Abstract
Transforming growth factor β (TGF-β) is a well characterized cytokine that appears to play a major role in directing the cellular response to injury, driving fibrogenesis, and, thus, potentially underlying the progression of chronic injury to fibrosis. In this study, we report the use of a novel TGF-β receptor antagonist to block fibrogenesis induced by ligation of the common bile duct in rats. The antagonist consisted of a chimeric IgG containing the extracellular portion of the TGF-β type II receptor. This “soluble receptor” was infused at the time of injury; in some experiments it was given at 4 days after injury, as a test of its ability to reverse fibrogenesis. The latter was assessed by expression of collagen, both as the mRNA in stellate cells isolated from control or injured liver and also by quantitative histochemistry of tissue sections. When the soluble receptor was administered at the time of injury, collagen I mRNA in stellate cells from the injured liver was 26% of that from animals receiving control IgG (P < 0.0002); when soluble receptor was given after injury induction, collagen I expression was 35% of that in control stellate cells (P < 0.0001). By quantitative histochemistry, hepatic fibrosis in treated animals was 55% of that in controls. We conclude that soluble TGF-β receptor is an effective inhibitor of experimental fibrogenesis in vivo and merits clinical evaluation as a novel agent for controlling hepatic fibrosis in chronic liver injury.
Transforming growth factor β (TGF-β), a protean regulator of cell growth and differentiation, is central to the injury response. Secreted as a latent precursor, the molecule is activated at sites of injury by mechanisms that have yet to be delineated clearly but may include extremes of pH or proteolysis by plasmin (1, 2). Active TGF-β binds to specific, high-affinity receptors present on most cells, initiating a signaling cascade that results in biological effects, including production of cytokines and inflammatory mediators, stimulation of extracellular matrix (ECM) synthesis, and inhibition of ECM degradation (3–9).
Two signaling receptors, termed type I and type II, mediate the biologic actions of TGF-β. The extracellular domain of the type II receptor (10) binds the ligand, causing formation of heteromeric complexes incorporating type I and type II receptors (11). The type II receptor then transphosphorylates the type I receptor, activating its kinase and initiating downstream signaling (11). Thus, the type II receptor appears to be essential for the biological activity of TGF-β in vivo (11–18).
In a number of epithelia, repeated or prolonged injury leads to progressive fibrosis and, ultimately, the development of excessive, unwanted scarring. The late stage of this process in the liver is termed cirrhosis. TGF-β appears to have a major regulatory role in this process, as shown both in animal models (19–24) and human hepatic injury (25–27). Similarly, transgenic mice overexpressing TGF-β1 and adenovirus-mediated gene transfer of TGF-β1 are characterized by fibrosis in many organs including the liver (28, 29). These studies suggest that inhibition of TGF-β signaling may be a useful therapeutic strategy for the treatment of hepatic fibrogenesis.
In the present study we examine the role of TGF-β inhibition on hepatic fibrogenesis induced by bile duct ligation in the rat, using a soluble receptor against the extracellular domain of the TGF-β type II receptor. The results indicate that this novel reagent inhibits stellate cell activation and hepatic fibrogenesis.
Materials and Methods
Materials.
Pronase and DNase were purchased from Boehringer Mannheim. Collagenase was from Serva. Ham’s F-12 medium, medium 199, DMEM, and fetal calf and donor horse sera were from Flow Laboratories. Eagle’s minimal essential medium without calcium was prepared by using amino acids purchased from Sigma. Accudenz was obtained from Accurate Chemicals; collagen I from rat tail tendon was prepared in the laboratory.
TRI reagent was from Molecular Research Center (Cincinnati). Acrylamide, bis-acrylamide, and agarose were from Bio-Rad. Ultrapure urea, agarose, trypsin-EDTA, and RNaseT2 were from GIBCO/BRL. T7 RNA polymerase, RQ1 DNase, and RNasin were from Promega. [α-32P]CTP (>800 Ci/mmol) was from Amersham-Pharmacia. Mink lung epithelial cells (CCL64) were obtained from the University of California at San Francisco Cell Culture Facility.
A mAb to smooth muscle α-actin (clone 1A4) was from Sigma. Biotinylated sheep anti-mouse IgG and the enhanced chemiluminescence Western blotting kit were from Amersham-Pharmacia. Anti-rat PI 3-kinase was from Upstate Biotechnology (Lake Placid, NY). Avidin-biotin complex (Vectastatin) was from Vector Laboratories. Purified TGF-β1 was purchased from R & D Systems. A 5-Bromo-2′-deoxyuridine labeling and detection kit II was from Boehringer Mannheim. Other chemicals including Direct Red 80 were from Sigma.
Soluble TGF-β Type II Receptor (sTGF-βR).
The TGF-β receptor fusion protein was synthesized by using the extracellular domain of the rabbit TGF-β type II receptor (amino acids 1–160), amplified by PCR from plasmid 3F11 (30). The products were ligated to a DNA fragment encoding the Fc domain of human IgG1 in the Biogen transient expression vector SAB132 and then cloned into a plasmid (pMSN001); DNA sequencing revealed a glycine-to-arginine mutation in the fourth amino acid of the signal peptide. This plasmid was used to stably transfect Chinese hamster ovary cells. Fusion protein secreted by the cells was purified on a protein A-Sepharose affinity column (31). The extracellular domain of the rabbit TGF-β type II receptor is 83% homologous to that of the rat.
