Abstract
We are interested in using recombinant adeno-associated viral vectors in the treatment of hemophilia A. Because of the size constraints of recombinant adeno-associated viral vectors, we delivered the heavy and light chains of the human factor 8 (hFVIII) cDNA independently by using two separate vectors. Recombinant AAV vectors were constructed that utilized the human elongation factor 1α promoter, a human growth factor polyadenylation signal, and the cDNA sequences encoding either the heavy or light chain of hFVIII. Portal vein injections of each vector alone, a combination of both vectors, or a hFIX control vector were performed in C57BL/6 mice. An ELISA specific for the light chain of hFVIII demonstrated very high levels (2–10 μg/ml) of protein expression in animals injected with the light chain vector alone or with both vectors. We utilized a chromogenic assay in combination with an antibody specific to hFVIII to determine the amount of biologically active hFVIII in mouse plasma. In animals injected with both the heavy and light chain vectors, greater than physiological levels (200–400 ng/ml) of biologically active hFVIII were produced. This suggests that coexpression of the heavy and light chains of hFVIII may be a feasible approach for treatment of hemophilia A.
Hemophilia A is an X-linked bleeding disorder caused by mutations in the gene for factor 8 (FVIII). Patients suffering from this disease experience spontaneous bleeding into the joints and soft tissue (1). The current treatment for this disease is protein replacement in response to bleeding episodes by using either plasma concentrates or recombinant FVIII protein (2). Prophylactic studies in Sweden have demonstrated that maintaining plasma FVIII levels above 1% of normal can dramatically improve the morbidity associated with the disease (3). However, widespread implementation of prophylactic regimens has not occurred in the United States because of the high cost of purified FVIII and the problems associated with repeated injections, including the high rate of catheter-related sepsis (4).
Somatic gene therapy offers an attractive alternative to the current treatment of hemophilia A. Ex vivo gene therapy initially was evaluated by using retroviral vectors for gene transfer of FVIII. Duration of expression after transplantation of transduced fibroblasts was short-lived, lasting approximately 1 week (5). In vivo gene therapy for delivery of FVIII by using adenoviral vectors has yielded more promising results. Initial studies using adenoviral vectors to deliver the FVIII cDNA by i.v. administration to mice resulted in the production of superphysiological plasma levels of FVIII within a week of injection. However, these levels were not sustained, because of toxicity associated with the vector (6). Improvements to the expression cassettes have allowed less toxic doses to be administered and have resulted in more sustained gene expression, although attenuation of expression is still observed over time (7, 8). In addition, phenotypic correction of the bleeding disorder in hemophilic mice and dogs has been demonstrated within the first week of vector administration (8, 9).
In contrast to retroviral and adenoviral vectors, recombinant adeno-associated viral (rAAV) vectors are extremely effective in providing sustained and stable gene expression, without evidence of toxicity. For example, this vector has been shown to produce therapeutic levels of FIX when delivered either intramuscularly or into the portal vasculature of mice and dogs (10–14). However, using rAAV to deliver the FVIII gene poses some challenges not encountered with the FIX gene, because of the large size of the FVIII cDNA and the size constraints of rAAV vectors (15). To overcome these problems, we considered delivering the FVIII cDNA by using two AAV vectors. The primary structure and processing of the protein have been elucidated in great detail. FVIII is synthesized as a single-chain polypeptide with a predicted molecular mass of 265 kDa and has a domain organization of A1-A2-B-A3-C1-C2 (16). Factor VIII is processed within the cell to yield a heterodimer comprised primarily of a heavy chain of 200 kDa containing the A1, A2, and B domains and an 80-kDa light chain containing the A3, C1, and C2 domains (17). Both the single-chain polypeptide and the heterodimer circulate in plasma as inactive precursors (18). Activation of FVIII in plasma initiates by thrombin cleavage between the A2 and B domains, which releases the B domain and results in a heavy chain consisting of the A1 and A2 domains (19). Consideration of the structure and processing of FVIII suggests that biologically active FVIII could be reconstituted if cells were engineered to coexpress both a heavy and light chain of FVIII. Indeed, several investigators (20, 21) demonstrated that biologically active FVIII could be produced after cotransfection of Chinese hamster ovary cells with plasmids encoding the heavy and light chains of FVIII. Because the DNA sequences encoding the FVIII heavy and light chains can be accommodated easily by an AAV vector, we reasoned that coinfection of liver cells with an AAV vector expressing the heavy chain of FVIII and one expressing the FVIII light chain would result in the production of biologically active FVIII in the plasma of injected mice. In this paper, we demonstrate in vivo production of biologically active FVIII at physiological levels for approximately 1 year after coinjection of both rAAV vectors, one expressing a B domain-deleted form of the heavy chain of FVIII and the other expressing the FVIII light chain.
Materials and Methods
Plasmid Construction, Vector Production, and Purification.
