summer trough. Among the ≤19 age group, sunshine hours and rainfall are positively associated with DU admissions (both p < 0.001; R2 = 0.565), representing a summer peak.
CONCLUSION
Meteorological variables are associated with DU exacerbations, although the potential role of nonsteroidal anti-inflammatory drug (NSAID) use because of seasonal acute respiratory illness cannot be ruled out. We recommend in-depth studies using chart reviews of DU patients admitted during peak and trough (incidence) months to clarify whether meteorological factors or the associated seasonal peaks of respiratory and other illnesses involving NSAID use are responsible for the observed seasonality.
KEY WORDS: duodenal ulcer, seasonality, climate
INTRODUCTION
Studies of disease seasonality using population-based data can uncover potential etiological factors, which could inform in-depth biomedical and prevention research. Recent documentation of hip fracture associations among the elderly with winter1–3 illustrates the utility of such studies for prevention. Seasonality studies can also assist the health system to plan for the needed care resources during the peak incidence months.
This study examines the seasonality of duodenal ulcer (DU) exacerbations using inpatient admissions as a proxy for exacerbation because 98.5% of admitted patients had either hemorrhage or perforation or both. We used population-based data from a single-payer, universal coverage, national health insurance system in Taiwan. Most documented studies of DU seasonality have used convenience samples, comprising patient panels of specific hospitals or surgeons.4,5 Such studies are liable to bias from various sources, e.g., specific hospital’s capacity to meet demands or indeterminate population base using the study hospital(s). Population-based studies are better equipped to draw generalizable conclusions, which can provide new directions for etiopathogenesis research.
We restrict to DU because, historically, its etiopathology, clinical course, and epidemiologic distribution are distinct from those of gastric ulcer. The incidence, persistence, and exacerbation of DU, including bleeding, are associated with increased gastric acid secretion6 and shown to be associated with Helicobacter pylori infection, smoking, and emotional stress.7–10 Classically, DUs used to be a disease of young adults, sharply declining after 50 years, parallel to the age-related differences in gastric acid levels.11 Because of widespread use of nonsteroidal anti-inflammatory drugs (NSAID) for chronic musculoskeletal pain conditions, DU has dramatically increased among older adults.5,12
Past documentation on DU seasonality shows mixed findings. A regional study in Spain found increased admissions for DU bleeding in autumn and winter, compared with spring and summer.13 At one hospital in Israel, peaking of acute DU admissions in July, November, and December were noted.4 In Norway, peaks in peptic ulcer perforation admissions in one administrative region during May to July and November to December were reported.14 At one hospital in Greece, reduced admissions for bleeding DUs (among patients without prior NSAID use) in winter and peaks in spring and autumn were noted.5 These studies were either single institution or region based and examined calendar month associations without examining the underlying meteorological variable levels, which may mediate the seasonal fluctuations. Seasonal combinations of temperature, humidity, atmospheric pressure, and sunshine hours vary greatly between temperate versus subtropical, coastal versus interior locations. Thus, “spring” or “winter” conditions may not generalize across regions. Evaluating calendar seasonality, concurrent with meteorological variables, enables identification of possible mediating variables.
Climatic variables, including seasonal ambient temperature and photoperiodicity, have been proposed as the operational mediators of DU seasonality.14 Our study represents a step forward, presenting evidence of its calendar-wise seasonality, concurrent with meteorological variables, using a nationwide, population-based database. Our study setting is Taiwan, a small subtropical island nation with relatively homogenous climate in its populated regions.
MATERIALS AND METHODS
Study Sample
We used 1997–2003 inpatient claims data from Taiwan’s National Health Insurance system, covering all 160,510 admissions for endoscopy-confirmed DU (ICD-9CM 532), after excluding 3,759 readmissions within 30 days (Table 1). Mean sample age was 55.8 (±18.4) years. We limit to inpatient admissions because 100% of these are endoscopy confirmed (outpatient cases may not have been confirmed by endoscopy). Further, because of universal health coverage and intense competition among providers for patients, exacerbations are most likely to result in admission to a hospital, to facilitate endoscopy and speedy institution of appropriate treatment. Because of the potential for serious consequences and availability of a third party payer source for inpatient care, providers rarely risk outpatient treatment for exacerbations. Thus, our sample includes most of the clinically significant exacerbation cases in Taiwan. Because of universal coverage of all its 23 million plus population with a single plan type, low copayments, and the government as the single payer, as well as a geographically well-distributed network of hospitals, access to care is uniform, and database enables calculation of population-based, monthly admission rates per 100,000.