The activity of sTGF-βR on the TGF-β receptor was tested by using mink lung epithelial cells, which are growth-inhibited by TGF-β (32). Mink lung cells (CCL64) were maintained in DMEM/10% FCS in 75-cm2 flasks. For the bioassay, subconfluent cultures between passages 3–10 were used. Cells were washed and trypsinized and seeded at a density of 1 × 104 cells per well in 96-well microtiter plates; the medium was DMEM/0.2% FCS/10 mM Hepes, pH 7.4, and the volume was 190 μl/well. A standard curve was constructed by using recombinant TGF-β1 (0–250 pg/ml). Serial dilutions of sTGF-βR (0–6,400 ng/ml) were incubated with a known amount of TGF-β1 (250 pg/ml) and added to the wells. After incubation at 37°C for 24 h, [3H]thymidine (0.1 μCi) was added for 4 hr. The cells then were lysed at −70°C, and the incorporated radioactivity was trapped on glass fiber filters by using a cell harvester (Cambridge Technology, Cambridge, MA). The radioactivity was measured in a liquid scintillation counter. Each assay was carried out in triplicate.
Animal Model of Liver Injury.
Injury was induced in male Sprague–Dawley rats (≈400 g body weight) by laparotomy and high ligation of the bile duct (33, 34). Histologically, the injury causes cholestasis, periductular inflammation, and fibrosis. Sham-operated animals were handled similarly but with bile duct manipulation only. At the time of laparotomy, some animals received sTGF-βR or normal human IgG by slow infusion into a femoral vein. The dose of sTGF-βR was established in pilot studies with 2.5, 5, 7.5, and 10 mg/kg of the chimeric IgG. A dose of 5 mg/kg markedly reduced collagen 1 expression in stellate cells and was more effective than 2.5 mg/kg, whereas doses >5 mg/kg had no additional effect. Thus, 5 mg/kg was used in the studies described. Animals receiving either sTGF-βR or control IgG were sacrificed 4 days later for cell isolation, and liver tissue was harvested for immunohistochemistry. In other experiments, animals that underwent bile duct ligation or sham operation were infused with the sTGF-βR (5 mg/kg) or control peptide on day 4. These animals were sacrificed 4 days later (day 8) for cell isolation.
Stellate Cell Isolation and Culture.
Hepatic stellate cells were isolated by in situ perfusion with pronase and collagenase and fractionation on a discontinuous gradient of Accudenz (15.6% and 8.2%) (35). Stellate cells were harvested from the media/8.2% interface, washed, and either plated on plastic tissue culture plates or harvested in Tri reagent (RNase protection assay) or in PBS (immunoblotting). Purity was assessed by microscopy by using as a marker the intrinsic fluorescence that is unique to this cell type (36); routinely, it was >98%. The culture medium was a modification of medium 199 (37) with 20% serum (10% calf/10% horse), insulin (4 milliunits/ml), and gentamicin (40 μg/ml).
Quantitation of mRNA.
Total RNA was isolated from purified stellate cells by using TRI reagent; all samples were examined by agarose/formaldehyde gel electrophoresis to verify integrity of the RNA. The radiolabeled probe for collagen I was prepared as described (38), and mRNA was quantified by RNase protection. The collagen a1(I) probe (33) protected a 350-bp fragment. Samples were assayed also for S-14 mRNA, which encodes a ribosomal protein (39). S-14 exhibits minimal variation after bile duct ligation (38) and was used to control for mRNA loading.
Probes were hybridized with either 10 μg [collagen a1(I)] or 5 μg (S14) of total RNA; the hybridization temperatures were 70°C and 55°C, respectively, for 16–18 hr. Unhybridized RNA was digested with ribonuclease T2 (33). The RNA–cRNA hybrids were extracted in phenol-chloroform, precipitated with ammonium acetate and ethanol, and then dissociated by boiling for 3 min in electrophoresis buffer containing 80% formamide. Samples were run on a 5% polyacrylamide/urea gel, dried, and autoradiographed (Kodak X-Omat AR-5). Specific bands were quantitated by scanning densitometry (Hoefer).
Quantification of α-Smooth Muscle Actin Expression by Stellate Cells.
Freshly isolated or cultured stellate cells were harvested in buffer (62.5 mM Tris⋅HCl, pH 6.8, containing 1% SDS, 10% glycerol, and 20 mM DTT), disrupted by sonication, and assayed for protein concentration. Extracts were boiled for 3 min, separated by 8% SDS/PAGE, and transferred to nitrocellulose. Nonspecific antibody binding was blocked by preincubation of the transfer in 0.5× PBS containing 0.15 M ammonium acetate, 5% skim milk, and 2% sheep serum. Blots then were incubated overnight in primary antibody (catalog no. A2547; Sigma) diluted 1:1,000 in PBS with 0.015 M ammonium acetate/0.1% skim milk. After washing, they were incubated for 2 hr with biotinylated sheep anti-mouse antibody. Bands were visualized by using either the Vectastatin ABC kit or the enhanced chemiluminescence kit per the manufacturer’s instructions.
Immunohistochemical Detection and Quantitation of Collagen in Liver Tissues.
Whole liver tissue was perfused under low pressure via the portal vein and then excised. The liver was cut into small pieces, fixed in 4% paraformaldehyde in PBS, and embedded in paraffin; 10-μm sections were mounted on glass slides. Sections were deparaffinized and the slides then were incubated for 30 min in a solution of saturated picric acid containing 0.1% direct red 80 and 0.1% Fast Green FCF (40). Stained slides were washed in running distilled water, dehydrated, mounted, and examined by light microscopy.
