Abstract
Defects in lymphocyte apoptosis may lead to autoimmune disorders and contribute to the pathogenesis of type 1 diabetes. Lymphocytes of nonobese diabetic (NOD) mice, an animal model of autoimmune diabetes, have been found resistant to various apoptosis signals, including the alkylating drug cyclophosphamide. Using an F2 intercross between the apoptosis-resistant NOD mouse and the apoptosis-susceptible C57BL/6 mouse, we define a major locus controlling the apoptosis-resistance phenotype and demonstrate its linkage (logarithm of odds score = 3.9) to a group of medial markers on chromosome 1. The newly defined gene cannot be dissociated from Ctla4 and Cd28 and in fact marks a 20-centimorgan region encompassing Idd5, a previously postulated diabetes susceptibility locus. Interestingly, we find that the CTLA-4 (cytotoxic T lymphocyte-associated antigen 4) and the CD28 costimulatory molecules are defectively expressed in NOD mice, suggesting that one or both of these molecules may be involved in the control of apoptosis resistance and, in turn, in diabetes susceptibility.
Immunologic tolerance is established and maintained by clonal deletion, active suppression, and silencing of potentially pathogenic lymphocytes (1). The development and regulation of the immune system is dependent on the daily suicide of lymphocyte clones and, hence, cell death by apoptosis plays a pivotal role in purging the body of dangerous lymphocytes. Indeed, inefficient elimination of lymphocytes can contribute to the pathogenesis of autoimmune diseases (2).
Nonobese diabetic (NOD) mice spontaneously develop autoimmune diabetes which closely resembles human type 1 (insulin-dependent) diabetes mellitus (IDDM) (3). Many loci have been mapped that govern the susceptibility to autoimmune diabetes both in man (IDDM1–8) (4) and mouse (Idd1–14) (5), but little is known about the nature of these genes or how alteration of gene activity contributes to diabetes susceptibility. One strategy to elucidate the function of these genes is to seek specific abnormalities in the immune system of NOD mice likely to contribute to onset of diabetes. Such NOD-defined traits can then be probed for linkage to the previously mapped diabetes susceptibility loci. The biological functions controlled by these loci may, in turn, provide a rational for a selective search of candidate genes (6). Persuing such an approach, we and others have recently shown that NOD lymphocytes display resistance to a number of apoptogenic mediators (7–9). We have also reported that the NOD allele at the Nod3 locus, in a distal region on chromosome 6 near the Idd6 locus, confers resistance to the thymocyte apoptosis induced by dexamethazone (10). Based on these observations we suggested that defects in apoptosis represents a contributor to the genesis of autoimmune diabetes by altering the selection of thymocyte populations.
NOD lymphocytes also display a reduced susceptibility to apoptosis induced by the alkylating drug cyclophosphamide (CY) (9). Here we analyze the genetic control of the resistance to CY-induced apoptosis of NOD lymphocytes in an (NOD × C57BL/6)F2 progeny.
MATERIALS AND METHODS
Induction and Measurement of Apoptosis After CY Treatment.
Mice were injected i.p. with 7.5 mg/mouse of CY (Orion, Espoo, Finland) dissolved in 300 μl of PBS and killed 16 h later. Inguinal and para-aortic lymph nodes were harvested, and cell suspensions were prepared by teasing the lymph nodes through a metal mesh. Cell suspensions were stained by the terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) method with the in situ cell death detection kit (Boehringer Mannheim) as described (9, 11). Apoptosis was assessed at the single cell level by flow cytometry sampling of 10,000 cells (FACScan and FACScalibur, Becton Dickinson), and expressed as the percentage of total cells. The staining specifically detects apoptotic cells upon terminal deoxynucleotidyltransferase (TdT) incorporation of fluorescein isothiocyanate (FITC)-labeled nucleotides into the single-strand DNA breaks of apoptotic cells. In negative control experiments, lymph node cells from mice left untreated (n = 6) were stained with TUNEL for assessing spontaneous apoptosis (1.7 ± 0.6%). In specificity control experiments, lymph node cells from treated mice were stained with TUNEL in absence of the TdT enzyme for detection of nonspecific labeling (see Fig. 1A). NOD, C57BL/6 (B6), BALB/c, C3H/Tif, DBA/2, (NOD × B6)F1, and (NOD × B6)F2 mice were bred and kept in conventional housing conditions at the animal facility of Umeå University. NOR mice were purchased from The Jackson Laboratory and let to adapt to the host housing conditions for at least 1 week before they were analyzed. All animals used were aged between 4 and 7 months.
