Abstract
CKII (formerly known as casein kinase II) is a ubiquitously expressed enzyme that plays an important role in regulating cell growth and differentiation. The β subunit of CKII (CKIIβ) is not catalytic but forms heterotetramers with the catalytic subunit α to generate an α2β2 holoenzyme. In Xenopus oocytes, CKIIβ also associates with another serine/threonine kinase, Mos. As a key regulator of meiosis, Mos is necessary and sufficient to initiate oocyte maturation. We have previously shown that the binding of CKIIβ to Mos represses Mos-mediated mitogen-activated protein kinase (MAPK) activation and that the ectopic expression of CKIIβ inhibits progesterone-induced Xenopus oocyte maturation. We have now used an antisense oligonucleotide technique to reduce the endogenous CKIIβ protein level in Xenopus oocytes, and we find that oocytes with a reduced content of CKIIβ are more sensitive to low doses of progesterone and show accelerated MAPK activation and germinal vesicle breakdown. Furthermore, ectopic expression of a Mos-binding fragment of CKIIβ suppressed the effect of antisense oligonucleotide. These results suggest that the endogenous CKIIβ normally sets a threshold level for Mos protein, which must be exceeded for Mos to activate the MAPK signaling pathway and induce oocyte maturation.
CKII, formerly known as casein kinase II, is a serine/threonine kinase that is ubiquitously expressed in different cell types and organisms (1, 2). It appears to signal through a second messenger-independent pathway and functions as a key regulatory enzyme in cell growth and differentiation (2, 3). The enzyme is found in both the nucleus and cytoplasm with the majority found in the nucleus. Purified CKII holoenzyme is a heterotetramer with CKIIα or CKIIα′ (an isoform of α) as the catalytic subunit and CKIIβ as the noncatalytic subunit. The β subunit of CKII can interact with another β subunit and link two α subunits together to form an α2β2 heterotetramer (4). The functional role of the β subunit is not clear. Studies in Schizosaccharomyces pombe have revealed that overexpression of Ckb1 (gene encoding CKIIβ) induces multiple septation and inhibits cell growth and cytokinesis without significantly affecting CKII kinase activity (5). Disruption of the Ckb1+ gene in fission yeast results in a cold-sensitive phenotype and abnormal cell shape (5). In mammalian fibroblasts, microinjection of an anti-CKIIβ antibody inhibits the mitogen-stimulated G1 to S transition, suggesting that this enzyme might be important for the mitotic cell cycle (6).
In addition to CKIIα, CKIIβ binds to another serine/threonine kinase, Mos (7). Mos is specifically expressed in germ cells and is required for normal Xenopus oocyte maturation (8). Fully grown Xenopus oocytes (stage VI) are arrested at the prophase of meiosis I, and they contain maternal mos mRNA but little Mos protein (9). Progesterone secreted from the surrounding follicle cells releases oocytes from the prophase arrest. As a result, oocytes undergo a series of events called maturation: germinal vesicle breakdown (GVBD), extrusion of the first polar body, progression into meiosis II, and metaphase arrest as unfertilized eggs (10). Mos protein is synthesized in response to progesterone, reaches its peak shortly after GVBD, and is degraded upon fertilization (8). Despite its transient appearance, Mos protein is necessary and sufficient to initiate meiosis and is essential for the metaphase arrest in meiosis II (9, 11, 12). These biological functions of Mos can be explained by its ability to stimulate a mitogen-activated protein kinase (MAPK) by phosphorylating and activating a MAPK kinase, MKK (13, 14). Like Mos, constitutively active forms of MKK and MAPK are able to induce GVBD in Xenopus oocytes and cause metaphase arrest in Xenopus embryos (15–17). The initial activation of MAPK coincides with the activation of a maturation promoting factor (MPF), which serves as an M phase regulator and drives meiotic progression (18, 19). A positive feedback loop has been demonstrated that connects Mos protein synthesis, MAPK activation, and MPF activation (20–22).