For quantitating collagen deposition, fibrils were counted manually by light microscopy. Fields were centered at ×20 magnification, with the central vein in the middle. Portal tracts were excluded because of the proliferation of bile ducts in portal areas; the ducts have ECM that is variable and independent of the injury stimulus. For similar reasons, the morphometric method was used in preference to the dye-elution method of Lopez-De Leon and Rojkind (40). For each animal, eight sections were examined in blinded fashion and collagen deposition in 10 microscopic fields was counted.
Immunocytochemical Detection of Stellate Cell Proliferation.
Proliferation of freshly isolated stellate cells from bile duct-ligated animals treated with either the sTGF-βR or control IgG was determined by using 5-bromo-2-deoxyuridine labeling as recommended by the manufacturer (Boehringer Mannheim).
Statistical Analysis.
Data are presented as mean ± SEM of at least three independent experiments. Differences between groups were analyzed by using the unpaired Student’s t test (two groups) or the one-factor ANOVA (multiple comparisons). The significance level for all tests was P < 0.05.
Results
Efficacy of sTGF-βR in Blocking the Actions of TGF-β.
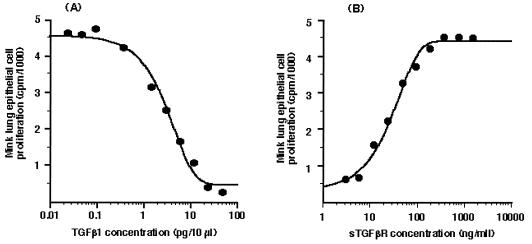
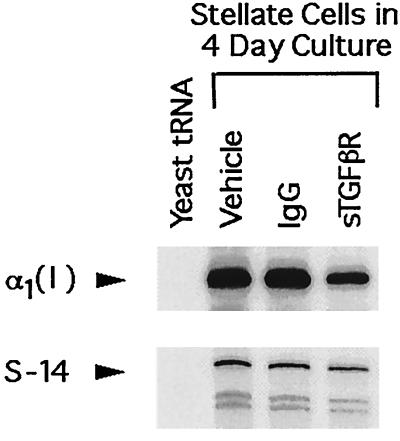
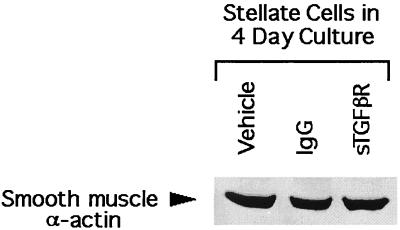
The efficacy of sTGF-βR was determined by using the mink lung epithelial cell assay as described earlier. Exogenous TGF-β1 produced a dose-dependent inhibition of mink lung cell proliferation (Fig. 1A). Addition of 400 ng/ml sTGF-βR completely abrogated the inhibitory effect of 250 pg/ml TGF-β1 (Fig. 1B). To confirm that sTGF-βR was effective also on native stellate cells, expression of type I collagen was examined in stellate cells isolated from normal rats and cultured in 20% serum-containing medium. The source of TGF-β in this study is both that present in serum and that produced by stellate cells in culture (32). The spontaneous, high-level expression of collagen I mRNA that occurs in culture (41) was decreased 35–40% in the presence of sTGF-βR (Fig. 2). Interestingly, however, another marker of stellate activation, smooth muscle α-actin, was unaffected by sTGF-βR (Fig. 3).
Figure 1.
Efficacy of sTGF-βR in blocking the actions of TGF-β. (A) Exogenous TGF-β produces a dose-dependent blockade of mink lung epithelial cell proliferation. (B) Dose-dependent inhibition of TGF-β (250 pg/ml) by soluble TGF-β receptor, measured as release of cells from the inhibitory effect of the cytokine. Serial dilutions of the sTGF-βR were incubated with a known amount of TGF-β1 (250 pg/ml) and added to the wells. Thymidine incorporation was determined over 4 hr.
Figure 2.
Representative RNase protection assay of collagen I and S-14 mRNA in stellate cells isolated from normal rats and placed in culture for 4 days in 20% serum-containing medium with the indicated additives. RNase protection was performed by using 10 μg of total RNA for collagen I and 5 μg for S-14. Yeast tRNA served as a negative control.
Figure 3.
Representative immunoblot of smooth muscle α-actin extracted from stellate cells. The cells were isolated from normal rats and placed in culture for 4 days in 20% serum-containing medium with the indicated additives. Smooth muscle α-actin expression was detected by using the mAb clone IA4 (Sigma).
sTGF-βR Infusion Reduces Collagen Expression in Vivo.
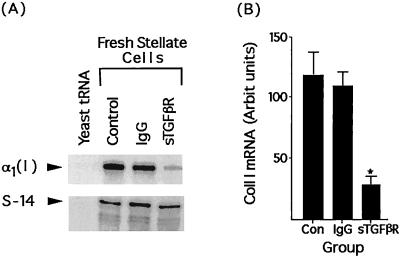
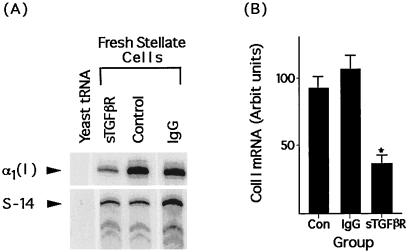
To determine the efficacy of sTGF-βR in inhibiting collagen gene expression in vivo, the sTGF-βR was infused at the time of bile duct ligation. Stellate cells were isolated 4 days later, and collagen I expression was determined by RNase protection. Collagen 1 mRNA expression in animals receiving the soluble TGF-β receptor was 26% of that in animals receiving control IgG (Fig. 4A) (P < 0.0002). There was no difference in collagen expression between animals receiving control IgG or vehicle (Fig. 4B).