Table 1.
Monthly Mean Values of DU Admissions (n = all 160,510 Cases, Excludes 30-Day Readmissions) and Meteorological Factors, 1997–2003
| Variable | Monthly mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| DU/100,000 persons aged 0–19 years | 0.55 | 0.14 | 0.26 | 0.88 |
| DU/100,000 persons aged 20–34 years | 4.25 | 0.67 | 3.05 | 5.72 |
| DU/100,000 persons aged 35–49 years | 8.48 | 1.78 | 4.45 | 13.53 |
| DU/100,000 persons aged ≥50 years | 25.66 | 4.28 | 15.15 | 39.15 |
| DU/100,000 persons | ||||
| Total | 8.61 | 1.16 | 5.56 | 12.34 |
| Male | 12.00 | 1.59 | 7.98 | 17.07 |
| Female | 5.05 | 0.74 | 3.04 | 7.35 |
Meteorological Data and Season Definitions
Mean monthly values were calculated using daily data on ambient temperature, relative humidity, atmospheric pressure, rainfall, and hours of sunshine across 19 weather observatories located in the populated regions of Taiwan. Seven mountain stations in sparsely populated areas were excluded. Being a relatively small island (total surface area = 36,188 km2), a single mean value for each calendar month across the island was judged appropriate.
Taiwan enjoys a subtropical climate, located between 21°45′N and 25°56′N, with the seasons ranging from cool to hot, and quite humid throughout the year. The Central Weather Bureau defines spring as March to May, summer June to August, autumn September to November, and winter December to February, with maximum humidity and rainfall during May to July. The average summer temperature is about 27.8°C, winter 17.7°C, spring 22.7°C, and fall 24.4°C, with very high humidity levels in summer. Mean duration of sunshine hours during summer is 7.6 hours/per day and during winter, 3.2 hours/per day.
Statistical Analysis
Monthly DU admission rates per 100,000 were calculated for 84 months, by gender and age group, ≤19, 20–34, 35–49, and ≥50 years. The age group of 50+ years distinguishes the classical, <50 aged DU population of the pre-NSAID era,14 from older age groups, who are also more likely to have exacerbating comorbidities. Distinct differences in seasonality among DU patients with and without NSAID use are also documented.13 We adjusted for arthritis (potential for nonsteroidal anti-inflammatory drug-induced ulcer/exacerbation), congestive heart failure, chronic obstructive pulmonary disease (COPD), and history of stroke/myocardial infarction (the latter, caused by long-term aspirin therapy). Increased risk of peptic ulcer bleeding in the above comorbidity groups is documented.15,16 Finally, although seasonal acute respiratory illnesses may be a common reason for NSAID use, which may confound seasonality findings, this factor could not be controlled for because of lack of data on prior illnesses in the claims.
We used autoregressive integrated moving average (ARIMA) multivariate modeling, coding calendar months as dummy variables, using January as the reference month. Time trend was a count variable, numbered 1 to 84, for each month of the time series. Meteorological variables were included in all age group models. Comorbidity controls were used only for the 35–49 and 50+ aged subsamples because there were no cases with these comorbidities in the <19 age group, and very few in the 19–34 age group. To select the models that best fit the data from the family of ARIMA models generated, we used the Akaike Information Criterion (AIC) and the Schwarz Criterion (lower values indicate better fit).17 A p value of 0.05 was used. To compare seasonality of DU admissions with any secular seasonal effects on admissions in general, we ran the ARIMA seasonality models with the same variables for all-cause admissions during this period, N = 18,608,100 admissions. The statistical package STATA (version 9.0, STATA Corporation) was used.
RESULTS
Admission Rates
During 1997–2003, DU admissions showed a steady, declining trend (from 25,983 to 19,866 in 2003). Monthly population-based rates ranged between 5.56/100,000 in May 2003 and 12.34 in January 1997 (mean monthly admissions = 8.61; SD = 1.16). Mean monthly admission rate for men was 12.00 and 5.05 for women, increasing significantly with age (Table 1). The mean ambient temperature was 23.2°C, mean relative humidity 278.4%, mean atmospheric pressure = 999.3 hPa, mean monthly rainfall 174.5 mm, and mean monthly total of sunshine hours was 169.3 hours. Figure 1 shows the time series plot of monthly DU admission rates for the total sample along with concurrent meteorological factors over the study period.
Figure 1.