The finding that endogenous CKIIβ binds to Mos in Xenopus egg extracts is intriguing because the binding of CKIIβ to Mos represses Mos-mediated MKK and MAPK activation in vitro (7). Ectopic expression of CKIIβ in Xenopus oocytes inhibits progesterone-induced maturation likely because it binds to Mos and inhibits Mos-dependent MAPK activation (7). However, it was not clear whether the function of endogenous CKIIβ was to restrict Mos activity. Here we report that the injection of CKIIβ antisense oligonucleotide reduced the endogenous CKIIβ protein level and stimulated Mos-dependent activation of MAPK, MPF, and GVBD in Xenopus oocytes treated with low concentrations of progesterone. We propose that the endogenous CKIIβ normally sets a threshold level for Mos protein, which must be exceeded for Mos to activate the MAPK signaling pathway and induce oocyte maturation.
MATERIALS AND METHODS
Oligonucleotides, RNA, and glutathione S-transferase (GST) Fusion Proteins.
The antisense and sense oligonucleotides were designed against the coding region of Xenopus CKIIβ cDNA (residues 175 to 182) (23). The sequence of the antisense oligonucleotide is 5′-GATTGGCAGGCCTCTTGGGCC-3′ and that of the sense oligonucleotide is 5′-GGCCCAAGAGGCCTGCCAATC-3′. The three phosphodiester links at the 3′ end of each oligonucleotide were replaced by phosphorothioate links (24). Myc-tagged CKIIβ (human) mRNA was in vitro transcribed using SP6 RNA polymerase (7). GST and GST-CKIIβ141–215 proteins were purified from E. coli as described (7).
Microinjection and GVBD Assays in Xenopus Oocytes.
Stage VI oocytes were isolated from female Xenopus ovaries using collagenase and cultured in L-15 medium (25). About 30 nl of 2 mg/ml antisense or sense oligonucleotide was microinjected into each oocyte followed by incubation at 18°C overnight before the addition of progesterone. In some experiments 1 mg/ml Myc-CKIIβ RNA was coinjected with the oligonucleotides. In the rescue experiment, 30–40 nl of 3 mg/ml GST or GST-CKIIβ141–215 protein was microinjected into each oocyte 12 h after the oligonucleotide injection. The injected oocytes were subsequently treated with progesterone. Twenty to thirty oocytes were used for each analysis. GVBD was scored 8–12 h after the addition of progesterone by the appearance of a white spot on the animal pole, and subsequently it was confirmed by dissection. Oocyte extracts were prepared as described (7).
Immunoblotting and Kinase Assays.
Anti-p42MAPK (Xenopus) antibody 1913.3 (1:10,000 dilution), anti-phospho-MAPK antibody (NEB, 1:1,000), anti-CKIIβ (human, 1:2,000) antibody, and anti-Mos (Xenopus K2, 1:1,000) antibodies were used respectively for immunoblotting. 9E10 hybridoma supernatant was used for immunoblotting Myc-tagged proteins (7). MAPK and MPF activities were assayed by the radiolabeled phosphate incorporation into myelin basic protein and histone H1, respectively. Extracts equivalent to half an oocyte were used for each kinase assay. The kinase reactions were performed in a 25-μl kinase buffer (10 mM MgCl2/10 mM Tris, pH 7.5/5 μM protein kinase A inhibitor/1 mM phenylmethysulfonyl fluoride/1 mM aprotinin/0.5 mM Na3VO4) containing 100 μM ATP (1,000 cpm/pmol) and 1.6 mg/ml myelin basic protein or histone H1 (Sigma). The reaction mix was incubated at 25°C for 15 min and resolved on SDS/PAGE.
RESULTS
Reduction of CKIIβ Protein Level in Xenopus Oocytes.