Figure 1.
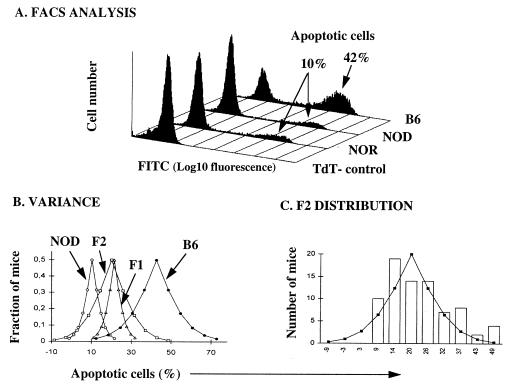
Apoptosis resistance trait scored by flow cytometry and analysis of phenotypic variance and distribution. (A) Apoptosis was induced by i.p. injection of CY. Lymph node cells were fixed and stained with TUNEL for detection of apoptosis, measured as percent of FITC positive cells. NOD and nonobese resistant (NOR) lymphocytes showed relative resistance, whereas B6 lymphocytes were four fold more sensitive. In absence of the terminal deoxynucleotidyltransferase (TdT) enzyme the nonspecific staining was <1%. The figure shows one representative of six independent experiments. (B) Phenotypic variance. T-distribution curves predicted by the apoptosis resistance scored in 8 NOD, 8 B6, 6 F1, and 78 F2 mice. (C) Phenotypic distribution. Comparison between the predicted T-distribution in the F2 progeny and the observed frequency distribution.
Genetic and Statistical Analysis of (NOD × B6)F2 Mice.
The 36 microsatellite markers used in this study discriminate between the NOD and B6 parental strains by virtue of polymorphism’s in simple sequence tandem repeats. The markers were purchased from Research Genetics; primers for the Bcl2 and Il2 markers were synthesized as described (10). According to a strategy proposed by Todd and coworkers (12), PCR amplifications from tail DNA were optimized for each microsatellite, varying the annealing temperature (51–55°C), the extension time (45 sec to 2 min), and the number of cycles (30–40). Amplification fragments were separated by gel electrophoresis in 4% agarose gels constituted of a mixture of 3% Nusieve GTG agarose (FMC) and 1% type II agarose (Sigma) or in 4% Metaphor agarose gel (FMC) and visualized by ethidium bromide staining. The genotypes of the F2 progeny were determined for each marker and the association of apoptosis resistance was sought by a χ2 test for goodness-of-fit against the expected distribution, as if the markers were not linked to the trait. Evidence of linkage was considered significant for P values < 0.001. Marker orders and recombination fractions were calculated by using the mapmaker/exp 3.0 qtl 1.1 software (13) using a three point analysis. Quantitative trait loci were analyzed by using the mapmaker qtl 1.1 software (14).
Sequencing of Cd28 and Ctla4 from NOD cDNA.
cDNA covering the entire coding region of the Cd28 and Ctla4 genes was prepared from total spleen RNA by conventional reverse transcription–PCR methods using primers hybridizing immediately upstream the first exon and downstream the second exon. PCR products were then cloned into the pT7Blue T vector (Novagen). Both strands of the inserts were sequenced by dye terminator cycle sequencing methodology (Perkin–Elmer) using an Applied Biosystems model 377 DNA sequencer.
Staining for CD28 and Cytotoxic T Lymphocyte-Associated Antigen 4 (CTLA-4).
Cell suspensions were prepared from spleens of 6- to 8-week-old B6 and NOD mice. Erythrocytes were depleted with Geys lysis buffer. Cells were cultured at 1 × 106/ml in RPMI 1640 medium containing 10% fetal calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, penicillin (100 units/ml), streptomycin (100 mg/ml), and 50 mM 2-mercaptoethanol. After 48 h of incubation with soluble anti-CD3 (clone 145 2C11) at 10 μg/ml, cells were harvested and centrifuged over a Ficoll–Hypaque gradient to remove dead cells. Cells (5–10 × 105) were incubated with 2.4G2 mAb to block Fc receptor prior to staining with phycoerythrin-conjugated anti-CTLA-4 (clone UC10-4F10-11), biotin-conjugated anti-CD28 (CD28.2), biotin-conjugated anti-CD69 (clone H1.2F3), FITC-conjugated anti-CD4, or anti-CD8 (all mAbs were purchased from PharMingen). For all antibody stainings controls were done with isotype matched antibodies. Biotin-coupled antibodies were revealed with avidin-phycoerythrin (Southern Biotechnology Associates). To restrict the analysis of CD69 expression on T cells, FITC-conjugated anti-Thy 1.2 antibody (Becton Dickinson) was used to electronically gate Thy 1.2+ T cells. Flow cytometry was performed on a FACScalibur (Becton Dickinson) and at least 5 × 103 events were acquired and analyzed with a cellquest software. The expression of CTLA-4 and CD28 on activated CD8+ and CD4+ cells was detected by gating large blast cells as determined by forward and side scatter profiles.