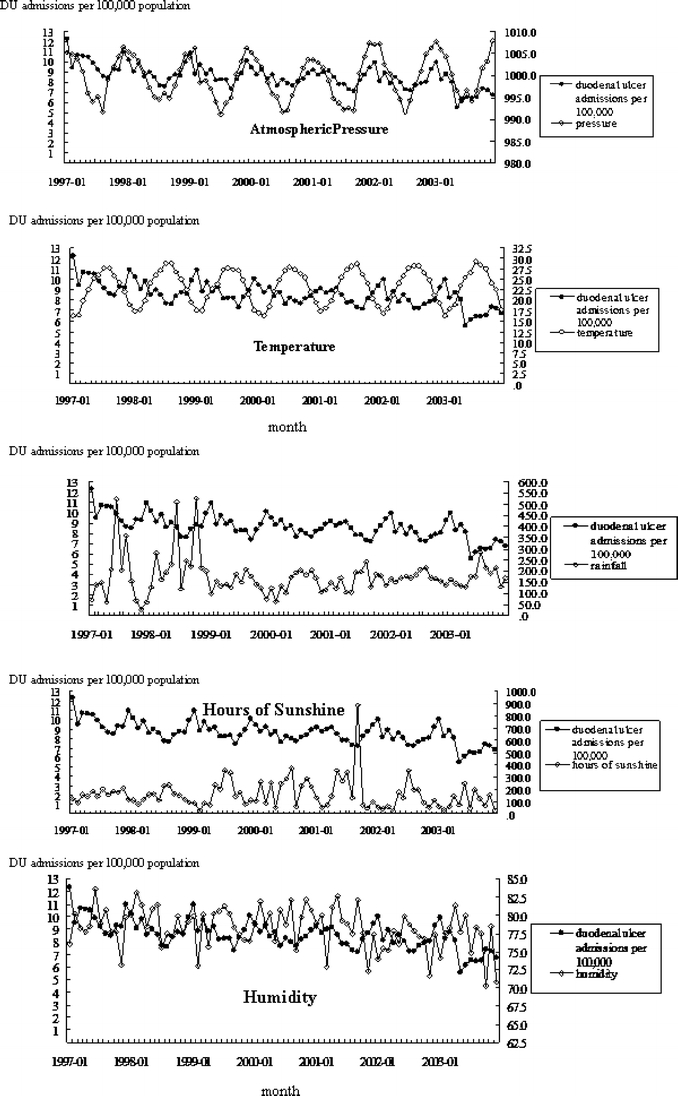
Time series of monthly DU admission rates and mean levels of meteorological variables
Table 2 presents the sample demographics and clinical typology; 71.3% were men, and 61.8% aged ≥50 years. Majority were acute or chronic DU with hemorrhage alone (ICD 532.40 or 532.0; 43.1%) and in-hospital mortality of 0.6%. Notably, DU with hemorrhage or perforation or both accounted for 98.5% (158,214 cases), indicating that most of our sample indeed represented DU exacerbations.
Table 2.
Demographic Characteristics and Type of Principal Diagnosis Among DU Inpatients in Taiwan, 1997–2003 (n = all 160,510, Excludes 30-Day Readmissions)
| Variable | n (%) |
|---|---|
| Gender | |
| Male | 114,503 (71.3) |
| Female | 46,007 (28.7) |
| Age group (years) | |
| ≤19 | 3,040 (1.9) |
| 20–34 | 20,196 (12.6) |
| 35–49 | 38,022 (23.7) |
| ≥50 | 99,252 (61.8) |
| Diagnosis (ICD-9-CM) | |
| DU (532) | 599 (0.4) |
| DU, acute with hemorrhage (5320) | 1,521 (1.0) |
| DU, acute with hemorrhage, without mention of obstruction (53200) | 33,672 (21.0) |
| DU, acute with hemorrhage, with obstruction (53201) | 800 (0.5) |
| DU, acute with perforation (5321) | 72 (0.0) |
| DU, acute with perforation, without mention of obstruction (53210) | 1,675 (1.0) |
| DU, acute with perforation, with obstruction (53211) | 107 (0.1) |
| DU, acute with hemorrhage and perforation, without mention of obstruction (53220) | 422 (0.3) |
| DU, acute with hemorrhage and perforation, with obstruction (53221) | 105 (0.1) |
| DU, acute without mention of hemorrhage or perforation (5323) | 273 (0.2) |
| DU, acute without mention of hemorrhage or perforation, without mention of obstruction (53230) | 9,189 (5.7) |
| DU, acute without mention of hemorrhage or perforation, with obstruction (53231) | 521 (0.3) |
| DU, chronic or unspecified with hemorrhage (5324) | 810 (0.5) |
| DU, chronic or unspecified with hemorrhage, without mention of obstruction (53240) | 67,521 (42.1) |
| DU, chronic or unspecified with hemorrhage, with obstruction (53241) | 1,276 (0.8) |
| DU, chronic or unspecified with perforation, without mention of obstruction (53250) | 6,922 (4.3) |
| DU, chronic or unspecified with hemorrhage and perforation (5326) | 235 (0.2) |
| DU, chronic or unspecified with hemorrhage and perforation, without mention of obstruction (53260) | 610 (0.4) |