Figure 4.
Soluble TGF-β receptors inhibit stellate cell collagen I mRNA expression in vivo. (A) Representative RNase protection assay of collagen I and S-14 mRNA in stellate cells isolated from rats 4 days after bile duct ligation. At the time of surgery, animals were infused with either the soluble TGF-β receptor (sTGF-βR, 5 mg/kg), control IgG (IgG), or vehicle (Con). RNA loading was as for Fig. 2. (B) Relative abundance of collagen I mRNA in stellate cells from animals treated similarly. Values represent the mean ± SEM of at least four separate experiments. The signal for collagen I has been corrected for the level of S-14 mRNA in the samples. ∗, P < 0.0002 vs. Con or IgG-treated rats. There was no difference in collagen I expression between control and IgG-treated animals.
We next examined the efficacy of the sTGF-βR administered to animals during active fibrogenesis as the scenario in which an antifibrotic agent most likely would be used in humans. Rats were subjected to bile duct ligation on day 0 and infused with sTGF-βR on day 4. Stellate cells were isolated on day 8, and collagen I mRNA expression was determined by RNase protection. As shown (Fig. 5), sTGF-βR treatment reduced collagen I mRNA by 64% (Fig. 5B, P < 0.05). The control IgG by itself had no effect on collagen I mRNA expression, relative to animals given vehicle only.
Figure 5.
Soluble TGF-β receptor reduces stellate cell collagen I mRNA expression when administered during progression of fibrosis. (A) Representative RNase protection assay of collagen I and S-14 mRNA in stellate cells isolated from rat bile duct ligated for 8 days. Animals were infused with either the sTGF-βR (5 mg/kg), control IgG (IgG), or vehicle (Con). RNA loading was as for Fig. 2. (B) Relative abundance of collagen I mRNA in stellate cells from animals treated similarly. Values represent the mean ± SEM of at least four separate experiments. The signal for collagen I has been corrected for the level of S-14 mRNA in the samples. ∗, P < 0.0001 vs. Con or IgG-treated rats. There was no difference in collagen I expression between control and IgG-treated animals.
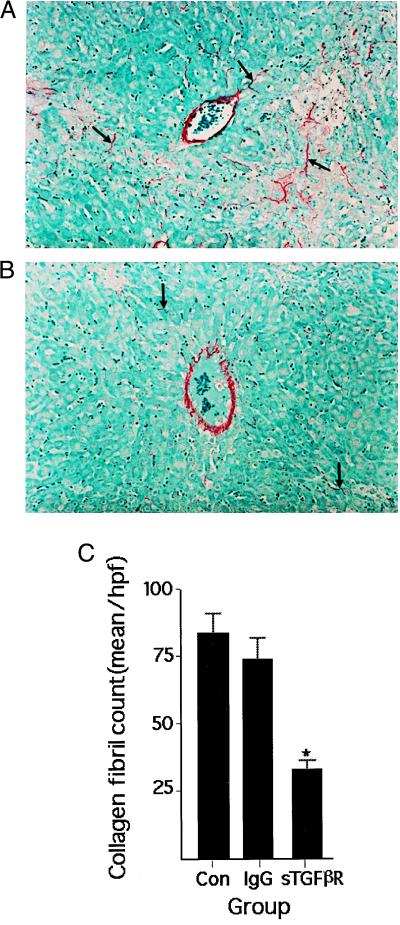
Collagen protein deposition after bile duct ligation was assessed by histochemistry using sirius red dye, which stains collagen specifically (40). The results mirrored the mRNA data, showing that treatment with sTGF-βR reduced significantly the number of collagen fibrils relative to controls receiving unmodified IgG or vehicle (Fig. 6).
Figure 6.
Collagen in histological sections of rat liver stained with sirius red. Representative photomicrographs of liver obtained from animals treated with either control IgG (IgG) (A) or sTGF-βR (B). Rats underwent bile duct ligation on day 0 and were infused with the sTGF-βR 4 days later. Liver was harvested on day 8. In sTGF-βR-treated animals, lobular collagen is reduced. (C) Quantitation of collagen deposition in similarly treated rats. Collagen fibrils were counted in 10 high-power fields per section. Values represent the mean ± SEM of at least four separate experiments. ∗, P < 0.0002 vs. Con or IgG-treated rats. There was no difference in collagen I deposition between control and IgG-treated animals.
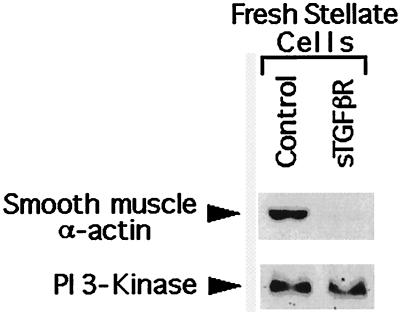
Infusion of sTGF-βR Inhibits Stellate Cell Activation.