RESULTS AND DISCUSSION
The Apoptosis Resistance Trait.
Peripheral lymphocyte susceptibility to apoptosis induced by CY was assessed in each mouse 16 h after i.p. administration of CY, a time point chosen upon previous kinetic studies (9). The phenotype was scored as the percentage of dead cells measured in each mouse by flow cytometry of TUNEL-stained lymph node cells (Fig. 1A). A group of NOD and B6 mice were used to set the reference values for the parental strains. The percentage of apoptotic cells in the lymph nodes was found to range between 4.5 and 18.2% in NOD mice (mean, 10.3%) and between 28.0 and 61.7% in B6 mice (mean, 42.6%) (Fig. 1B). These data confirmed our previous observation that lymph node and spleen cells from NOD mice display a relative resistance to CY-induced apoptosis (Fig. 1A and ref. 9).
The (NOD × B6)F1 progeny showed an intermediate phenotype in that the percentage of apoptotic cells ranged between 15.3 and 25.1% (mean, 21.4%) (Fig. 1B). The phenotype in the F2 progeny (n = 78) spanned from low apoptosis values (NOD-like) to high values (B6-like), indicating that the alleles controlling this trait were segregating in the cross. Nevertheless, the phenotypic distribution in both the F1 and F2 progenies was found to be slightly but unmistakably biased toward the parental NOD phenotype, suggesting an incomplete dominant mode of inheritance (Fig. 1B). An estimation of nongenetic variance based on the analysis of the parental strains and the F1 progeny (15) ascribed 42% of the variance observed in the F2 progeny to genetic factors. The F2 mice were individually scored as NOD-like if displaying an apoptosis score <18.8% and as B6-like if >22.8%, the limits representing the parental mean values ±1.75 × SD. We excluded nine mice from the analysis because they were found to display an intermediate phenotype within the region of the parental overlap represented by the probability curves shown in Fig. 1B.
Mapping of Lymphocyte Apoptosis-Resistance Trait to Idd5.
The CY-treated F2 mice were genotyped at 36 microsatellite markers covering the Idd loci 1–10. Association between each marker and the apoptosis resistance phenotype was sought by χ2 tests and significant evidence for linkage was taken for P values < 0.001. Association of the apoptosis-resistance trait was found to a medial region of chromosome 1 included between markers D1Mit322 and D1Mit181, the most strongly linked marker being D1Mit21 (χ2 = 18.7, P = 0,00009, see Table 1). All of the 13 F2 mice scored as homozygous for the NOD allele at D1Mit21 were scored as NOD-like (Table 1), strongly indicating that the NOD allele mediates apoptosis resistance. The locus controlling the peripheral lymphocyte apoptosis resistance to CY is included in the diabetes susceptibility region Idd5 (16–18). No evidence for significant linkage was found to any of the other markers studied.
Table 1.