The human factor VIII sequences used were derived from the full-length cDNA clone, pEV-85.24, which was kindly provided by Bayer (Berkeley, CA). The DNA sequences encoding the heavy and light chains of human FVIII (hFVIII) were constructed as described in Yonemura et al. (21) and cloned into expression cassettes in AAV vectors (Fig. 1). Both vectors contain the promoter and the first noncoding intron (from −573 to +985) of the human elongation factor 1α (EF1α) gene (22, 23). Each vector also contains the first 57 bp of the FVIII heavy chain that encodes the 19-aa signal sequence. The heavy chain construct encodes the A1 and A2 domains and 5 aa from the amino terminus of the B domain. The light chain vector encodes 85 aa of the carboxyl-terminal B domain, in addition to the complete A3, C1, and C2 domains. Both vectors utilize the human growth hormone (hGH) polyadenylation signal. The expression cassettes were inserted between AAV inverted terminal repeats. The initial cloning step involved deleting 854 bp of EF1α sequences between the SpeI and XcmI sites of pVm4.1e-hFIX (12) and religating to create pVm4.1eδD-hFIX. This construct then was digested with EcoRI, which released the hFIX cDNA, and was ligated to an oligonucleotide containing MfeI ends (EcoRI-compatible) and an internal ClaI restriction site, creating pVm4.1eδD-linker. The heavy and light chain fragments, including the hGH polyadenylation sequences, were isolated from pVm4.1cFVIII-HC and pVm4.1cFVIII-LC, respectively, as ClaI-BstEII fragments. These fragments were cloned between the corresponding sites in pVm4.1eδD-linker, creating plasmids pVm4.1eδD-FVIII-HC and pVm4.1eδD-FVIII-LC. In addition, the hFIX cDNA was cloned into pVm4.1eδD-linker, creating pVm4.1eδD-hFIX.
Figure 1.
Map of rAAV-hFVIII-HC and rAAV-hFVIII-LC vectors. Upper line in each illustration represents the gene structure of the vectors, and the lower line in each represents the structure of the hFVIII protein domains encoded by the vectors. ITR, AAV inverted terminal repeat; EF1α Pro/Intron 1, human polypeptide elongation factor 1α gene promoter and first intron; hFVIII-HC, human FVIII cDNA (hFVIII base pairs 1–2292); hFVIII-LC, human FVIII cDNA (hFVIII base pairs 1–57 and 4744–7053); hGH PA, human growth hormone polyadenylation signal; SS, human FVIII signal sequence; A1, A2, “B”, A3, C1, C2, complete and incomplete (“‘) protein domains of the hFVIII protein.
The triple transfection and purification methods described by Matsushita et al. (24) were employed for rAAV vector production, with minor modifications. The vector-production process involved cotransfection of 293 cells (approximately 1 × 107 plated per T 225 flask) with 20 μg of each of the following plasmids: the rAAV vector plasmid, the AAV helper plasmid (pHLP19), and the adenovirus helper plasmid (pladeno5), using the calcium phosphate method (25) for a period of 6 hr. After the transfection period, the media was replaced, and the cells were harvested 3 days later. The cell pellets then were subjected to three cycles of freeze–thaw lysis. The cell debris was removed by centrifugation, and the supernatant was treated with Benzonase (EM Industries, Hawthorne, NY) (200 units/ml) at 37°C for 1 hr. After incubation, the supernatant was made 25 mM in CaCl2 and was placed on ice for 1 hr. The resulting precipitate was removed by centrifugation (Beckman JA-12 rotor, 8,000 rpm, 15 min) and discarded. The supernatant then was made 10% in PEG 8,000 and was placed on ice for 3 hr. The precipitate was collected by centrifugation (300 × g, 30 min) and resuspended in 4 ml of 50 mM Hepes/150 mM NaCl/25 mM EDTA, pH 8.0, per 20 T225 flasks. Solid CsCl was added to produce a density of 1.4 g/ml, and the sample was centrifuged at 45,000 rpm for 23 hr in a Beckman TI70 rotor. rAAV-containing fractions were pooled, adjusted to a density of 1.4 g/ml CsCl, and centrifuged at 65,000 rpm for 5 hr in the same rotor. The fractions containing rAAV were then dialyzed, concentrated, and sterile-filtered. Three rAAV vectors were prepared: rAAV-hFVIII-HC, rAAV-hFVIII-LC, and rAAV-hFIX. The physical titers were determined by DNA dot-blot hybridization, and dosing was performed on the basis of the physical titer.
Transduction of 293 Cells.
293 cells were seeded in six-well plates at a density of 5 × 105 cells per well. When the monolayers reached 80–90% confluence, they were infected with 3 × 109 or 3 × 1010 vector genomes of rAAV-hFVIII-HC, rAAV- hFVIII-LC, an equal ratio of rAAV-hFVIII-HC and rAAV-hFVIII-LC, or rAAV-hFIX. Eighteen hours postinfection the media was replaced with DME/10% heat-inactivated FBS and was collected 24 hr later for analysis. Culture supernatants were analyzed by ELISA for hFVIII light chain antigen levels and by the ChromZ FVIII coagulation activity (FVIII-c) assay (Helena Laboratories) for biological activity (see below).
Animal Experiments.
