Abstract
Epithelial defensins provide an active defense against the external microbial environment. We investigated the distribution and expression of this class of antimicrobial peptides in normal cattle and in animals in varying states of disease. β-defensin mRNA was found to be widely expressed in numerous exposed epithelia but was found at higher levels in tissues that are constantly exposed to and colonized by microorganisms. We observed induction in ileal mucosa during chronic infection with Mycobacterium paratuberculosis and in bronchial epithelium after acute infection with Pasteurella haemolytica. It has been proposed that expression of antimicrobial peptides is an integral component of the inflammatory response. The results reported here support this hypothesis and suggest that epithelial defensins provide a rapidly mobilized local defense against infectious organisms.
Antibiotic peptides are a key component of innate immunity. Their distribution throughout the animal kingdom is ubiquitous, reflecting the importance of these molecules in host defense (1). Among the best studied of the these peptides are the mammalian defensins. Defensins are cationic, cysteine-rich peptides that display broad spectrum antimicrobial activity. They are found in the granules of inflammatory cells, where they play a role in nonoxidative microbial killing (2). Their structure is characterized by a conserved cysteine motif that forms three disulfide linkages, imposing a characteristic β-sheet structure (3, 4). Associated with this structure is an amphiphilic charge distribution that enables the defensins to interact with and disrupt target cell membranes (5). They are believed to carry out this function by forming channels in the target membrane, leading to cell lysis and eventual death (6).
Although defensins were originally discovered in neutrophil and monocyte granules, they have more recently been identified as part of the antimicrobial barrier of mucosal surfaces. In mouse and human, defensin RNA has been localized to the Paneth cell of the small intestine, a specialized epithelial cell located at the crypt base (7, 8). The associated peptide has been localized within secretory granules of the Paneth cell and in the lumen of the small intestine, suggesting a role for defensins as part of the host gut defense (9). Defensins also have been found in bovine tracheal and lingual and human respiratory epithelium. Tracheal antimicrobial peptide (TAP), a β-defensin isolated from bovine tracheal mucosa, was localized to the ciliated columnar epithelial cells of the trachea and bronchi (10, 11). Lingual antimicrobial peptide (LAP), also a β-defensin, was found in bovine lingual mucosa, and demonstrated to be present in the stratified squamous epithelium of the tongue (12). The epithelial defensins are distinctly different gene products than the β-defensins produced in the bovine neutrophil (13). Most recently, human β-defensin-1 was demonstrated to be present in the epithelium of the trachea and bronchi, as well as submucosal gland and alveolar epithelium (14, 15).
There are considerable data indicating that epithelial defensins are up-regulated in response to infection. In cultured tracheal epithelial cells, TAP message is induced in response to bacterial lipopolysaccharide (LPS) exposure (16). This response was blocked by an antibody to CD14, suggesting that epithelial cells provide an active, inducible antimicrobial defense. In our previous work on bovine tongue, we found that LAP message was induced at sites of injury (12). This increase was contemporaneous with signs of acute and chronic inflammation, providing evidence that epithelial antibiotics are an integral component of the mammalian inflammatory response.
The goal of the current study was to investigate the expression of β-defensins in bovine epithelial tissue and determine how their expression is affected during acute and chronic infection. We initially looked at the distribution of β-defensin mRNA in various exposed epithelia and defined its cellular distribution at these sites. We then tested for inducibility of β-defensins during bacterial infection. Initially, we focused on bovine paratuberculosis, a chronic inflammatory disease of the intestinal tract. Subsequently, we determined the kinetics of induction in response to acute respiratory infection by Pasteurella haemolytica. Understanding the expression characteristics of β-defensins should help further our understanding of their regulation and ultimately define their biological role.
MATERIALS AND METHODS
In Situ Hybridization.
In situ hybridization was performed as described (12). The probe was prepared from a full length LAP cDNA clone. Transcripts were synthesized from the T7 and T3 RNA polymerase promoters of the pBluescript plasmid template using 35S-UTP. Tissue sections were hybridized with 5 × 106 cpm/ml of 35S antisense or sense probes for 16–18 h at 42°C. Slides were developed after 4–6 weeks and analyzed by bright and dark field microscopy.
Collection of Paratuberculosis-Infected Tissue.
Paratuberculosis-infected tissue was obtained from four cows testing positive for Mycobacterium paratuberculosis by culture of feces and samples of tissue from the ileocecal lymph node and ileum (17). Paratuberculosis-free tissue was collected from two cows obtained from herds certified to test negative for the disease.
Experimental Infection with P. haemolytica.
Two cows were inoculated intrabronchially with P. haemolytica as described (18). The right cranial lobe received P. haemolytica, and the left cranial lobe received pyrogen-free saline. Cows were killed 4 h postinoculation, and tissue was collected.