Association of the apoptosis-resistance phenotype with microsatellite marker loci on chromosome 1
| φ | Marker locus | χ2 (2 df) | B6-like, nn: nb: bb | NOD-like, nn: nb: bb | n | P < 0.001 |
|---|---|---|---|---|---|---|
| D1Mit411 | 5.7 | 3: 10: 15 | 11: 19: 11 | 69 | ||
| 0.086 | D1Mit322 | 14.5 | 1: 10: 17 | 12: 21: 8 | 69 | 0.0007 |
| 0.066 | D1Mit478 | 16.1 | 0: 12: 16 | 11: 23: 7 | 69 | 0.0003 |
| 0.033 | D1Mit21 | 18.7 | 0: 12: 16 | 13: 22: 6 | 69 | 0.00009 |
| 0.019 | D1Mit181 | 14.8 | 0: 14: 14 | 13: 21: 7 | 69 | 0.0006 |
| 0.073 | D1Mit8 | 11.4 | 1: 12: 15 | 11: 22: 8 | 69 | |
| 0.145 | Bcl2 | 6.3 | 3: 14: 11 | 13: 21: 7 | 69 | |
| 0.013 | D1Mit26 | 6.7 | 3: 14: 11 | 12: 22: 6 | 68 | |
| 0.108 | D1Mit498 | 2.4 | 5: 13: 10 | 13: 19: 9 | 69 |
The 69 F2 CY-treated mice were classified as B6-like if highly responsive to CY (≥22.8% TUNEL-positive cells) or as NOD-like if apoptosis resistant (<18.8% TUNEL-positive cells). φ, Recombination fraction; n, number of animals genotyped; df, degree of freedom; nn, NOD homozygote; nb, heterozygote; bb, B6 homozygote. No significant evidence for association was found between the phenotype and the other 27 markers tested: D1Mit430, D1Mit117, D3Mit164, Il-2, D3Mit95, D3Mit96, D3Mit40, D3Mit19, D4Mit180, D6Mit184, D6Mit17, D6Mit129, D6Mit9, D6Mit218, D6Mit194, D6Mit198, D6Mit14, D6Mit15, D7Mit76, D7Mit55, D9Mit162, D11Mit164, D11Mit39, D11Mit199, D14Mit11, D17Mit28, and D17Mit34.
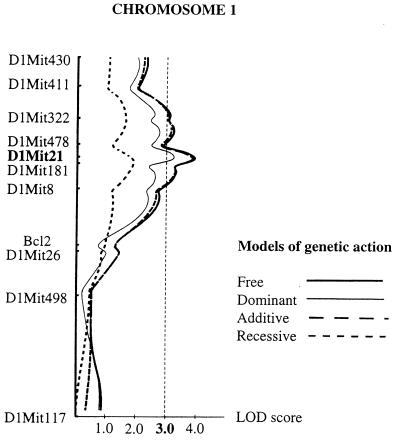
To evaluate the evidence obtained for linkage of apoptosis resistance to the medial region of chromosome 1, we next analyzed the phenotypic effect of this locus with the quantitative trait locus (QTL) analysis (13), a method that does not require an arbitrary setting of phenotypic values to classify the segregating progeny. Therefore the QTL analysis was extended to 78 F2 mice. Using the interval mapping method implemented in the mapmaker/qtl software, we scanned for QTLs for the apoptosis resistance trait on chromosome 1 (Fig. 2). The lod scores confirmed linkage to the region spanning the segment from D1Mit322 to D1Mit8, containing the Idd5 locus, and reached a maximum of 3.9 over the D1Mit21 marker. This value does not meet the stringent criteria suggested by Lander and Kruglyak (19) to declare a significant linkage in an F2 cross, but suggests the presence of a QTL linked to D1Mit21 that controls the apoptosis resistant trait. The lod score profiles recomputed under three different models of genetic action did not allow to discriminate confidently between an additive or a dominant mode of action of the NOD allele (Fig. 2). However, this does not overturn the conclusion reached earlier on the basis of the phenotypic variance and frequency distribution observed in the parental strains and in the F1 and F2 progenies (Fig. 1 B and C), which suggests an incomplete dominance of the NOD allele.
Figure 2.
QTL analysis of the lymphocyte apoptosis resistance in the F2 intercross. The logarithmic of odds (lod) score curve along chromosome 1 represents the maximum likelihood estimates for the presence of a QTL at each point. The dotted line indicates a threshold level for statistical significance of lod score >3.0. The fitness of the QTL to four genetic modes of action of the NOD allele is indicated. Plots were generated by using the qtl 1.1 software (14). The locus accounts for 21% of the phenotypic variation observed, which would explain 50% of the genetic variance in the F2 progeny.
Narrowing the Linkage Region Using Recombinant Congenic NOR Mice.