C57BL/6 mice were obtained from Charles River Breeding Laboratories. Mice were injected via the portal vein (12) with either 3 × 1011 particles of rAAV-hFVIII-HC (n = 5), 3 × 1011 particles of rAAV-hFVIII-LC (n = 5), 3 × 1011 particles of both rAAV-hFVIII-HC and rAAV-hFVIII-LC (n = 5), or 3 × 1011 particles of rAAV-hFIX (n = 4). Blood samples were collected in sodium citrate via the retroorbital plexus at biweekly intervals for the first 2 months and at monthly intervals until 6 months. Additional samples were assayed at 11 months. Samples were centrifuged and plasma was aliquoted and stored frozen. Plasma was analyzed by ELISA for hFVIII antigen and by a chromogenic assay for biological activity.
FVIII Assays.
An ELISA specific for the light chain of hFVIII was used to determine hFVIII light chain antigen levels in the injected animals. NUNC Maxisorb 96-well plates were coated with a hFVIII light chain-specific mAb, N77110 (Biodesign International, Kennebunkport, ME). After incubation overnight at 4°C, the plate was washed with wash buffer (PBS/0.05% Tween 20) and blocked with 200 μl of blocking buffer (PBS/10% horse serum/0.05% Tween 20) at room temperature for 1 hr. The plate was washed and standards and samples were applied. Bioclate recombinant human FVIII (Centeon, Kankakee, IL) was used as the standard and was diluted in blocking buffer to concentrations ranging from 320 ng/ml to 10 ng/ml. For analysis of transduced culture supernatants, the standards contained 50% media, and for analysis of mouse plasma, the standards were made 10% in normal pooled mouse plasma (Sigma). A standard assay reference plasma (SARP; Helena Laboratories) also was included in the assay and was used to normalize the interassay ELISA results of plasma samples. The plates were incubated at room temperature for 2 hr and then washed. Horseradish peroxidase-conjugated light chain-specific antibody, ESH8-HRP (American Diagnostica, Greenwich, CT), was added (100 μl/well), and the plates were incubated for 1 hr at room temperature. The plates were washed and the antigen was detected by using an ABTS peroxidase substrate kit (Bio-Rad) according to the manufacturer’s instructions.
The FVIII-c assay (Helena Laboratories) was used to detect biologically active FVIII. Bioclate recombinant human FVIII (Centeon) was used as a standard to analyze transfected culture supernatants. The standards were diluted in plasma dilution buffer (supplied in kit) in the range of 10 ng/ml to 0.313 ng/ml and were made 2.5% in media. Because this assay can detect both human and murine FVIII-c activity, it was modified to deplete biologically active human FVIII in the mouse plasma. Mouse plasma was preincubated with an antibody specific for human FVIII before performing the assay. The difference in FVIII activity between the untreated plasma sample and the antibody-treated sample represents the amount of biologically active human FVIII in the plasma. Normal pooled human plasma (FACT) obtained from George King Biomedical (Overland Park, KS) was used as a standard to analyze mouse plasma samples. Serial dilutions of FACT were made in human FVIII-deficient plasma (George King Biomedical) in the range of 200 ng/ml (undiluted) to 6.25 ng/ml. The FACT standards (10 μl) were incubated at 37°C for 15 min in the presence or absence of 2 μl of antibody N77110 (1 mg/ml). Similarly, mouse plasma samples were diluted in FVIII-deficient plasma, and 10 μl of these diluted samples was incubated in the presence or absence of 2 μl of N77110 at 37°C for 15 min and immediately placed on ice. The antibody-adsorbed and nonadsorbed FACT standards and mouse plasma samples were diluted 1:20 in plasma dilution buffer provided in the ChromZ kit. Thus, the final concentration of the FACT standards used in the assay ranged from 10 ng/ml to 0.313 ng/ml. Twenty-five microliters of these dilutions was added to a chilled, 96-well plate. With the plate on ice, 25 μl of FIXa reagent and 50 μl of FX were added, and the plate was incubated at 37°C for 15 min. Substrate (50 μl) was added and the plate was incubated for an additional 3 min at 37°C. The reaction was stopped with the addition of 25 μl of 50% acetic acid, and the OD at 405 nm was measured.
Immunofluorescent Staining of FVIII Heavy and Light Chains.
293 cells were plated on rat tail collagen-coated culture slides at a density of 4 × 105 cells per well. Forty-eight hours later, cells were transduced at a multiplicity of infection of 3 × 104 particles per cell with rAAV-hFVIII-HC and rAAV-hFVIII-LC. Forty-eight hours posttransduction, the cells were fixed in situ with acetone, blocked with 2% BSA, and stained with a fluorescently labeled anti-hFVIII light chain antibody (green; 1:250) and a fluorescently labeled anti-hFVIII heavy chain antibody (red; 1:1,000). The anti-hFVIII light chain antibody used was ESH-4 mAb (American Diagnostica) and was fluorescently labeled with alexa-488 (Molecular Probes) according to the manufacturer’s instructions. The anti-hFVIII heavy chain antibody used was MAS530P mAb (Accurate Chemicals) and was fluorescently labeled with alexa-594 (Molecular Probes) according to the manufacturer’s instructions. The cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Images were collected by using a Zeiss Axioskop fluorescence microscope equipped with filters for DAPI, FITC, and rhodamine, and a charge-coupled device camera. Image analysis was performed by using quips imaging software (Vysis, Downer Grove, IL).
DNA and RNA Analysis.