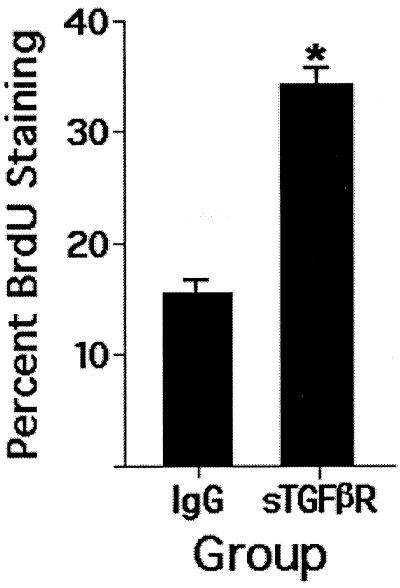
In vivo, stellate cell activation involves a multifaceted change in the cell phenotype: increased expression of collagen I is prominent but is only one feature. Other markers include de novo expression of α-smooth muscle actin and increased proliferation, both appearing early in the injury response. Using these markers, we sought to determine whether the effect of sTGF-βR was relatively selective or involved activation broadly. As shown in Fig. 7, treatment of rats with a single dose of the sTGF-βR was associated with markedly reduced expression of α-smooth muscle actin expression 4 days later. To examine stellate cell proliferation in vivo, we infused rats with either the soluble receptor or control IgG on day 1 after induction of injury; stellate cells were isolated on day 4 and placed in culture overnight for labeling with BrdUrd (1:1,000 dilution of BrdUrd reagent in 10% serum-containing medium; 4 hr incubation). The assumption is that the proliferative activity of the cells in vivo carries over to early primary culture. This labeling procedure was chosen so that the proliferating cells could be identified unequivocally. The difference in proliferation between the group treated with sTGF-βR and that receiving control IgG was highly significant (Fig. 8; P = 0.0002).
Figure 7.
Representative immunoblot for smooth muscle α-actin in fresh stellate cells isolated from 4-day bile duct-ligated rats. Animals were infused at the time of surgery with either the sTGF-βR or control peptide (IgG). The antibody used was as in Fig. 3. Rat PI 3-kinase served as an internal control for protein loading.
Figure 8.
Stellate cell proliferation in response to sTGF-βR infusion in vivo as determined by BrdUrd labeling. Rats underwent bile duct ligation and infusion of sTGF-βR on day 0; stellate cells were isolated 4 days later. BrdUrd labeling was performed soon after cell isolation as described in Materials and Methods. The percentage of stained cells was determined by counting 10 randomly selected fields in each plate. P = 0.0002 (n = 4).
Discussion
Fibrosis is closely associated with recurring or unremitting injury, often leading to clinically apparent malfunction or failure of internal epithelial tissues. An example is chronic hepatitis C, which affects an estimated 4 million Americans (42). The virus itself causes relatively low-grade inflammation, little cellular necrosis, and limited repair activity. However, the damage is persistent and, over time, results in pathological scarring and, eventually, in cirrhosis. Portal hypertension ensues with its secondary manifestations of gastrointestinal bleeding, fluid retention, and encephalopathy.
Substantial evidence now points to TGF-β as an important, if not the preeminent, driver of the fibrogenic response to injury (19, 20, 24, 25, 43–46). The initial work demonstrated direct effects of this factor on ECM production by cells in culture (6). Border et al. (47, 48) showed that TGF-β-sequestering reagents prevent experimental renal fibrosis. As details of TGF-β activation, receptor binding, and intracellular signaling have come to light (49), manipulation of these pathways has provided evidence for the fibrogenic activity of TGF-β in vivo. Munger et al. (50) have shown that activation of latent TGF-β in the lung airway epithelium requires formation of a complex of the TGF-β receptor and αvβ6 integrin. Mice lacking this integrin respond to injury with reduced bronchiolar fibrosis even though inflammation is comparable to that in wild-type mice (50). Although β6 appears to be absent from liver (51), the findings illustrate the critical fibrogenic role of TGF-β in epithelial fibrosis. Also, Qi and colleagues have looked at the effects of delivering a dominant-negative TGF-β type II receptor to the liver by using an adenoviral vector (52), showing that fibrosis induced by dimethylnitrosamine is attenuated in the transfected liver. In short, although multiple cytokines unquestionably help orchestrate the repair response, TGF-β appears to have a decisive role.
These and other studies create a strong rationale for an antifibrotic strategy in which the principal objective is blocking TGF-β. The present work evaluates a “soluble receptor” antagonist of the type II receptor for this cytokine. The antagonist consists of the extracellular portion of the receptor spliced into mouse IgG, forming a chimeric molecule that presumably competes for the ligand at the level of the endogenous receptor. The results indicate that one dose of the soluble receptor blocks fibrogenesis for at least 4 days, whether given at the time of injury or during progression of fibrosis, as would be the case typically in the treatment of human liver disease. Collagen I was selected as the marker of fibrogenesis because it is the principal component of a fibrotic scar (53). The reduction in collagen I mRNA correlated well with diminished lobular fibrosis as viewed histologically. It should be noted that bile duct ligation induces not only lobular fibrosis but also marked proliferation of biliary ductules in portal areas and, with this, a substantial increase in periductular ECM, which may derive from biliary epithelial cells. The regulation of ECM production in this location is unknown; by histological evaluation, it was unaffected by sTGF-βR (data not shown).