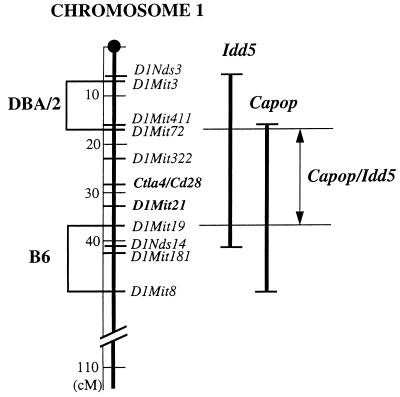
To better define the region of linkage, we tested the apoptosis resistance in NOR mice, a recently developed recombinant congenic strain (20). NOR mice possess a tripartite genome deriving from NOD, B6 and DBA/2 strains of mice. Despite the fact that about 88% of their genome, including the H-2 locus, is of NOD origin, they do not develop diabetes, most likely because some of the recombinant chromosomal regions encompass nonpermissive alleles at some Idd loci (Idd5 on chromosome 1, Idd13 on chromosome 2, Idd9 on chromosome 4, and Idd4 on chromosome 11) (21). In particular, chromosome 1 contains two discrete portions of the medial region that are of DBA/2, and B6 origin (21). The intervening region spanning a 20-centimorgan (cM) interval between D1Mit72 and D1Mit19 is of NOD origin and partially overlaps the 40-cM region previously shown to include the Idd5 locus (16–18) as well as the region identified here to encompass the locus controlling apoptosis resistance (Fig. 3). Peripheral lymphocytes of DBA/2 mice were found to be high responders to CY-induced apoptosis (data not shown), whereas lymphocytes of NOR mice (n = 4) were found to be indistinguishable from NOD lymphocytes, implying that the intervening region of NOR chromosome 1 includes the NOD allele, thus conferring apoptosis resistance. Based on these results, we further narrowed down the region of linkage to ≈20 cM between D1Mit72 and D1Mit19, which is included in the 40-cM region of chromosome 1 encompassing the Idd5 locus. Furthermore, the most strongly linked marker to the apoptosis-resistance trait, D1Mit21, colocalizes with D1Mit5, which in turn has been identified as the marker most closely linked to the Idd5 locus. We therefore propose that our newly identified locus contributes to diabetes pathogenesis by rendering NOD peripheral lymphocytes resistant to apoptosis, thus explaining the diabetogenic effect of the Idd5 locus.
Figure 3.
Chromosomal locations of the regions including the apoptosis-resistant trait locus and the Idd5 locus. The two regions partially overlap the two recombinant segments that in the NOR chromosome 1 are of DBA/2 and B6 origin. NOR lymphocytes display the same apoptosis resistance as NOD lymphocytes (Fig. 1A), whereas the B6 and DBA/2 lymphocytes are highly susceptible to apoptosis. Therefore, the locus controlling apoptosis resistance must be included in the 20 cM intervening region (from D1Mit72 to D1Mit19) that in NOR mice is composed of NOD alleles. Such a region encompasses both Ctla4 and Cd28. Capop, CY-apoptosis resistance.
CY is known to induce an accelerated form of diabetes in NOD mice but not in other strains of mice (17, 22). The resistance of NOD lymphocytes to CY-induced apoptosis and its association with the Idd5 locus may have implications for the understanding of the diabetogenic properties of CY. Thus, we propose that the NOD allele of the Idd5 locus mediates an abnormal survival of peripheral lymphocytes, leading to the accumulation of autoaggressive clones that contributes to the onset of diabetes over a time frame of 12–30 weeks. CY-induced lymphocyte depletion would select for apoptosis resistant cells that in a lymphopenic environment may instead need only 2–4 weeks to expand to numbers large enough to precipitate diabetes.
Defective Expression of CTLA-4 and Reduced Up-Regulation of CD28 in NOD T Cells.
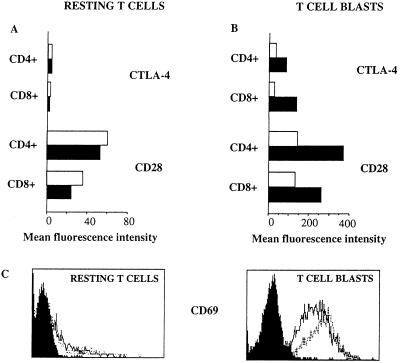
The 20-cM region of chromosome 1 set out here encompasses the two genes Ctla4 and Cd28, both known to be involved in the control of immune responses. Thus, CD28 is a surface antigen that is able to transduce costimulatory signals in T cells (23), whereas CTLA-4 has been suggested to mediate negative regulation (24–26). This makes Ctla4 and Cd28 plausible candidate genes for mediating the observed effects. We therefore analyzed the structure and the surface expression of these genes in the NOD mouse. The cDNA sequences of the NOD alleles of both Ctla4 and Cd28 were found to be identical (data not shown) to those obtained from the B6 alleles (27). In contrast, expression of both CTLA-4 and CD28 was found to be defective in NOD peripheral lymphocytes (Fig. 4). Thus, while CD28 is expressed in the majority of T cells of both NOD and B6 mice (Fig. 4A), the up-regulation of the receptor normally observed upon activation of T cells is reduced in NOD mice as compared with B6 and BALB/c mice (Fig. 4B and data not shown). However, the levels of up-regulation of CD28 in C3H/Tif and DBA/2 mice were not consistently higher than NOD mice (data not shown). Similarly, NOD T cells fail to up-regulate the expression of CTLA-4 to the level observed in B6, BALB/c, C3H/Tif and DBA/2 T cells (Fig. 4B and data not shown). The impaired up-regulation of CTLA-4 and CD28 is not due to a previously reported failure to activate NOD T cells (28) because the lower levels of expression are evident also in the selected population of activated T cell blasts.