DNA was extracted from liver, kidney, spleen, and heart tissue and analyzed by Southern blotting (26). Twenty micrograms of DNA was digested with BglII, separated on a 1% agarose gel, and hybridized with a 32P-labeled, 1,126-bp AlwNI fragment encoding the A1 and A2 domains of hFVIII (heavy chain probe) or a 32P-labeled, 1,456-bp NdeI-EcoRI fragment encoding the A3, C1, and C2 domains of hFVIII (light chain probe). Copy number controls were generated by spiking BglII-digested naïve mouse liver DNA with BglII-digested heavy or light chain plasmid DNA at ratios of 10, 5, 1, 0.1, and 0.01 copies per diploid genome. The hybridized membranes were analyzed by using a Storm 860 PhosphorImager (Molecular Devices), and quantitation of vector copy number was evaluated by using imagequant software (Molecular Devices). Autoradiography of the hybridized membranes also was performed.
Total RNA was isolated from liver tissue by using the RNA Stat extraction kit (Tel-Test, Friendswood, TX). Northern blot analysis (26) was performed on 10 μg RNA by using the 32P-labeled probes specific to the heavy and light chains of hFVIII described above, and autoradiography was performed on the hybridized membranes.
Results
In Vitro Production of Biologically Active FVIII.
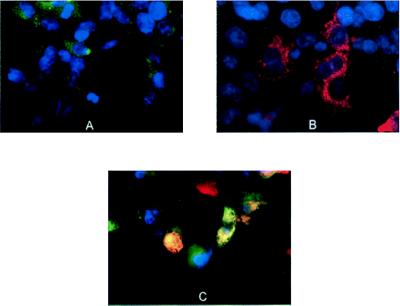
rAAV vectors encoding either a B domain-deleted heavy chain of hFVIII or the hFVIII light chain, expressed from an EF1α promoter (Fig. 1), were constructed and used to test the feasibility of producing biologically active FVIII after delivery of the individual chains. 293 cells were infected with either rAAV-hFVIII-HC, rAAV-hFVIII-LC, equal amounts of both vectors, or a rAAV-hFIX vector. The level of FVIII light chain antigen was determined by ELISA, and the amount of biologically active protein was assessed by using the ChromZ assay. As shown in Table 1, background levels of hFVIII antigen were detected when cells were transduced with either rAAV-hFVIII-HC or rAAV-hFIX. Transduction of cells with rAAV-hFVIII-LC resulted in the production of hFVIII light chain antigen in a dose-dependent manner, but no biologically active protein was observed. However, when cells were coinfected with both rAAV-FVIII-HC and rAAV-hFVIII-LC, biologically active protein was produced. When cells were cotransduced with 3 × 1010 vector genomes, 440 milliunits/ml of active hFVIII or approximately 88 ng/ml was produced. This represents approximately 75% of the total amount of hFVIII light chain produced, as measured by ELISA. These results demonstrate that biologically active FVIII can be produced by codelivery of two rAAV vectors to cells in vitro. Fig. 2 shows visually the production of the hFVIII chains in transduced 293 cells. Infection of cells with either rAAV-hFVIII-LC (Fig. 2A) or rAAV-hFVIII-HC (Fig. 2B) followed by staining with antibodies to both chains results in production of the individual chains of hFVIII. Immunofluorescent staining of cells coinfected at a multiplicity of infection of 3 × 104 with both vectors resulted in approximately 40% cotransduction (Fig. 2C).
Table 1.
In vitro production of biologically active hFVIII from two rAAV vectors
| Vector | No. of particles | ELISA, ng/ml | ChromZ
|
|
|---|---|---|---|---|
| mu/ml | ng/ml | |||
| AAV-FVIII-HC and AAV-FVIII-LC | 3 × 109 | 24 | 35 | 7.1 |
| AAV-FVIII-HC and AAV-FVIII-LC | 3 × 1010 | 121 | 440 | 87.9 |
| AAV-FVIII-HC | 3 × 109 | 0 | 0 | 0 |
| AAV-FVIII-HC | 3 × 1010 | 0 | 0 | 0 |
| AAV-FVIII-LC | 3 × 109 | 20.5 | 0 | 0 |
| AAV-FVIII-LC | 3 × 1010 | 96.9 | 0 | 0 |
| AAV-hFIX | 3 × 109 | 0 | 0 | 0 |
| AAV-hFIX | 3 × 1010 | 0 | 0 | 0 |
| No vector | 0 | 0 | 0 | |
293 cells were seeded in six-well plates in DMEM containing 10% heat-inactivated FBS. When the cells reached a density of approximately 1 × 106 cells per well, they were infected with 3 × 109 or 3 × 1010 particles of either rAAV-hFVIII-HC, rAAV-hFVIII-LC, both rAAV-hFVIII-HC and rAAV-hFVIII-LC, rAAV-hFIX, or no vector. The medium was replaced 18 hr after the addition of vector. Approximately 42 hr postinfection, the medium was collected and analyzed by ELISA for hFVIII light chain and by the ChromZ assay for biologically active FVIII. For both assays, duplicate wells were transduced and medium from each well was assayed in triplicate. Data are reported as the mean of duplicate wells. The activity units were converted to nanograms by using the definition of one unit is equal to the amount of FVIII in 1 ml of normal-pooled human plasma, or 200 ng.
Figure 2.