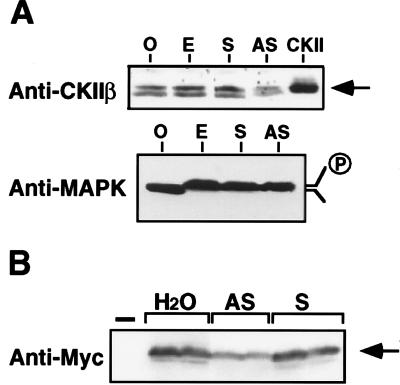
The protein level of CKIIβ was almost the same in extracts of Xenopus oocytes and eggs (Fig. 1A Upper). To reduce the amount of CKIIβ, we microinjected antisense oligonucleotides into stage VI oocytes. The 21-mer oligonucleotides were modified in linkages at the 3′ end to increase stability (24). A control, sense oligonucleotide had little effect on CKIIβ protein level in oocytes. By contrast, an antisense oligonucleotide significantly decreased the endogenous CIIβ protein by 30–40% (Fig. 1A Upper). Importantly, the antisense oligonucleotides did not affect the amounts of other proteins such as p42 MAPK (Fig. 1A Lower). We consistently observed two bands that ran at a position similar to the control CKII in CKIIβ immunoblot (Fig. 1A Upper). It was not clear whether they were due to protein degradation, or phosphorylation, or a yet to be identified CKIIβ gene product. The CKIIβ antisense oligonucleotide was not only effective on the endogenous CKIIβ protein but also on an ectopically expressed Myc-tagged CKIIβ. When Myc-CKIIβ RNA was coinjected with antisense CKIIβ oligonucleotide into oocytes, the accumulation of Myc-CKIIβ protein was reduced by 35–40% (Fig. 1B).
Figure 1.
The effect of antisense and sense oligonucleotides on CKIIβ protein level. Xenopus CKIIβ antisense and sense oligonucleotides (60–80 ng) were injected into stage VI Xenopus oocytes. O, uninjected oocyte; E, uninjected egg; S, sense oligonucleotide; AS, antisense oligonucleotide. (A) The injected oocytes were treated with 500 ng/ml progesterone for 8 h and analyzed by anti-CKIIβ or anti-MAPK immunoblotting. CKII prepared from recombinant baculovirus was used as a positive control. (B) Oocytes were coinjected with Myc-CKIIβ mRNA (1 mg/ml) mixed with H2O, antisense, or sense oligonucleotides. After incubation at room temperature for 4 h, 50 ng/ml progesterone was added and the incubation was continued for 12 h. Myc-CKIIβ protein was detected by immunoblotting duplicate samples. —, control oocytes lacking Myc-CKIIβ.
Stimulation of GVBD in Oocytes with a Reduced Level of CKIIβ Protein.
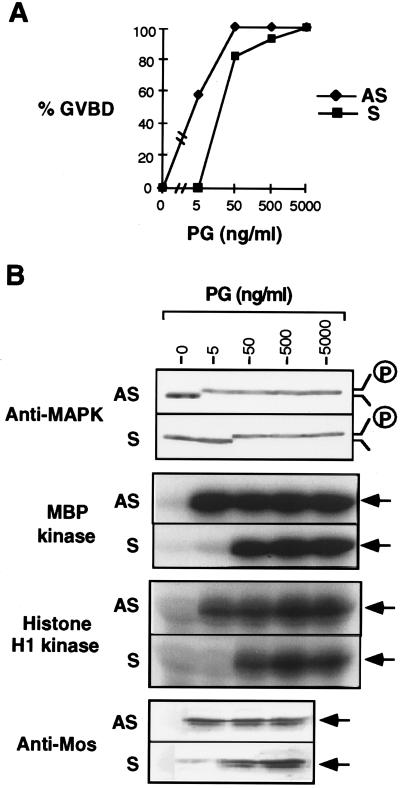
During meiotic maturation, one hallmark of a mature Xenopus oocyte is the formation of a white spot on the animal pole after GVBD. We studied the effects of CKIIβ sense and antisense oligonucleotides on oocyte maturation by scoring the number of oocytes that underwent GVBD in response to progesterone. Oocytes injected with the CKIIβ sense oligonucleotide or another antisense oligonucleotide that failed to reduce the amount of endogenous CKIIβ protein behaved the same as the control oocytes injected with water (data not shown). By contrast, oocytes injected with the effective CKIIβ antisense oligonucleotide had increased sensitivity to progesterone: 8- to 10-fold less progesterone was needed to induce 50% GVBD by 8 h compared with control oocytes (Fig. 2A). Strikingly, at a very low concentration of progesterone (5 ng/ml), 