In animals given sTGF-βR, we found also reduced expression of smooth-muscle α-actin in stellate cells, although this was not observed in cultured stellate cells incubated with the soluble receptor. The in vivo–in culture discrepancy does not have an obvious explanation but adds to previous data (54) indicating that the changes seen in stellate cells during adaptation to culture do not necessarily mimic all features of activation in vivo, as sometimes has been asserted. The down-regulation of smooth muscle α-actin in vivo suggests that the effect of the soluble receptor is broad, involving activation, and includes a decrease in collagen I. This fits well with earlier findings, which showed that stellate activation was directed in part by the EIIIA isoform of fibronectin, which appears very early in the injury response and derives from sinusoidal endothelial cells. Endothelial cells are directly adjacent to stellate cells and, thus, in a position to modify their pericellular matrix (38). TGF-β regulates the production of EIIIA fibronectin by the sinusoidal endothelium and, through this action, may help initiate the repair sequence (38). Other data indicate that TGF-β also may directly activate stellate cells (55), which have been shown to display the appropriate receptors (56). Thus, the demonstrated efficacy of the antagonist may be related to its blocking both direct and indirect effects of TGF-β.
A similar approach has been used to block tumor necrosis factor α (TNF-α) and has undergone successful clinical testing in chronic inflammatory diseases such as rheumatoid arthritis (57). As IgGs, these inhibitors have a half-life of several days in the circulation and, in humanized form, are unlikely to elicit an immune response. Thus, they offer significant advantages over DNA-based strategies requiring adenoviral vectors. Expression of genes transfected with adenovirus to the liver typically is short-lived. Moreover, readministration may be ineffective because of a vigorous immune response to the vector (58). Although immunosuppression may be used to blunt the immune response, these drugs are toxic and may exacerbate diseases such as hepatitis C (59).
TGF-β is expressed by a variety of normal cell types and tissues, suggesting a role in normal homeostasis. In the normal liver, the mRNA and protein for TGF-β1 are present at relatively high levels in sinusoidal endothelial cells, Kupffer cells, and biliary epithelial cells, and in injury this expression is unchanged. In stellate cells, the mRNA is low but increases strikingly with fibrogenic injury induced by bile duct ligation (32). The change clearly is timed to conversion of these cells to an activated, fibrogenic form, suggesting autocrine stimulation (32). The current view is that the type II receptor transmits the signal from all isoforms of TGF-β (11–13), and, thus, its blockade could have multiple effects. In this regard, the findings in mice with complete deletion of the individual isoforms may be noted. In the absence of TGF-β1, mice exhibit progressive infiltration of the liver and other tissues by mononuclear cells; they have no additional cellular or structural hepatic abnormality, although detection of lesions may be precluded by the shortened survival of these animals (60). Deletion of TGF-β2 or TGF-β3 leads to developmental abnormalities involving bone and neuroectodermal structures (61, 62). The liver appears not to be involved, although, again, early mortality may not allow time for the expression of relatively subtle abnormalities. The lack of obvious effects on the liver notwithstanding, these data raise concerns about side-effects that would need to be addressed before clinical use of a type II receptor antagonist could be envisioned.
TGF-β is antiproliferative as well as profibrogenic, with effects not only on the immune system, as revealed by the TGF-β1 knockout, but also on epithelial cells including hepatocytes (63). Recent work from this laboratory has shown that expression of the type II receptor on stellate cells decreases during the injury response (56), correlating with increased proliferation. Interestingly, the fibrogenic effects of TGF-β are unaffected by this change in the receptor. The present work shows that blockade of the type II receptor to a degree that reduces fibrogenesis is associated with an additional increase in proliferation of stellate cells. The question of whether this is deleterious over the long term in the context of an ongoing inflammatory stimulus (such as hepatitis) will require further study.
A related question involving the role of TGF-β in growth control concerns the finding that some epithelial cancers are resistant to TGF-β; the mechanisms vary from a mutated receptor to alteration of TGF-β-signaling pathways (64). This points to the need for extended observation of animals receiving the soluble receptor, with attention particularly to evidence for dysplastic growth. It is possible that cytokines involved in the maintenance of homeostasis are tightly confined, largely autocrine, and, therefore, inaccessible to exogenous inhibitors. In injury, on the other hand, production of TGF-β increases locally (32) as does expression of receptors related to TGF-β activation (50). These changes may reflect, and contribute to, a disruption in homeostasis that allows large, circulating molecules including IgG access to the cell surface. If this is the case, the effects of the soluble TGF-β antagonist may be relatively restricted to the site of injury, mitigating concerns about altered growth regulation in normal tissues. It should be noted that the inhibitory effect of TGF-β is incomplete: the present data indicate that 50–70% suppression of fibrogenesis was about the maximum achievable. At this level, there was no grossly detectable failure of repair of the surgical wound created to ligate the bile duct. Finally, the results with the TNF-α-soluble receptor provide some reassurance. Although TNF-α, like TGF-β, has a well defined role in growth regulation (65), abnormal cell proliferation has not been observed to date in experimental animals or in patients receiving this reagent (57).
In conclusion, the use of sTGF-βR during hepatic injury induced by bile duct ligation is very effective in blocking fibrogenesis and otherwise has characteristics that make it attractive as a therapeutic agent. Long-term studies in animal models are the next step to establish its safety and clinical potential.
Acknowledgments
J.G. was an Australian National Health and Medical Research Council Neil Hamilton Fairley Fellow; he also received a postdoctoral fellowship from the American Liver Foundation. The work was supported by Grants DK31198 and DK26743 from the U.S. National Institutes of Health and by RO3 TW00717 from the Fogarty International Center, National Institutes of Health. The authors also gratefully acknowledge the assistance of the Culture and Microscopy Core Facilities of the University of California at San Francisco Liver Core Center.