Cotransduction of 293 cells with rAAV-hFVIII-HC and rAAV-hFVIII-LC. 293 cells were plated on culture slides and, 48 hr later, were transduced at a multiplicity of infection of 3 × 104 particles per cell with rAAV-hFVIII-LC (A), rAAV-hFVIII-HC (B), or both vectors (C). Forty-eight hours posttransduction, the cells were fixed and stained with a fluorescently labeled anti-hFVIII light chain antibody (green; 1:250) and a fluorescently labeled anti-hFVIII heavy chain antibody (red; 1:1,000) and counterstained with 4′,6-diamidino-2-phenylindole. Colocalization of both the heavy and light chains of hFVIII is evident by the yellow staining.
In Vivo Production of Biologically Active FVIII.
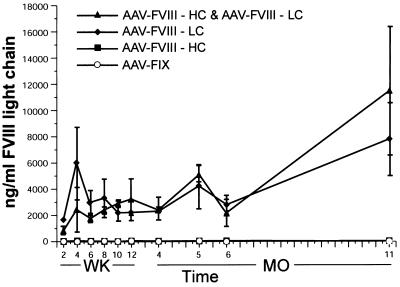
To test the feasibility of this approach in vivo, we delivered the AAV vectors to the livers of C57BL/6 animals. It has been demonstrated that this strain of mice does not elicit an immune response to human FVIII when the gene is delivered to the portal vasculature via an adenoviral vector (7). Three groups of five animals were injected via the portal vein with 3 × 1011 particles of rAAV-hFVIII-HC, 3 × 1011 particles of rAAV-hFVIII-LC, or 3 × 1011 particles of both vectors. In addition, a group of four animals was injected with 3 × 1011 particles of rAAV-hFIX. Fig. 3 demonstrates that very high levels of hFVIII light chain were expressed in animals injected with AAV-hFVIII-LC alone or with both vectors. These levels have been sustained for approximately 1 year, with no evidence of waning. It should be noted that one of the five animals coinjected with both the heavy and light chain vector did not express FVIII (presumably because of an inefficient injection) and was omitted from the analysis. To assess the amount of biologically active human FVIII produced in the animals, we modified the ChromZ assay. Because this assay detects both human and murine FVIII, we determined the amount of FVIII present in the plasma before and after adsorption to an antibody specific to human FVIII. The amount of FVIII remaining in the plasma after adsorption represents the amount of active murine FVIII, and the difference represents the amount of active human FVIII. Control experiments demonstrated that the antibody could remove 80–90% of the human FVIII from a mouse plasma sample when the sample was spiked with up to 32 ng of hFVIII (data not shown). The modified ChromZ assay indicated that only those animals injected with both vectors produced biologically active FVIII. Table 2 shows the ELISA and ChromZ results obtained from plasma collected 8 weeks postinjection (similar results were obtained 10 weeks and 5 months postinjection, data not shown). Animals injected with both vectors produce more than 2 μg/ml hFVIII light chain as measured by ELISA. The ChromZ assay indicated that 3–4 units of active FVIII was present in these plasma samples. The contribution from murine FVIII was approximately 2–2.7 units, indicating that approximately 1.2–2.2 units or 230–430 ng/ml active hFVIII was present in the plasma. Although this represents only a fraction of the total amount of protein synthesized, greater than physiological levels of the active protein are produced. Similar analyses performed on animals injected with the light chain vector alone, the heavy chain vector alone, or the hFIX vector demonstrated no biologically active human FVIII in the plasma of these animals (Table 2). These data demonstrate the feasibility of producing biologically active FVIII by using two AAV vectors to independently deliver the heavy and light chains of FVIII.
Figure 3.
Expression of human FVIII in mouse plasma. C57BL/6 mice were injected via the portal vein with either 3 × 1011 particles of rAAV-hFVIII-HC, 3 × 1011 particles of rAAV-hFVIII-LC, 3 × 1011 particles of both rAAV-hFVIII-HC and rAAV-hFVIII-LC, or 3 × 1011 particles of rAAV-hFIX. Blood samples were collected via the retroorbital, and plasma was analyzed by ELISA for the level of hFVIII light chain antigen.
Table 2.