| DU, chronic without mention of hemorrhage or perforation (5327) | 347 (0.2) |
| DU, chronic without mention of hemorrhage or perforation, without mention of obstruction (53270) | 6,589 (4.1) |
| DU, unspecified as acute or chronic, without mention of hemorrhage or perforation (5329) | 1,169 (0.7) |
| DU, unspecified as acute or chronic, without mention of hemorrhage or perforation, without mention of obstruction (53290) | 23,599 (14.7) |
| DU, unspecified as acute or chronic, without mention of hemorrhage or perforation, with obstruction (53291) | 1,544 (1.0) |
| DU, chronic without mention of hemorrhage or perforation, with obstruction (53271) | 752 (0.5) |
| Secondary diagnoses | |
| Arthritis | 356 (0.2) |
| COPD | 677 (0.4) |
| Congestive heart failure | 1,664 (1.0) |
| Diabetes | 18,466 (11.5) |
| Stroke | 5,338 (3.3) |
| Myocardial infarction | 631 (0.4) |
| Inpatient mortality | 942 (0.6%) |
Calendar Month Variations
Seasonal variations by age group and gender (Figs. 2 and 3) show a declining time trend. Figure 2 shows increasing sharpness of spikes and troughs with increasing age. Figure 3 shows similar seasonal patterns in the pooled sample and by gender, a significant peak in December–January (early and middle winter), a sharp decrease in February, and a trough during June to August (summer).
Figure 2.
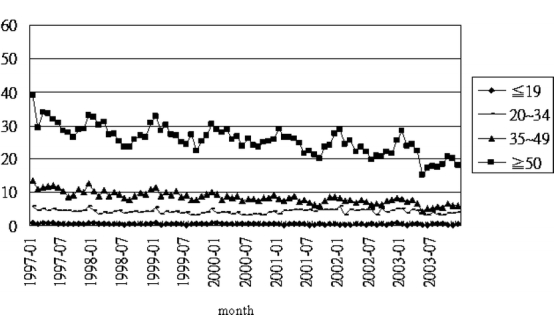
Time series plot of monthly DU admissions/100,000 population by age group
Figure 3.
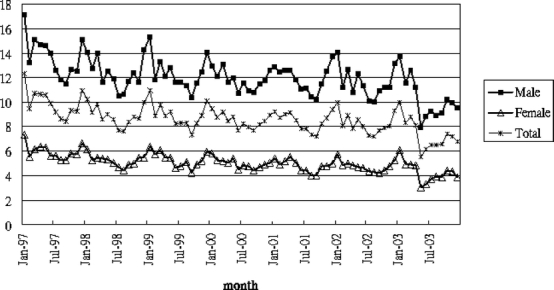
Time series plot of monthly DU admissions/100,000 population by gender
Adjusted Month and Meteorological Associations
Tables 3 and 4 present the ARIMA model estimates of significant calendar month and weather factor effects, adjusted for comorbidities. Gender-wise analysis results are not presented because of essentially similar findings. The models distinguish the specific effect of the climate variables, and calendar months, adjusted for comorbidities and other variables. R2 for all models is generally high, 0.876 for the full sample model, 0.876 for the 35–49 age group, 0.920 for the 50+ age group, 0.565 for the ≤19 age group, and 0.547 for the 20–34 age group. Seasonality is significant for all age groups (p < 0.05 for all SAR12) except 35–49 age group, with February showing significantly less (1.096/100,000 less in the total sample) admissions than January in all age groups, after adjusting for meteorological variables.
Table 3.