Abbreviations
- TGF-β
transforming growth factor β (refers to the TGF-β1 isoform unless otherwise specified)
- ECM
extracellular matrix
- sTGF-βR
soluble type II TGF-β receptor
- TNF-α
tumor necrosis factor α
References
- 1.Lyons R M, Keski-Oja J, Moses H L. J Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flaumenhaft R, Abe M, Mignatti P, Rifkin D B. J Cell Biol. 1992;118:901–909. doi: 10.1083/jcb.118.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kehrl J H, Wakefield L M, Roberts A B, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn M B, Fauci A S. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsunawaki S, Sporn M, Ding A, Nathan C. Nature (London) 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 5.Roberts A B, Sporn M B, Assoian R K, Smith J M, Roche N S, Wakefield L M, Heine U I, Liotta L A, Falanga V, Kehrl J H, et al. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ignotz R A, Massague J. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- 7.Balza E, Borsi L, Allemanni G, Zardi L. FEBS Lett. 1988;228:42–44. doi: 10.1016/0014-5793(88)80580-5. [DOI] [PubMed] [Google Scholar]
- 8.Edwards D R, Murphy G, Reynolds J J, Whitham S E, Docherty A J, Angel P, Heath J K. EMBO J. 1987;6:1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laiho M, Saksela O, Keski-Oja J. J Biol Chem. 1987;262:17467–17474. [PubMed] [Google Scholar]
- 10.Lin H Y, Moustakas A, Knaus P, Wells R G, Henis Y I, Lodish H F. J Biol Chem. 1995;270:2747–2754. doi: 10.1074/jbc.270.6.2747. [DOI] [PubMed] [Google Scholar]
- 11.Wrana J L, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang X F, Massague J. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki M, Moustakas A, Lin H Y, Lodish H F, Carr B I. Proc Natl Acad Sci USA. 1993;90:5359–5363. doi: 10.1073/pnas.90.11.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhushan A, Lin H Y, Lodish H F, Kintner C R. Mol Cell Biol. 1994;14:4280–4285. doi: 10.1128/mcb.14.6.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ten Dijke P, Yamashita H, Ichijo H, Franzen P, Laiho M, Miyazono K, Heldin C H. Science. 1994;264:101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]
- 15.Bassing C H, Yingling J M, Howe D J, Wang T, He W W, Gustafson M L, Shah P, Donahoe P K, Wang X F. Science. 1994;263:87–89. doi: 10.1126/science.8272871. [DOI] [PubMed] [Google Scholar]
- 16.Franzen P, ten Dijke P, Ichijo H, Yamashita H, Schulz P, Heldin C H, Miyazono K. Cell. 1993;75:681–692. doi: 10.1016/0092-8674(93)90489-d. [DOI] [PubMed] [Google Scholar]
- 17.Attisano L, Carcamo J, Ventura F, Weis F M, Massague J, Wrana J L. Cell. 1993;75:671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- 18.Carcamo J, Weis F M, Ventura F, Wieser R, Wrana J L, Attisano L, Massague J. Mol Cell Biol. 1994;14:3810–3821. doi: 10.1128/mcb.14.6.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czaja M J, Weiner F R, Flanders K C, Giambrone M A, Wind R, Biempica L, Zern M A. J Cell Biol. 1989;108:2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuoka M, Tsukamoto H. Hepatology. 1990;11:599–605. doi: 10.1002/hep.1840110412. [DOI] [PubMed] [Google Scholar]
- 21.Nakatsukasa H, Nagy P, Evarts R P, Hsia C C, Marsden E, Thorgeirsson S S. J Clin Invest. 1990;85:1833–1843. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieder H, Armbrust T, Meyer zum Buschenfelde K H, Ramadori G. Hepatology. 1993;18:937–944. doi: 10.1002/hep.1840180427. [DOI] [PubMed] [Google Scholar]
- 23.Friedman S L, Yamasaki G, Wong L. J Biol Chem. 1994;269:10551–10558. [PubMed] [Google Scholar]
- 24.Knittel T, Janneck T, Muller L, Fellmer P, Ramadori G. Hepatology. 1996;24:352–360. doi: 10.1053/jhep.1996.v24.pm0008690404. [DOI] [PubMed] [Google Scholar]
- 25.Castilla A, Prieto J, Fausto N. New Engl J Med. 1991;324:933–940. doi: 10.1056/NEJM199104043241401. [DOI] [PubMed] [Google Scholar]
- 26.Casini A, Pinzani M, Milani S, Grappone C, Galli G, Jezequel A M, Schuppan D, Rotella C M, Surrenti C. Gastroenterology. 1993;105:245–253. doi: 10.1016/0016-5085(93)90033-9. [DOI] [PubMed] [Google Scholar]
- 27.Bedossa P, Peltier E, Terris B, Franco D, Poynard T. Hepatology. 1995;21:760–766. [PubMed] [Google Scholar]
- 28.Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts A B, Sporn M B, Thorgeirsson S S. Proc Natl Acad Sci USA. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clouthier D E, Comerford S A, Hammer R E. J Clin Invest. 1997;100:2697–2713. doi: 10.1172/JCI119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.di Clemente N, Wilson C, Faure E, Boussin L, Carmillo P, Tizard R, Picard J Y, Vigier B, Josso N, Cate R. Mol Endocrinol. 1994;8:1006–1020. doi: 10.1210/mend.8.8.7997230. [DOI] [PubMed] [Google Scholar]