58% of oocytes injected with the antisense oligonucleotide showed GVBD and all of them had full MAPK activity, whereas GVBD did not occur and MAPK was inactive in the control oocytes (Fig. 2 A and B). MPF activity, as monitored by the histone H1 kinase assay, was also strongly increased by the antisense oligonucleotide at 5 ng/ml progesterone (Fig. 2B). Higher progesterone concentration could overcome the effect of the antisense oligonucleotide (Fig. 2, 50 ng/ml progesterone). This is likely due to an elevated Mos protein level in response to increased progesterone (Fig. 2B, Anti-Mos). Interestingly, much less Mos protein was accumulated in the control oocytes than in the antisense oligonucleotide injected oocytes, when 5 ng/ml progesterone was used (Fig. 2B). This is consistent with the hypothesis that there is positive feedback from activated MAPK and MPF to Mos protein synthesis or accumulation (20–22), although we cannot rule out the possibility that CKIIβ might reduce Mos protein stability.
Figure 2.
CKIIβ antisense oligonucleotide promoting oocyte maturation. The antisense and sense oligonucleotides were injected into oocytes followed by progesterone treatment at various concentration for 8 h. (A) The percentage of GVBD was scored. (B) Oocyte extracts were prepared from A and analyzed by anti-MAPK immunoblotting, myelin basic protein (MBP) kinase assay, histone H1 kinase assay, as well as anti-Mos immunoblotting.
The Acceleration of MAPK Activation and GVBD by CKIIβ Antisense Oligonucleotide.
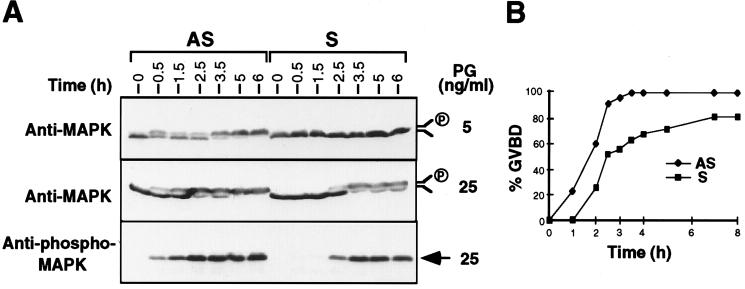
Since Mos-dependent MAPK activation is essential for GVBD (15, 26), we examined the time course of MAPK phosphorylation during meiotic maturation. In the presence of 5 ng/ml progesterone, MAPK bandshift was detected by 30 min and completed by 5 h in oocytes injected with the antisense oligonucleotide, whereas MAPK was completely inactive in the control oocytes even after 6 h (Fig. 3A Top). A higher concentration of progesterone (25 ng/ml) allowed MAPK phosphorylation to be first detected in control oocytes at 2.5 h. Under the same conditions, antisense oligonucleotide-injected oocytes showed detectable MAPK phosphorylation at the first 30 min, and the phosphorylation reached 70% by 2.5 h (Fig. 3A Middle). Bandshifts of MAPK correlated well with the phosphorylation of MAPK as assayed by phospho-MAPK immunoblotting (Fig. 3A Bottom). Furthermore, GVBD was accelerated in the CKIIβ antisense oligonucleotide-injected oocytes (Fig. 3B). After 2 h of progesterone treatment (50 ng/ml), more than 50% of oocytes exhibited GVBD, and by 3.5 h, 100% of oocytes completed GVBD. In contrast, only 26% of oocytes injected with sense oligonucleotide underwent GVBD within 3 h of progesterone stimulation, and even after 7 h, only 80% of them showed GVBD (Fig. 3B). These results suggest that lowering the concentration of CKIIβ not only sensitizes oocytes to low progesterone concentrations, but also accelerates the maturation process.