ARIMA Regression Analysis Showing Seasonal and Meteorological Effects on Monthly DU Admission Rates, by Age Groups (≤19 and 20–34)
| Age (yr) | Total (n = 160,510) | ≤19 (n = 3,040) | 20–34 (n = 20,196) | |||
|---|---|---|---|---|---|---|
| Independent variable | β | t value | â | t value | â | t value |
| Intercept | −39.7268 | −0.60 | −17.2793 | −1.35 | −15.8640 | −0.22 |
| AR1 | −0.5833 | −0.58 | −0.5483 | −1.69* | 0.9241 | 10.57*** |
| SAR12 | −0.4921 | −2.19* | −0.2610 | −1.80* | −0.3460 | −2.36** |
| MA1 | 1.2410 | 0.981 | 0.7727 | 3.17*** | −0.6005 | −3.58*** |
| Atmospheric pressure | 0.0480 | 0.75 | 0.0169 | 1.34 | 0.0204 | 0.29 |
| Ambient temperature | −0.1575 | −2.43* | 0.0108 | 0.47 | −0.1688 | −1.51 |
| Relative humidity | 0.0664 | 2.78** | 0.0093 | 1.51 | 0.0349 | 1.11 |
| Rainfall | 0.0003 | 0.45 | 0.0007 | 3.54*** | 0.0007 | 0.72 |
| Hours of sunshine | 0.0000 | 0.16 | 0.0004 | 2.80*** | 0.005 | 0.66 |
| February | −1.0960 | −5.03*** | −0.2163 | −4.45*** | −0.8899 | −4.41*** |
| May | 0.6822 | 2.27* | ||||
| Oct | ||||||
| Nov | ||||||
| Dec | ||||||
| Comorbidities | ||||||
| Arthritisa | 0.4908 | 0.54 | ||||
| COPDa | 0.1837 | 0.51 | ||||
| Congestive heart failurea | −0.2264 | −0.98 | ||||
| Diabetesa | −0.1092 | −1.28 | ||||
| History of strokeb | 0.1649 | 0.95 | ||||
| History of myocardial infarctionb | 0.5063 | 1.55 | ||||
| Trend | −0.0142 | −2.14* | −0.0019 | −3.08*** | 0.0010 | 0.07 |
| AIC | 1.5336 | −1.5022 | 1.7693 | |||
| Schwarz criterion (SC) | 2.4330 | −0.8330 | 2.4386 | |||
| R2 | 0.876 | 0.565 | 0.547 | |||
Selection of the final parameters was based upon the lowest AIC and SC.
AR1 Autoregressive lag 1, MA1 moving average lag 1, SAR12 seasonal correlation lag 12
*p < 0.05
**p < 0.01
***p < 0.001
aComorbidities that adversely impact DU exacerbations through pathophysiological mechanisms related to DU. The ARIMA model adjusts for the prevalence of the respective comorbidity among the admissions during each month. Age groups of <19 and 20–34 are not subjected to these adjusted variables due to almost nil or very few numbers of these comorbidities in these groups.
bComorbitities that are adjusted for to account for possible long-term aspirin medication for stroke/myocardial infarction prophylaxis.
Table 4.
ARIMA Regression Analysis Showing Seasonal and Meteorological Effects on Monthly DU Admission Rates, by Age Group (35–49 and ≥50)
| Age (yr) | 35–49 (n = 38,022) | ≥50 (n = 99,252) | ||
|---|---|---|---|---|
| Independent variable | â | t value | â | t value |
| Intercept | −16.9645 | −0.21 | −199.510 | −1.08 |
| AR1 | 0.4624 | 0.85 | −0.5560 | −3.83*** |
| SAR12 | −0.0840 | −0.52 | −0.4219 | −2.47* |
| MA1 | −0.2843 | −0.46 | 0.9646 | 41.01*** |
| Atmospheric pressure | 0.0269 | 0.34 | 0.2260 | 1.25 |
| Ambient temperature | −0.1744 | −2.02* | −0.4122 | −2.35* |
| Relative humidity | 0.0623 | 1.39 | 0.1671 | 2.39* |
| Rainfall | −0.0007 | −0.50 | 0.0010 | 0.40 |
| Hours of sunshine | −0.0014 | −1.45 | 0.0014 | 1.02 |
| February | −1.2858 | −3.82*** | −2.8200 | −4.69*** |
| May | ||||
| Oct | ||||
| Nov | −1.7385 | −2.73** | ||
| Dec | −1.4262 | −2.08* | ||
| Comorbidities | ||||
| Arthritisa | −0.2644 | −0.38 | 1.7185 | 1.05 |
| COPDa | −0.2214 | −0.44 | −0.0792 | −0.12 |
| Congestive heart failurea | −0.2384 | −0.43 | 0.4031 | 0.92 |
| Diabetesa | 0.0511 | 0.65 | −0.2781 | −1.74 |
| History of strokeb | 0.0334 | 0.14 | 0.3658 | 1.33 |
| History of myocardial infarctionb | −0.0652 | −0.12 | 2.3059 | 2.76** |
| Trend | −0.0578 | −9.53*** | −0.0952 | −5.33*** |
| AIC | 2.1796 | 3.6120 | ||
| Schwarz criterion (SC) | 3.0082 | 4.4406 | ||
| R2 | 0.8745 | 0.9200 | ||
Selection of the final parameters was based upon the lowest AIC and SC.