Figure 4.
Defective expression of CTLA-4 and reduced up-regulation of CD28 in activated NOD T cells. Splenic T cells of NOD and B6 mice were stimulated in vitro with soluble anti-CD3. Fresh resting T cells (A) and 48-h cultured splenocytes (B) were stained for either CTLA-4 or CD28. Mean fluorescence values are shown for CD4+ and CD8+ T cells of resting cells and electronically gated blasts of both NOD mice (open bars) and B6 mice (filled bars). (C) The expression of the activation marker CD69 on resting and activated T cell blasts is similar in NOD (dotted line) and B6 (solid line). Filled histograms show background levels of fluorescence detected with an isotype matched control antibody. Data are representative of two independent experiments.
Because both the CD28 and the CTLA-4 receptors are involved in the regulation of T cell activity and because they colocalize to the same chromosomal region, neither of them can be ruled out as candidate gene for diabetes susceptibility and apoptosis resistance. Ctla4 represents a particularly attractive candidate in view of its demonstrated role in the negative regulation of immune activity (24–26). On the other hand, up-regulation of the CTLA-4 expression upon activation has been reported to be dependent on CD28 (29, 30). Moreover, the presence of a disrupted Cd28 gene in NOD mice has recently been reported to lead to an increase in the incidence of diabetes (31). A simplified overall picture could imply that the locus controlling apoptosis resistance is the Idd5 gene. Thus, the diabetes susceptibility conferred by the Idd5 is the result of a mutation in the NOD allele of this locus affecting the expression of the Ctla4 and/or the Cd28 genes. The resulting aberrant expression of CTLA-4 and CD28 would impair the control of T-cell activity contributing to the autoimmune attack leading to diabetes.
Acknowledgments
We wish to dedicate this work to the memory of Dr. Tommy Meo. We thank Drs. T. Meo and K. Forsman for discussions, Dr. S. Tuck for critical reviewing of the manuscript, and Dr. E. Lander for providing the mapmaker/qtl software package. F.C. was partly supported by a grant from the Kempe Foundation, and C.M.C. is a recipient of a fellowship from the “Instituto Pasteur–Fondazione Cenci-Bolognetti.” This work was supported by grants from The Juvenile Diabetes Foundation, the NOVO Nordisk Foundation, and the Swedish Medical Research Council.