Figure 3.
CKIIβ antisense oligonucleotide accelerating MAPK phosphorylation and GVBD. A batch of oocytes was injected with oligonucleotides and treated with progesterone for various times. (A) Five oocytes were taken and frozen in liquid N2 for further analysis by anti-MAPK or anti-phospho-MAPK immunoblotting. (B) Progesterone (50 ng/ml) was added to the injected oocytes, and GVBD was scored at the indicated times.
The Rescue of CKIIβ Antisense Oligonucleotide Effects by GST-CKIIβ141–215.
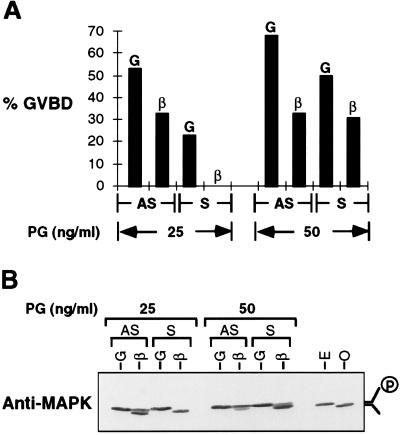
Ectopic expression of CKIIβ in Xenopus oocytes inhibits progesterone-induced oocyte maturation likely by the binding of CKIIβ to Mos and repression of Mos-mediated MAPK activation (7). A fragment of CKIIβ that does not bind to Mos, CKIIβ1–160, had no effect (7). If the effect of CKIIβ antisense oligonucleotide on oocyte maturation was due to the reduction in endogenous CKIIβ protein level, we reasoned that ectopic expression of CKIIβ should be able to rescue the phenotype. For this purpose, we first microinjected sense or antisense oligonucleotide and then injected a GST fusion protein containing the C-terminal 75 amino acids of CKIIβ, GST-CKIIβ141–215, which has a higher affinity than full-length GST-CKIIβ for binding to Mos (7). As a control for the fusion protein, some oocytes were injected with GST. When challenged with 25 ng/ml progesterone, 53% of oocytes injected with antisense oligonucleotide followed by GST underwent GVBD, compared with 33% of oocytes injected with antisense oligonucleotide followed by GST-CKIIβ141–215. By contrast, 23% of oocytes injected with sense oligonucleotide and GST underwent GVBD (Fig. 4A Left). When a higher concentration of progesterone (50 ng/ml) was used, more control oocytes completed GVBD and the antisense effect was less pronounced, but GST-CKIIβ141–215 again inhibited GVBD (Fig. 4A Right). MAPK phosphorylation in both antisense and sense oligonucleotide-injected oocytes was inhibited by GST-CKIIβ141–215 (Fig. 4B). Because the ectopic expression of CKIIβ fusion protein can rescue the phenotype of the CKIIβ antisense oligonucleotide, we conclude that the effects of the CKIIβ antisense oligonucleotide on progesterone-induced oocyte maturation is very likely due to the specific reduction in endogenous CKIIβ protein level.
Figure 4.
The rescue of CKIIβ antisense oligonucleotide effect by GST-CKIIβ141–215. Twelve hours after the oligonucleotide injection, oocytes were reinjected with GST or GST-CKIIβ141–215 protein followed by progesterone treatment. (A) The percentage of GVBD was scored 8 h later. (B) Oocyte extracts were prepared from A and analyzed by anti-MAPK immunoblotting. G, GST; β, GST-CKIIβ141–215.