Biological activity of hFVIII in vivo
| Mouse | ELISA ng/ml | Chrom Z FVIII biological activity
|
|||
|---|---|---|---|---|---|
| Total FVIII (−Ab), units | mFVIII (+Ab), units | hFVIII, units | hFVIII, ng/ml | ||
| AAV-FVIII-HC and AAV-FVIII-LC | |||||
| 3-L | 2,144 ± 15 | 3.1 ± 0.2 | 1.92 ± 0.16 | 1.2 | 234 |
| 3-R | 2,407 ± 14 | 4.5 ± 0.17 | 2.7 ± 0.08 | 1.8 | 359 |
| 6-B | 2,314 ± 160 | 4.2 ± 0.24 | 2.0 ± 0.09 | 2.2 | 434 |
| AAV-FVIII-LC | |||||
| 2-O | 1,331 ± 79 | 0.64 ± 0.05 | 0.65 ± 0.04 | 0 | 0 |
| 2-L | 4,472 ± 626 | 1.5 ± 0.06 | 1.8 ± 0.08 | 0 | 0 |
| 2-R | 3,397 ± 187 | 2.0 ± 0.01 | 2.4 ± 0.16 | 0 | 0 |
| AAV-FVIII-HC | |||||
| 1-O | 0 | 2.8 ± 0.07 | 2.7 ± 0.26 | 0* | 0* |
| 1-L | 0 | 1.7 ± 0.09 | 1.8 ± 0.09 | 0 | 0 |
| 1-B | 0 | 0.4 ± 0.09 | 0.37 ± 0.02 | 0* | 0* |
| AAV-FIX | |||||
| 4-O | 0 | 2.0 ± 0.12 | 1.8 ± 0.11 | 0.2 | 40 |
| 4-R | 0 | 2.3 ± 0.14 | 2.4 ± 0.12 | 0 | 0 |
| 4-B | 0 | 1.5 ± 0.08 | 1.65 ± 0.15 | 0 | 0 |
C57BL/6 mice were injected via the portal vein with either 3 × 1011 particles of rAAV-hFVIII-HC, 3 × 1011 particles of rAAV-hFVIII-LC, 3 × 1011 particles of both rAAV-hFVIII-HC and rAAV-hFVIII-LC, or 3 × 1011 particles of rAAV-hFIX. Blood samples were collected via the retroorbital plexus 8 weeks postinjection, and plasma was analyzed by ELISA for the level of hFVIII light chain antigen and by the ChromZ assay for FVIII biological activity. Mouse plasma was incubated with or without an antibody to human FVIII before performing the assay. The difference in FVIII activity between the untreated plasma sample and the antibody-treated sample represents the amount of biologically active human FVIII in the murine plasma. The activity units were converted to nanograms by using the definition of one unit is equal the amount of FVIII in 1 ml of normal-pooled human plasma, or 200 ng. For both assays, plasma samples were analyzed in triplicate; data are reported as mean ± SD.
Value is 0 if consider SD.
Gene Transfer and Expression of Vector in Liver.
Evidence of gene transfer to the liver was obtained by isolating DNA from animals sacrificed 8 weeks postinjection. After digestion of liver DNA with BglII and hybridization with a hFVIII light chain probe, a band at the predicted size of 3,015 bp was detected in animals injected with rAAV-hFVIII-LC or both the heavy and light chain vectors (Fig. 4A). This band was not observed in the DNA of animals injected with the heavy chain vector alone or the hFIX vector. Phosphoimage analysis revealed that the light chain vector was present at approximately 2.4 and 1.5 copies per diploid genome in animals injected with the light chain vector alone or both vectors, respectively. When BglII-digested DNA was hybridized with a hFVIII heavy chain probe, the expected band of 2,318 bp was observed in animals injected with the heavy chain vector alone or both vectors, but was not detected in animals injected with the light chain vector alone or the hFIX vector (Fig. 4B). The copy number in animals injected with the heavy chain vector alone and both vectors was 1.1 and 1.7 vector copies per diploid genome, respectively. DNA also was extracted from spleen, kidney, and heart tissue of an animal injected with both rAAV-hFVIII-HC and rAAV-hFVIII-LC. Hybridization with either a hFVIII light chain probe or a heavy chain probe revealed that these tissues contain less than 1 copy of vector sequences per 10 diploid genomes, demonstrating that the vector distributes primarily to the liver after intraportal injection (data not shown).
Figure 4.
Southern blot analysis of liver DNA. Liver DNA (20 μg) extracted 8 weeks postinjection from mice injected via the portal vein with 3 × 1011 particles of rAAV-hFVIII-LC (lane 1), rAAV-hFIX (lane 2), rAAV-hFVIII-HC (lane 3), or both rAAV-hFVIII-HC and rAAV-hFVIII-LC (lane 4) was digested with BglII and hybridized with a probe specific for the light chain of hFVIII (A) or the heavy chain of hFVIII (B). Copy number controls were generated by spiking BglII-digested naïve mouse liver DNA with the corresponding plasmids at ratios of 10, 5, 1, 0.1, and 0.01 copies per diploid genome (lanes 5–9).
Human FVIII gene expression in the liver was assessed by performing Northern blot analysis on RNA isolated from animals sacrificed 8 weeks postinjection. As shown in Fig. 5A, hFVIII light chain transcripts of the predicted size (2.7 kb) were observed in animals injected with the light chain vector alone or both vectors. Similarly, the expected hFVIII heavy chain transcripts (2.7 kb) were detected in animals that were injected with the heavy chain vector alone or both vectors. Because the heavy and light chain DNA sequences were shown by Southern blot to be present at approximately the same copy number (1.7 and 1.5 copies per diploid genome, respectively) in an animal injected with both vectors, these results demonstrate that both the heavy and light chains of hFVIII are expressed in the liver at approximately equivalent amounts.
Figure 5.
Northern blot analysis of liver RNA. Liver RNA (10 μg) extracted 8 weeks postinjection from uninjected mice (lane 1) or mice injected via the portal vein with 3 × 1011 particles of rAAV-hFVIII-LC (lane 3), rAAV-hFVIII-HC (lane 4), both rAAV-hFVIII-HC and rAAV-hFVIII-LC (lane 2), or rAAV-hFIX (lane 5) was hybridized with a probe specific for the light chain of hFVIII (A) or the heavy chain of hFVIII (B).