- 31.Sanicola M, Hession C, Worley D, Carmillo P, Ehrenfels C, Walus L, Robinson S, Jaworski G, Wei H, Tizard R, et al. Proc Natl Acad Sci USA. 1997;94:6238–6243. doi: 10.1073/pnas.94.12.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bissell D M, Wang S S, Jarnagin W R, Roll F J. J Clin Invest. 1995;96:447–455. doi: 10.1172/JCI118055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maher J J, McGuire R F. J Clin Invest. 1990;86:1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bienkowski R S, Cowan M J, McDonald J A, Crystal R G. J Biol Chem. 1978;253:4356–4363. [PubMed] [Google Scholar]
- 35.Friedman S L, Roll F J. Anal Biochem. 1987;161:207–218. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- 36.Friedman S L. Prog Liver Dis. 1996;14:101–130. [PubMed] [Google Scholar]
- 37.Bissell D M, Guzelian P S. Ann N Y Acad Sci. 1980;349:85–98. doi: 10.1111/j.1749-6632.1980.tb29518.x. [DOI] [PubMed] [Google Scholar]
- 38.Jarnagin W R, Rockey D C, Koteliansky V E, Wang S S, Bissell D M. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhoads D D, Dixit A, Roufa D J. Mol Cell Biol. 1986;6:2774–2783. doi: 10.1128/mcb.6.8.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-De Leon A, Rojkind M. J Histochem Cytochem. 1985;33:737–743. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- 41.Friedman S L, Roll F J, Boyles J, Arenson D M, Bissell D M. J Biol Chem. 1989;264:10756–10762. [PubMed] [Google Scholar]
- 42.Alter M J. Hepatology. 1997;26:62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 43.Okuda S, Languino L R, Ruoslahti E, Border W A. J Clin Invest. 1990;86:453–462. doi: 10.1172/JCI114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broekelmann T J, Limper A H, Colby T V, McDonald J A. Proc Natl Acad Sci USA. 1991;88:6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah M, Foreman D M, Ferguson M W. Lancet. 1992;339:213–214. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- 46.Frank S, Madlener M, Werner S. J Biol Chem. 1996;271:10188–10193. doi: 10.1074/jbc.271.17.10188. [DOI] [PubMed] [Google Scholar]
- 47.Border W A, Okuda S, Languino L R, Sporn M B, Ruoslahti E. Nature (London) 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 48.Border W A, Noble N A, Yamamoto T, Harper J R, Yamaguchi Y, Pierschbacher M D, Ruoslahti E. Nature (London) 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 49.Kretzschmar M, Massague J. Curr Opin Genet Dev. 1998;8:103–111. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- 50.Munger J S, Huang X, Kawakatsu H, Griffiths M J, Dalton S L, Wu J, Pittet J F, Kaminski N, Garat C, Matthay M A, et al. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 51.Breuss J M, Gillett N, Lu L, Sheppard D, Pytela R. J Histochem Cytochem. 1993;41:1521–1527. doi: 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- 52.Qi Z, Atsuchi N, Ooshima A, Takeshita A, Ueno H. Proc Natl Acad Sci USA. 1999;96:2345–2349. doi: 10.1073/pnas.96.5.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rojkind M, Giambrone M A, Biempica L. Gastroenterology. 1979;76:710–719. [PubMed] [Google Scholar]
- 54.Racine-Samson L, Rockey D C, Bissell D M. J Biol Chem. 1997;272:30911–30917. doi: 10.1074/jbc.272.49.30911. [DOI] [PubMed] [Google Scholar]
- 55.Matsuoka M, Pham N T, Tsukamoto H. Liver. 1989;9:71–78. doi: 10.1111/j.1600-0676.1989.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 56.Roulot D, Sevcsik A M, Coste T, Strosberg A D, Marullo S. Hepatology. 1999;29:1730–1738. doi: 10.1002/hep.510290622. [DOI] [PubMed] [Google Scholar]
- 57.Weinblatt M E, Kremer J M, Bankhurst A D, Bulpitt K J, Fleischmann R M, Fox R I, Jackson C G, Lange M, Burge D J. New Engl J Med. 1999;340:253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 58.Kay M A, Liu D, Hoogerbrugge P M. Proc Natl Acad Sci USA. 1997;94:12744–12746. doi: 10.1073/pnas.94.24.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Martino V, Saurini F, Samuel D, Gigou M, Dussaix E, Reynes M, Bismuth H, Feray C. Hepatology. 1997;26:1343–1350. doi: 10.1053/jhep.1997.v26.pm0009362382. [DOI] [PubMed] [Google Scholar]
- 60.Shull M M, Ormsby I, Kier A B, Pawlowski S, Diebold R J, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Nature (London) 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanford L P, Ormsby I, Gittenberger-de Groot A C, Sariola H, Friedman R, Boivin G P, Cardell E L, Doetschman T. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaartinen V, Voncken J W, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 63.Russell W E, Coffey R J, Ouellette A J, Moses H L. Proc Natl Acad Sci USA. 1988;85:5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markowitz S D, Roberts A B. Cytokine Growth Factor Rev. 1996;7:93–102. doi: 10.1016/1359-6101(96)00001-9. [DOI] [PubMed] [Google Scholar]
- 65.Yamada Y, Kirillova I, Peschon J J, Fausto N. Proc Natl Acad Sci USA. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]