DISCUSSION
CKIIβ is likely to be a physiological repressor of Mos based on the following evidence. First, CKIIβ binds to Mos and represses Mos-dependent MAPK phosphorylation both in vivo and in vitro (7). Second, ectopic expression of CKIIβ in Xenopus oocytes inhibits progesterone-induced, Mos-dependent MAPK phosphorylation and oocyte maturation (7). Third, endogenous CKIIβ is bound to Mos synthesized from stored mRNA during oocyte maturation (7). Moreover, as we report here, whereas Mos protein level increases during Xenopus oocyte maturation, the amount of CKIIβ protein remains almost the same. Reducing the endogenous CKIIβ protein level by using an antisense oligonucleotide increases the activities of MAPK and MPF, increases Mos protein levels, and promotes GVBD in oocytes treated with a low concentration of progesterone. At a higher progesterone concentration, reducing the content of CKIIβ accelerates MAPK phosphorylation and GVBD. These effects are specific and can be overruled by injecting oocytes with a C-terminal fragment of CKIIβ that binds to Mos and artificially restores the in vivo CKIIβ protein level.
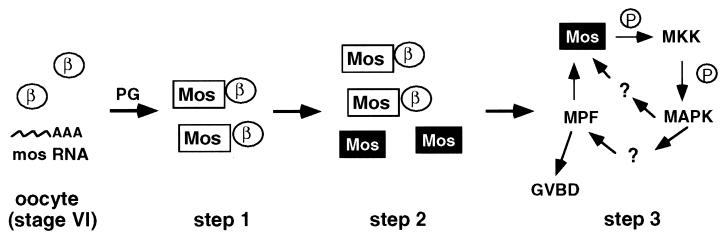
Based on these results, we propose a threshold model to explain the endogenous function of CKIIβ. As shown in Fig. 5, upon progesterone treatment of oocytes, the initially synthesized Mos protein associates with CKIIβ and remains inactive (step 1). As meiotic maturation progresses, the amount of Mos protein reaches and then exceeds the threshold level determined by CKIIβ (step 2). Excess Mos molecules phosphorylate and activate MKK, which in turn activates MAPK. At this time a positive feedback loop involving MAPK activation, MPF activation, and Mos protein synthesis is established (20–22), which leads to GVBD (step 3). Ectopic expression of CKIIβ increases the threshold level of Mos and inhibits progesterone-induced, Mos-dependent MAPK activation and GVBD. By contrast, a decrease in the threshold level by lowering the concentration of CKIIβ means that less Mos protein is needed to initiate meiosis and oocytes undergo GVBD at a low concentration of progesterone. Our results do not exclude the possibility that CKIIβ may also limit Mos protein accumulation by, for example, targeting Mos for degradation. As in the proposed model, stimulation of Mos degradation would also set a threshold level for the accumulation of Mos before GVBD can commence.
Figure 5.
A proposed model for the CKIIβ function during Xenopus oocyte maturation. The initiation of oocyte maturation can be divided into three steps. Progesterone stimulates Mos protein synthesis. The newly synthesized Mos protein (represented as a box) binds to CKIIβ (represented as a circle) and is inactive (step 1). As meiosis progresses, the amount of Mos protein reaches and exceeds that of CKIIβ (step 2). Free Mos molecules are active and can phosphorylate MKK. The activated MKK goes on to activate its substrate, MAPK. Activated MAPK is important for MPF activation and for further stimulating Mos protein synthesis. The activation of MPF leads to GVBD (step 3).
CKIIβ was originally identified as a noncatalytic subunit of CKII that binds to the catalytic subunit, CKIIα (27, 28). Mammalian CKIIβ has 215 residues in two separate, functional domains (3). The β-β homodimerization domain is localized between residues 20 and 145 and the α-β heterodimerization domain is localized in the C-terminal 45 amino acids (29, 30). The C terminus of CKIIβ also binds to Mos (7). In addition, CKIIβ contains clusters of acidic amino acids at the N terminus, basic amino acids at the C terminus, four cysteines that might form a zinc finger, and a cyclin destruction box-like domain that might target the protein for degradation (3). The stoichiometry of CKIIβ and CKIIα is not clear in vivo; however, in several cell lines CKIIβ is synthesized more rapidly than CKIIα and the excess is rapidly degraded (31).