ABBREVIATIONS
- NOD
nonobese diabetic
- B6
C57BL/6
- lod
logarithmic of odds
- CY
cyclophosphamide
- NOR
nonobese resistant
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end labeling
- FITC
fluorescein isothiocyanate
- CTLA-4
cytotoxic T lymphocyte-associated antigen 4
- QTL
quantitative trait locus
- cM
centimorgan
References
- 1.Kisielow P, von Boehmer H. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J J, Duke R C, Fadok V A, Sellins K S. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 3.Castano L, Eisenbarth G S. Annu Rev Immunol. 1991;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 4.Vyse T J, Todd J A. Cell. 1996;85:311–318. doi: 10.1016/s0092-8674(00)81110-1. [DOI] [PubMed] [Google Scholar]
- 5.Wicker L S, Todd J A, Peterson L B. Annu Rev Immunol. 1995;13:179–200. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- 6.Holmberg D, Lejon K, Penha-Gonçalves C, Cilio M C, Colucci F, Bergman M-L. Immunologist. 1996;4:128–130. [Google Scholar]
- 7.Garchon H J, Luan J J, Eloy L, Bedossa P, Bach J F. Eur J Immunol. 1994;24:380–384. doi: 10.1002/eji.1830240217. [DOI] [PubMed] [Google Scholar]
- 8.Leijon K, Hammarstrom B, Holmberg D. Int Immunol. 1994;6:339–345. doi: 10.1093/intimm/6.2.339. [DOI] [PubMed] [Google Scholar]
- 9.Colucci F, Cilio C M, Lejon K, Penha Gonçalves C, Bergman M-L, Holmberg D. J Autoimmunity. 1996;9:271–276. doi: 10.1006/jaut.1996.0034. [DOI] [PubMed] [Google Scholar]
- 10.Penha Gonçalves C, Leijon K, Persson L, Holmberg D. Genomics. 1995;28:398–404. doi: 10.1006/geno.1995.1167. [DOI] [PubMed] [Google Scholar]
- 11.Gavrieli Y, Sherman Y, Ben Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Love J M, Knight A M, McAleer M A, Todd J A. Nucleic Acids Res. 1990;18:4123–4130. doi: 10.1093/nar/18.14.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lander E S, Green P, Abrahamson J, Barlow A, Daly M J, Lincoln S E, Newburg L. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- 14.Paterson A H, Lander E S, Hewitt J D, Peterson S, Lincoln S E, Tanksley S D. Nature (London) 1988;335:721–726. doi: 10.1038/335721a0. [DOI] [PubMed] [Google Scholar]
- 15.Lander E S, Botstein D. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todd J A, Aitman T J, Cornall R J, Ghosh S, Hall J R, Hearne C M, Knight A M, Love J M, McAleer M A, Prins J B, Rodriques N, Lathrop H, Pressey A, DeLarato N H, Peterson L B, Wicker L S. Nature (London) 1991;351:542–547. doi: 10.1038/351542a0. [DOI] [PubMed] [Google Scholar]
- 17.Cornall R J, Prins J B, Todd J A, Pressey A, DeLarato N H, Wicker L S, Peterson L B. Nature (London) 1991;353:262–265. doi: 10.1038/353262a0. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, Palmer S M, Rodrigues N R, Cordell H J, Hearne C M, Cornall R J, Prins J B, McShane P, Lathrop G M, Peterson L B, Wicker L S, Todd J A. Nat Genet. 1993;4:404–409. doi: 10.1038/ng0893-404. [DOI] [PubMed] [Google Scholar]
- 19.Lander E, Kruglyak L. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 20.Prochazka M, Serreze D V, Frankel W N, Leiter E H. Diabetes. 1992;41:98–106. doi: 10.2337/diab.41.1.98. [DOI] [PubMed] [Google Scholar]
- 21.Serreze D V, Prochazka M, Reifsnyder P C, Bridgett M M, Leiter E H. J Exp Med. 1994;180:1553–1558. doi: 10.1084/jem.180.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harada M, Makino S. Diabetologia. 1984;27:604–606. doi: 10.1007/BF00276978. [DOI] [PubMed] [Google Scholar]
- 23.Linsley P S, Brady W, Grosmaire L, Aruffo A, Damle N K, Ledbetter J A. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walunas T L, Lenschow D J, Bakker C Y, Linsley P S, Freeman G J, Green J M, Thompson C B, Bluestone J A. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 25.Tivol E A, Borriello F, Schweitzer A N, Lynch W P, Bluestone J A, Sharpe A H. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 26.Waterhouse P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K P, Thompson C B, Griesser H, Mak T W. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 27.Harper K, Balzano C, Rouvier E, Mattei M G, Luciani M F, Golstein P. J Immunol. 1991;147:1037–1044. [PubMed] [Google Scholar]
- 28.Zipris D, Lazarus A H, Crow A R, Hadzija M, Delovitch T L. J Immunol. 1991;146:3763–3771. [PubMed] [Google Scholar]
- 29.Linsley P S, Greene J L, Tan P, Bradshaw J, Ledbetter J A, Anasetti C, Damle N K. J Exp Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindsten T, Lee K P, Harris E S, Petryniak B, Craighead N, Reynolds P J, Lombard D B, Freeman G J, Nadler L M, Gray G S, Thompson C B, June C H. J Immunol. 1993;151:3489–3499. [PubMed] [Google Scholar]
- 31.Lenschow D J, Herold K C, Rhee L, Patel B, Koons A, Qin H Y, Fuchs E, Singh B, Thompson C B, Bluestone J A. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]