AR1 Autoregressive lag 1, MA1 moving average lag 1, SAR12 seasonal correlation lag 12
*p < 0.05
**p < 0.01
***p < 0.001
aComorbidities that adversely impact DU exacerbations through pathophysiological mechanisms related to DU, Weiner et al 2000. The ARIMA model adjusts for the prevalence of the respective comorbidity among the admissions during each month.
bComorbitities that are adjusted for to account for possible long-term aspirin medication for stroke/myocardial infarction prophylaxis.
Ambient temperature is significant in the total sample model, with increasing temperature associated with decreasing admissions; a 0.158 decline per degree Celsius increase for the total sample, 0.174 for the 35–49 age group, and 0.412/100,000 among 50+ age group (all p < 0.05). These estimates represent a winter-associated increase in DU exacerbations in the 35–49 and 50+ age groups. Relative humidity is significantly positively associated in the total sample and 50+ group (0.06/100,000 higher with each percentage increase in relative humidity and 0.167 higher among the 50+ age group; p < 0.01 and <0.05, respectively). Among the ≤19 age group, increasing sunshine hours and rainfall are significantly associated with increasing admissions (0.0007 higher with each millimeter increase in rainfall and 0.0004 per hour of daily sunshine; both p < 0.001), representing a summer-associated increase in this age group. May–June is the most humid and highest rainfall period in Taiwan. The trend estimate is significant, indicating a steady decline in admissions over the 84-month study period; for the pooled sample, 0.014 monthly decline. ARIMA modeling of all-cause admissions in Taiwan also shows a 9.5% dip in February (table not presented).
DISCUSSION
This study presents a higher order of clarity on DU seasonality relative to past studies because of its use of multivariate ARIMA analysis, concurrently modeling calendar months and meteorological correlates, while adjusting for many DU-relevant comorbidities (arthritis, caused by NSAID use, and other documented DU exacerbation risk factors,15,16 namely, COPD, congestive heart failure, diabetes, and history of stroke and myocardial infarction). Another major strength is its population-based data source, endoscopy-confirmed diagnosis, and setting of a single payer system with universal health coverage and low copayments. The latter features ensure virtually 100% coverage of all cases in the country.
A potential study limitation is the use of inpatient admission as a proxy for DU exacerbation, considering that some exacerbations are doubtless, managed on outpatient basis. Including outpatients in the study sample could overcome this handicap. However, endoscopy is rarely, if ever, done in an outpatient setting, which raises questions about the diagnostic accuracy among outpatients. By contrast, all inpatients with a DU diagnosis are endoscopy confirmed. Another reason to restrict our sample to inpatients was that 98.5% had either hemorrhage or perforation or both, complications that affirm an ongoing exacerbation. Nevertheless, exclusion of ambulatory DU exacerbations remains a study limitation that limits the population-wide generalizability of our findings.
We observe a February trough in all age groups (effect size = 12.73%, 1.096 less than the mean monthly admission rate of 8.61/100,000; Table 3). This is consistent with the general avoidance of elective admissions during the Chinese New Year month of February, when hospitals work with a skeletal staff to provide care for emergency cases. This explanation is substantiated by significantly higher emergent severity of February admissions (23.78% of February cases had no hemorrhage/perforation/obstruction, 26.2% in other months; p < 0.001). All-cause admissions during the study period also showed a similar February dip of 9.5%.
Our key finding is a significant negative association with ambient temperature in DU exacerbations among older adults aged 35–50 years and 50+, with this effect increasing with age. We noted an even bigger effect among the 65+ age group (table not presented). When using only calendar months in the ARIMA models, we find peak DU admissions in January, the peak winter month, a decline into summer, and rise in fall, among all age groups beyond 35 years (table not presented). Most authors (from Israel, Spain, and Norway4,13,14) report winter peaks, whereas the study from Greece5 found a trough in winter. Tsai et al. also reported a winter increase in DU admissions (November through March) during 1989–1996, using one hospital’s database.18 Past documentations on spring and autumn associations are mixed. Our findings are consistent with the winter DU admission peaks reported by most authors.