Despite much effort, the regulation of CKII activity is still poorly understood. One observation suggests that CKIIβ may be regulated during mitosis and meiosis. Using an expression-cloning strategy in HeLa S3 cells, Matsumoto-Taniura et al. (32) have found that CKIIβ is recognized by MPM2, an antibody that stains some M phase phosphorylation sites. Therefore, CKIIβ is an MPM2 antigen that is phosphorylated at M phase of the cell cycle, possibly by MAPK or another unidentified MPM2 kinase.
The regulation of Mos is achieved partially through the increased translation when stored maternal mos mRNA becomes polyadenylated (33). In addition, serine-3 phosphorylation has been shown to be important for Mos protein stability and its interaction with the substrate MKK (25, 34). Then what is the benefit of a stoichiometric inhibitor, CKIIβ, as opposed to other restrictions on Mos quantity or phosphorylation? Biochemical analysis using Xenopus oocyte extracts has suggested that the three-kinase cascade of Mos, MKK, and MAPK allows a large change in signal output (MAPK activity) in response to a small change in signal input (Mos protein quantity) (35). This results from cooperativity and near-saturation “zero-order ultrasensitivity” (36, 37). A stoichiometric inhibitor further increases the sensitivity of a system (37). As the quantity of a kinase increases above the level of an inhibitor, kinase activity follows a sharp, hyperbolic stimulus–response curve to generate a switch-like output (37). This may explain the observation that MAPK activation, MPF activation, and GVBD all occur within a narrow time window starting several hours after progesterone stimulation. Together with the positive feedback loop linking Mos protein synthesis, MAPK activation, and MPF (20), this ensures the coordinated activation of MAPK and MPF that commits oocytes irreversibly to GVBD, chromosome condensation, suppression of DNA replication, entry into meiosis II, and the subsequent metaphase arrest.
Acknowledgments
We thank Noriyuki Sagata for the Mos antibody and Dongxia Li for the CKIIβ antibody and CKII protein. We are grateful to Yukiko Gotoh and James Ferrell for discussions and suggestions. This work was supported by National Service Award T32 GM07270 from the National Institute of General Medical Sciences to M.C. and by American Cancer Society Grants BE115 and CB201 to J.A.C.
ABBREVIATIONS
- GST
glutathione S-transferase
- MAPK
mitogen-activated protein kinase
- GVBD
germinal vesicle breakdown
- MPF
maturation promoting factor
References
- 1.Pinna L A. Biochim Biophys Acta. 1990;1054:267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- 2.Krebs E G, Eisenman R N, Kuenzel E A, Litchfield D W, Lozeman F J, Luscher B, Sommercorn J. Cold Spring Harbor Symp Quant Biol. 1988;53:77–84. doi: 10.1101/sqb.1988.053.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Allende J E, Allende C C. FASEB J. 1995;9:313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 4.Gietz R D, Graham K C, Litchfield D W. J Biol Chem. 1995;270:13017–13021. doi: 10.1074/jbc.270.22.13017. [DOI] [PubMed] [Google Scholar]
- 5.Roussou I, Draetta G. Mol Cell Biol. 1994;14:576–586. doi: 10.1128/mcb.14.1.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenz P, Pepperkok R, Ansorge W, Pyerin W. J Biol Chem. 1993;268:2733–2739. [PubMed] [Google Scholar]
- 7.Chen M, Li D, Krebs E G, Cooper J A. Mol Cell Biol. 1997;17:1904–1912. doi: 10.1128/mcb.17.4.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagata N. BioEssays. 1997;19:13–21. doi: 10.1002/bies.950190105. [DOI] [PubMed] [Google Scholar]