Discussion
The data presented demonstrate the feasibility of producing biologically active human FVIII by cotransducing liver with two vectors: one encoding a B domain-deleted form of the hFVIII heavy chain and one encoding the light chain of hFVIII. Extremely high levels (2–10 μg/ml) of hFVIII light chain were detected by ELISA after injection of 3 × 1011 particles of the hFVIII light chain vector or both the heavy and light chain vectors. Whereas expression of each chain alone resulted in production of inactive protein, 200–400 ng/ml of biologically active protein was produced in mice that were coinjected with both vectors. Although the amount of biologically active protein was only a fraction of the total amount of light chain antigen produced, it represents greater than normal physiological levels of FVIII and would be expected to completely correct the bleeding disorder in humans. Because the ELISA measures only light chain antigen, it is not possible to calculate the specific activity or the percentage of the total protein that is active. However, Northern blot analysis demonstrated that the heavy and light chain RNA transcripts were expressed at approximately equivalent amounts in a coinfected animal. Thus, if one assumes that the amount of hFVIII heavy chain produced and secreted into the plasma of animals injected with both vectors is equivalent to the amount of light chain produced, then approximately 5–10% of the total hFVIII protein produced is biologically active. In fact, the amount of secreted heavy chain may be lower because it has been shown that unassociated heavy chain is rapidly degraded in vitro (17). There are several explanations to account for the low percentage of active protein. First, it is possible that production of biologically active hFVIII requires cotransduction of liver cells by both vectors. This may be necessary for efficient heterodimer formation between the heavy and light chains. It has been shown in Chinese hamster ovary cells that cleavage of the single-chain polypeptide into the heavy and light chains occurs intracellularly, and small amounts of the light chain are associated with the heavy chain in the cell (17). If heterodimer formation requires cotransduction of cells, it is possible that we have achieved 5–10% cotransduction and, hence, 5–10% active FVIII. A recent report by Snyder et al. (13) concluded that after transduction of liver with 4.8 × 1010 particles of an AAV-hFIX vector, the average copy number per transduced cell was 3.5, but only 5% of the hepatocytes showed evidence of gene expression. This suggested that each transduced hepatocyte contained approximately 70 copies of rAAV. Additional cotransduction experiments revealed that 30–40% of these transduced hepatocytes were cotransduced (M. Kay, personal communication). If we assume that at a dose of 3 × 1011 vector genomes we get three times more transduction than what was observed with 4.8 × 1010 (based on a comparison of hFIX expression/vector dose in the different labs) (12, 13), then 15% of the hepatocytes were transduced, and 30–40% of these, or 6%, were cotransduced. Thus, our results that approximately 5–10% of the total human FVIII protein is biologically active is consistent with a requirement for cotransduction and the estimated level of cotransduction.
Although heterodimer formation between the heavy and light chains of FVIII may occur intracellularly, there are several reports demonstrating that reconstitution of FVIII activity from isolated subunits does occur at high FVIII concentrations and in the presence of Mn2+ or Ca2+ ions (27, 28). In addition, von Willebrand factor (vWF) also has been shown to promote reconstitution of FVIII activity from dissociated heavy and light chain subunits, suggesting that vWF may act to promote stable assembly of FVIII subunits at the site of secretion (27, 29). These results suggest that the heavy and light chains of FVIII may be able to associate after secretion from independently transduced cells under defined conditions. Thus, it may not be essential for cells to be cotransduced, but transduction of neighboring cells with either vector would be required to provide a high local concentration of both chains, facilitating heterodimer formation.
Another possible explanation for the observation that only 5–10% of the total FVIII is active is that the chains are processed inefficiently. However, Yonemura et al. (21), using identical FVIII constructs, showed that most of the products secreted from Chinese hamster ovary cells were processed at the native cleavage sites.
It is not clear what the ramifications of production and secretion of inactive FVIII chains are in vivo. However, many mild and moderate hemophilia patients secrete nonfunctional FVIII antigen at levels comparable to those in normal plasma (30). It should also be pointed out that no untoward effects were observed over the course of 1 year in the mice injected with rAAV-hFVIII-HC, rAAV-hFVIII-LC, or both vectors. Nevertheless, one would like to increase the proportion of active FVIII being synthesized. Modifications of the present dual-vector strategy or alternative strategies to deliver FVIII using rAAV vectors are being considered. Construction of a single-chain hFVIII vector that is under the packaging limit of rAAV vectors may be possible if minimal, but efficient, genetic regulatory elements can be identified.
In summary, the data presented support the use of rAAV vectors as gene transfer vehicles to treat hemophilia A. Further support for the dual-vector approach will come from the use of hemophilia A knockout mice (31) to demonstrate phenotypic correction of the bleeding defect.