Beyond calendar month associations, we find complex relationships with temperature and humidity. Our finding of an adverse, independent association with humidity among older age groups calls for caution in generalizing calendar month “seasonality” across countries, unless climate variables are accounted for. Studies are needed in other countries and climate zones to verify our finding of temperature and humidity associations with DU exacerbation.
Winter DU peaks in a subtropical island with mild winters appear puzzling. However, authors in subtropical Israel also document a winter peak. This may reflect the role of melatonin circannual variations in DU patients, invoked by photoperiodicity changes with the seasons, documented by some authors,19 rather than the “stress” of harsh winters. In our models, for older age groups, season is represented by ambient temperature rather than sunshine hours. Our finding of a winter association may suggest the need for further biomedical research into chronobiological pathways that may be involved in ulcer–climate relationships.
A major study limitation is the lack of data on prior NSAID use, a known risk factor for DU exacerbations.13 There are no data in the claims on prior NSAID use, as there is no code for it in the ICD-9CM system. One explanation for the winter peaks in DU exacerbation could well be arthritis exacerbations or upper respiratory illnesses causing higher NSAID use. Hawley et al. noted a winter peak and summer dip in arthritis symptom severity among patients with osteoarthritis and rheumatoid arthritis.20 In this study, we controlled for arthritis. Acute respiratory illnesses are also generally seasonal, but the claims system does not accommodate documentation of antecedent illness. Therefore, we are unable to account for NSAID use because of seasonal respiratory infections, which could potentially affect our findings.
A potential factor in DU associations with winter and humidity among older adults could be smoking. Piper et al. documented that each increase in smoking by 10 cigarettes increased the risk of DU recurrence by 40%. Anecdotal evidence suggests that, in Taiwan, smokers tend to smoke more during winter and the rainy season. The male-to-female DU admissions ratio of 2.48 may also be partly because of this factor; traditionally, more smokers are men, particularly heavy smokers. The potential seasonality of smoking behavior also needs further investigation.
Contrary to older age groups, among ≤19 age group, there is a small but significant peak in summer. We submit that this may be caused by emotional stress among teenagers aged 15–18 nationwide, associated with high school and college entrance tests during June–July, and continuing until admissions in early August. Students compete intensely for admissions to top-ranking high schools and colleges. Another potential angle to be investigated is the interaction of high circulating sex hormone levels with photoperiodicity and other climatic conditions.
Consistent with the global trend of DU incidence, our data also show a steady temporal decline over the study period. This is attributed to the worldwide trend of aggressive treatment to eradicate H. pylori, a major etiological agent for DU. Studies have shown that H. pylori can be eradicated in more than 90% of patients with peptic ulcer and that it reduces ulcer recurrences and bleeding rates.21,22
A potential study limitation is its reliance on administrative data, raising questions about accuracy of comorbidity documentation. In our data, 0.6% of patients aged over 50 years have a documented secondary diagnosis of arthritis/chronic musculoskeletal problem. This could be construed as documentation bias. However, the National Health Insurance (NHI) Bureau’s checks and balances generally encourage providers to document comorbidities. This is because the NHI Bureau randomly samples each institution’s inpatient records to assess patient severity profiles, with higher comorbidity index qualifying for higher reimbursement rates. Therefore, systematic deficiencies in comorbidity documentation are unlikely.
Our study suggests directions for in-depth research to verify the role of unaccounted factors such as the seasonality of acute respiratory infections mediating the associations. Studies involving in-depth chart review of cases and controls during peak and trough months are needed to isolate true meteorological associations, if any, from faux associations caused by seasonal comorbidities that may mediate the observed climate–DU relationship.
Acknowledgment
This study is based on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, Taiwan and managed by the National Health Research Institutes. The interpretations and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health or the National Health Research Institutes.
Conflict of interest This study used publicly available, de-identified data published by the National Health Insurance Research Database for research purpose. Authors declare that there was no conflict of interest involved in conducting this research or publishing the work.