- 9.Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude G F. Nature (London) 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- 10.Masui Y, Clarke H J. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- 11.Sagata N, Watanabe N, Vande Woude G F, Ikawa Y. Nature (London) 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- 12.Sagata N, Daar I, Oskarsson M, Showalter S D, Vande Woude G F. Science. 1989;245:643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- 13.Posada J, Yew N, Ahn N G, Vande Woude G F, Cooper J A. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Resing K A, Mansour S J, Hermann A S, Johnson R S, Candia J M, Fukasawa K, Vande Woude G F, Ahn N G. Biochemistry. 1995;34:2610–2620. doi: 10.1021/bi00008a027. [DOI] [PubMed] [Google Scholar]
- 15.Gotoh Y, Masuyama N, Dell K, Shirakabe K, Nishida E. J Biol Chem. 1995;270:25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- 16.Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller J L. Science. 1993;262:1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- 17.Haccard O, Lewellyn A, Hartley R S, Erikson E, Maller J L. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- 18.Posada J, Sanghera J, Pelech S, Abersold R, Cooper J A. Mol Cell Biol. 1991;11:2517–2528. doi: 10.1128/mcb.11.5.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrell J E, Wu M, Gerhart J C, Martin G S. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotoh Y, Nishida E. Prog Cell Cycle Res. 1995;1:287–297. doi: 10.1007/978-1-4615-1809-9_23. [DOI] [PubMed] [Google Scholar]
- 21.Roy L M, Haccard O, Izumi T, Lattes B G, Lewellyn A L, Maller J L. Oncogene. 1996;12:2203–2211. [PubMed] [Google Scholar]
- 22.Matten W T, Copeland T D, Ahn N G, Vande Woude G F. Dev Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- 23.Jedlicki A, Hinrichs M V, Allende C C, Allende J E. FEBS Lett. 1992;297:280–284. doi: 10.1016/0014-5793(92)80556-v. [DOI] [PubMed] [Google Scholar]
- 24.Baker C, Holland D, Edge M, Colman A. Nucleic Acids Res. 1990;18:3537–3543. doi: 10.1093/nar/18.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Cooper J A. Mol Cell Biol. 1995;15:4727–4734. doi: 10.1128/mcb.15.9.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosako H, Gotoh Y, Nishida E. EMBO J. 1994;13:2131–2138. doi: 10.1002/j.1460-2075.1994.tb06489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meggio F, Pinna L A. Eur J Biochem. 1984;145:593–599. doi: 10.1111/j.1432-1033.1984.tb08598.x. [DOI] [PubMed] [Google Scholar]
- 28.Cochet C, Chambaz E M. J Biol Chem. 1983;258:1403–1406. [PubMed] [Google Scholar]
- 29.Kusk M, Bendixen C, Duno M, Westergaad O, Thomsen B. J Mol Biol. 1995;253:703–711. doi: 10.1006/jmbi.1995.0584. [DOI] [PubMed] [Google Scholar]
- 30.Marin O, Meggio F, Boldyreff B, Issinger O-G, Pinna L A. FEBS Lett. 1995;363:111–114. doi: 10.1016/0014-5793(95)00295-k. [DOI] [PubMed] [Google Scholar]
- 31.Luscher B, Litchfield D W. Eur J Biochem. 1994;220:521–526. doi: 10.1111/j.1432-1033.1994.tb18651.x. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto-Taniura N, Pirollet F, Monroe R, Gerace L, Westendorf J M. Mol Biol Cell. 1996;7:1455–1469. doi: 10.1091/mbc.7.9.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheets M D, Wu M, Wickens M. Nature (London) 1995;374:511–516. doi: 10.1038/374511a0. [DOI] [PubMed] [Google Scholar]
- 34.Nishizawa M, Okazaki K, Furuno N, Watanabe N, Sagata N. EMBO J. 1992;11:2433–2446. doi: 10.1002/j.1460-2075.1992.tb05308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C Y, Ferrell J E. Proc Natl Acad Sci USA. 1996;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldbeter A, Koshland D E., Jr Proc Natl Acad Sci USA. 1981;78:6840–6844. doi: 10.1073/pnas.78.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrell J E. Trends Biochem Sci. 1996;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]