Abbreviations
- rAAV
recombinant adeno-associated viral vector
- hFVIII
human factor 8
- EF1α
elongation factor 1α
References
- 1.Sadler J, Davie E W. Hemophilia A, Hemophilia B, and von Willebrand’s Disease. Philadelphia: Saunders; 1987. [Google Scholar]
- 2.Furie B, Limentani S, Rosenfield C. Blood. 1994;84:3–9. [PubMed] [Google Scholar]
- 3.Lofqvist T, Nilsson I M, Berntorp E, Pettersson H. J Int Med. 1997;241:395–400. doi: 10.1046/j.1365-2796.1997.130135000.x. [DOI] [PubMed] [Google Scholar]
- 4.Ragni M V, Hord J D, Blatt J. Haemophilia. 1997;3:90–95. doi: 10.1046/j.1365-2516.1997.00100.x. [DOI] [PubMed] [Google Scholar]
- 5.Varavani D, Belloni P, Nijjar T, Smith J, Couto L, Rabier M, Clift S, Berns A, Cohen L. Proc Natl Acad Sci USA. 1995;92:1023–1027. doi: 10.1073/pnas.92.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connelly S, Smith T, Dhir G, Gardner J, Mehaffey M, Zaret K, McClelland A, Kaleko M. Hum Gene Ther. 1995;6:185–193. doi: 10.1089/hum.1995.6.2-185. [DOI] [PubMed] [Google Scholar]
- 7.Connelly S, Gardner J, Lyons R, McClelland A, Kaleko M. Blood. 1996;87:4671–4677. [PubMed] [Google Scholar]
- 8.Connelly S, Andrews J, Gallo A, Kayda D, Qian J, Hoyer L, Kadan M, Gorziglia M, Trapnell B, McClelland A, Kaleko M. Blood. 1998;91:3273–3281. [PubMed] [Google Scholar]
- 9.Connelly S, Mount J, Mauser A, Gardner J, Kaleko M, McClelland A, Lothrop C. Blood. 1996;88:3846–3853. [PubMed] [Google Scholar]
- 10.Herzog R W, Hagstrom J N, Kung S H, Tai S J, Wilson J M, Fisher K J, High K A. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herzog R, Yang E, Couto L, Hagstrom J N, Elwell D, Fields P, Burton M, Bellinger D, Read M, Brinkhous K, et al. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 12.Nakai H, Herzog R W, Hagstrom J N, Walter J, Kung S-H, Yang E Y, Tai S J, Iwaki Y, Kurtzman G J, Fisher K J, et al. Blood. 1998;91:4600–4607. [PubMed] [Google Scholar]
- 13.Snyder R, Miao C, Patijn G, Spratt S, Danos O, Nagy D, Gown A, Winther B, Meuse L, Cohen L, et al. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 14.Snyder R, Miao C, Meuse L, Tubb J, Donahue B, Lin H-F, Stafford D, Patel S, Thompson A, Nichols T, et al. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 15.Dong J-Y, Fan P-D, Frizzell R. Hum Gene Ther. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 16.Vehar G, Keyt B, Eaton D, Rodriguez H, O’Brien D, Rotblat F, Oppermann H, Keck R, Wood W, Harkins R, et al. Nature (London) 1984;312:337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman R, Wasley L, Dorner A. J Biol Chem. 1988;263:6352–6362. [PubMed] [Google Scholar]
- 18.Ganz P, Tackaberry E, Palmer D, Rock G. Eur J Biochem. 1988;170:521–528. doi: 10.1111/j.1432-1033.1988.tb13731.x. [DOI] [PubMed] [Google Scholar]
- 19.Eaton D, Rodriguez H, Vehar G. Biochemistry. 1986;25:505–512. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- 20.Burke R, Pachl C, Quiroga M, Rosenberg S, Haigwood N, Nordfang O, Ezban M. J Biol Chem. 1986;261:12574–12578. [PubMed] [Google Scholar]
- 21.Yonemura H, Sugawara K, Nakashima K, Nakahara Y, Hamamoto T, Mimaki I, Yokomizo K, Tajima Y, Masuda K, Imaizumi A, et al. Protein Eng. 1993;6:669–674. doi: 10.1093/protein/6.6.669. [DOI] [PubMed] [Google Scholar]
- 22.Uetsuki T, Naito A, Nagata S, Kaziro Y. J Biol Chem. 1989;264:5791–5798. [PubMed] [Google Scholar]
- 23.Kim D, Uetsuki T, Kaziro Y, Yamaguchi N, Sugano S. Gene. 1990;91:217–223. doi: 10.1016/0378-1119(90)90091-5. [DOI] [PubMed] [Google Scholar]
- 24.Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman G, Iwaki Y, Colosi P. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 25.Wigler M, Perucho M, Kurtz D, Dana S, Pellice A, Axel R, Silverstein S. Proc Natl Acad Sci USA. 1980;77:3567–3570. doi: 10.1073/pnas.77.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 27.Fay P. Arch Biochem Biophys. 1988;262:525–531. doi: 10.1016/0003-9861(88)90404-3. [DOI] [PubMed] [Google Scholar]
- 28.Fay P, Haidaris P, Smudzin T. J Biol Chem. 1991;266:8957–8962. [PubMed] [Google Scholar]
- 29.Wise R, Dorner A, M, K, Pittman D, Kaufman R. J Biol Chem. 1991;266:21948–21955. [PubMed] [Google Scholar]
- 30.Lazahchick J, Hoyer L. J Clin Invest. 1978;62:1048–1052. doi: 10.1172/JCI109209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bi L, Lawler A M, Antonarakis S E, High K A, Gearhart J D, Kazazian H H. Nat Genet. 1995;10:119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]