References
- 1.Jacobsen SJ, Goldberg J, Miles TP, et al. Seasonal variation in the incidence of hip fracture among white persons aged 65 years and older in the United States, 1984–1987. Am J Epidemiol. 1991;133:996–1004. [DOI] [PubMed]
- 2.Mirchandani S, Aharonoff GB, Hiebert R, et al. The effects of weather and seasonality on hip fracture incidence in older adults. Orthopedics. 2005;28:149–55. [DOI] [PubMed]
- 3.Lin HC, Xirasagar S, Tang YC. Seasonality of hip fractures and estimates of season-attributable effects: a multivariate ARIMA analysis of population-based data. Osteoporos Int. 2006;17:795–806. [DOI] [PubMed]
- 4.Braverman DZ, Morali GA, Patz JK, et al. Is duodenal ulcer a seasonal disease? A retrospective endoscopic study of 3105 patients. Am J Gastroenterol. 1992;87:1591–93. [PubMed]
- 5.Thomopoulos KC, Katsakoulis EC, Margaritis VG, et al. Seasonality in the prevalence of upper gastrointestinal bleeding. J Clin Gastroenterol. 1997;25:576–9. [DOI] [PubMed]
- 6.Wagner S, Gladziwa U, Gebel M, et al. Circadian pattern of intragastric acidity in duodenal ulcer patients: a study of variations in relation to ulcer activity. Gut. 1991;32:1104–9 [DOI] [PMC free article] [PubMed]
- 7.Taylor DN, Blaser MJ. The epidemiology of Helicobacter pylori infection. Epidemiol Rev. 1991;13:42–59. [DOI] [PubMed]
- 8.Piper DW, McIntosh JH, Hudson HM. Factors relevant to the prognosis of chronic duodenal ulcer. Digestion. 1985;31:9–16. [DOI] [PubMed]
- 9.McIntosh JH, Nasiry RW, Frydman M, et al. The personality pattern of patients with chronic peptic ulcer. Scand J Gastroenterol. 1983;18:945–50. [DOI] [PubMed]
- 10.Levenstein S, Prantera C, Varvo V, et al. Patterns of biologic and psychologic risk factors in duodenal ulcer patients. J Clin Gastroenterol. 1995;21:110–7. [DOI] [PubMed]
- 11.Stanchev I, Dinkov I, Minchev M. Age related aspects of gastric secretion in duodenal ulcer during the exacerbation phase. Vutr Bloes. 1979;18:41–5. [PubMed]
- 12.Ohmann C, Imhof M, Ruppert C, et al. Time trends in the epidemiology of peptic ulcer bleeding. Scand J Gastroenterol. 2005;40:914–20. [DOI] [PubMed]
- 13.Tenias Burillo JM, Llorente Melero MJ, Zaragoza Marcet A. Epidemiologic aspects on non-variceal upper gastrointestinal bleeding in a Mediterranean region: incidence and socio-geographic and temporal fluctuations. Rev Esp Enferm Dig. 2001;93:96–105. [PubMed]
- 14.Svanes C, Sothern RB, Sorbye H. Rhythmic patterns in incidence of peptic ulcer perforation over 5.5 decades in Norway. Chronobiol Int. 1998;15:241–64. [DOI] [PubMed]
- 15.Weil J, Langman MJ, Wainwright P, Lawson DH, et al. Peptic ulcer bleeding: accessory risk factors and interactions with non-steroidal anti-inflammatory drugs. Gut. 2000;46:27–31. [DOI] [PMC free article] [PubMed]
- 16.Cheng HC, Chuang SA, Kao AW, et al. Increased risk of rebleeding of peptic ulcer bleeding in patients with comorbid illness receiving omeprazole infusion. Hepatogastroenterol. 2003;50:2270–3. [PubMed]
- 17.Greene WH. Econometric Analysis. New Jersey: Pearson Education, Inc; 2003.
- 18.Tsai CJ, Lin CY. Seasonal changes in symptomatic duodenal ulcer activity in Taiwan: a comparison between subjects with and without hemorrhage. J Intern Med. 1998;244:405–10. [DOI] [PubMed]
- 19.Malinovskaya NK, Komarov FI, Rapoport SI, et al. Melatonin production in patients with duodenal ulcer. Neuro Endocrinol Lett. 2001;22:109–17. [PubMed]
- 20.Hawley DJ, Wolfe F, Lue FA. Seasonal symptom severity in patients with rheumatic diseases: a study of 1,424 patients. J Rheumatol. 2001;28:1900–9. [PubMed]
- 21.Rokkas T, Mavrogeorgis A, Liatsos C, Rallis E, Kalogeropoulos N. Optimal dose of omeprazole in combination with amoxicillin in eradicating H. pylori and preventing relapses in duodenal ulcer patients. Hepatogastroenterol. 1995;42:842–6. [PubMed]
- 22.Labenz J, Borsch G. Highly significant clinical course of relapsing and complicated peptic ulcer disease after cure of H pylori infection. Am J Gastroenterol. 1994;89:1785–8. [PubMed]