RESULTS
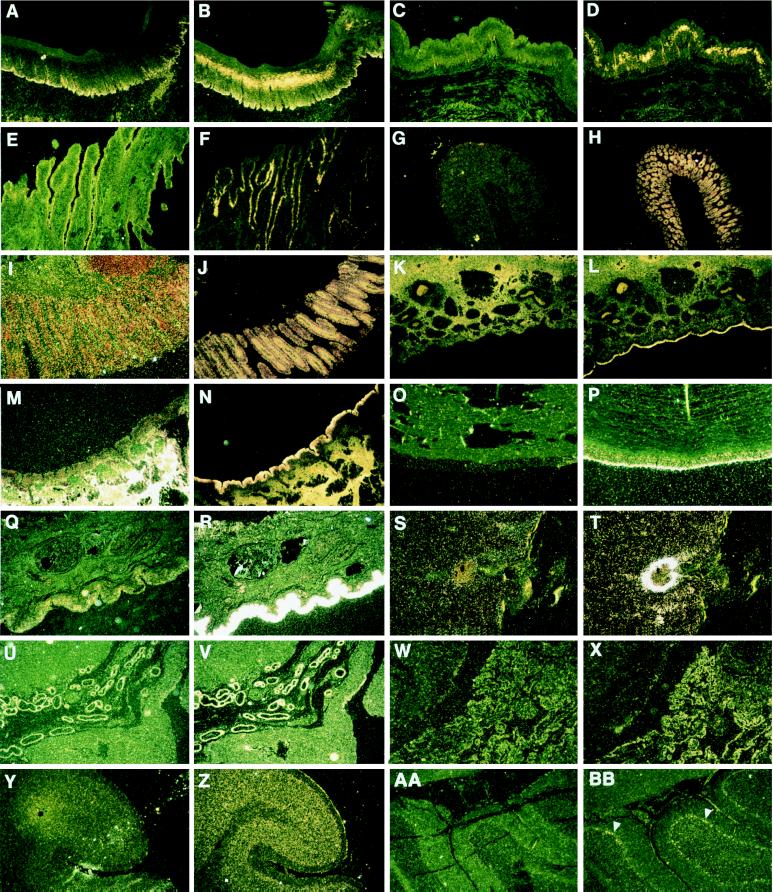
We examined the cellular distribution of bovine β-defensin mRNA in selected tissues by in situ hybridization (Fig. 1). Hybridization conditions and probes were chosen that detect mRNA encoding TAP, LAP, or a very closely related RNA sequence; the known myeloid β-defensins are not visualized under these conditions (12). Hybridization of LAP antisense probe to bovine epithelial tissue revealed abundant expression of LAP or closely related β-defensin species in palate, esophogus, stomach, colon, rectum, nares, trachea, conjunctiva, and skin. In tissues with stratified squamous epithelium such as esophagus and palate, β-defensin transcript was seen in the middle layers of the epithelium. β-defensin message also was present in the columnar epithelium of the intestinal crypts, in the meninges and choroid plexus of the brain, and in the placenta. No signal was visible when tissues were hybridized with LAP sense probe. Presence of β-defensin message in such diverse epithelial tissues strongly supports a general role for these molecules in host defense. In addition, RNA for LAP, or a close relative, was detected in cerebral cortex and cerebellar Purkinje cells. The reason for its expression at these sites is not clear.
Figure 1.
In situ hybridization of LAP probe to normal and abnormal bovine tissues. The first panel of each pair represents tissue probed with the sense negative control; the second is hybridized with the antisense LAP probe. Palate (A, B), esophagus (C, D), reticulum (stomach) (E, F), colon (G, H), rectum (I, J), nares (K, L), trachea (M, N), conjunctiva (O, P), inflamed skin (Q, R), dermal abscess (S, T), placenta (embryonic epithelium) (U, V), choroid plexus (W, X), cortex of brain (granular layer and meninges) (Y, Z), and Purkinje cells (arrows: AA, BB). (Magnification ×20.)
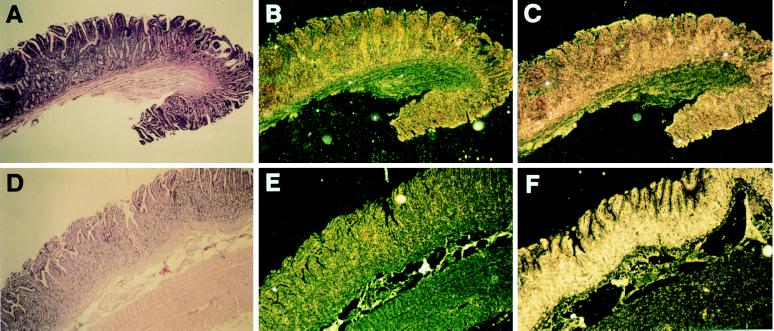
We next examined the effect of various disease states on β-defensin expression levels (Fig. 2). We studied expression in the intestinal epithelium of animals suffering from bovine paratuberculosis, a chronic inflammatory disease caused by infection of the ileum with M. paratuberculosis (19). In situ hybridization was performed on ileal tissue from four cows testing positive for paratuberculosis by stool and organ culture, and two cows from a paratuberculosis-free herd testing negative for the disease. Cows testing positive had clinical signs indicating chronic infection, including progressive weight loss, emaciation, and chronic diarrhea. Tissues derived from these animals also displayed characteristics indicating chronic infection such as the presence of inflammatory cells, mucosal thickening, and submucosal granulomas. Culture of samples of tissue from the ileum and adjacent lymph nodes yielded large numbers of M. paratuberculosis colonies, indicating an advanced stage of the disease. Hybridization of LAP antisense probe to ileal tissue from infected cows revealed intense signal in an area corresponding to the mucosal epithelium (Fig. 2D–F). In contrast, hybridization with tissue derived from disease-free cows (Fig. 2A–C) showed relatively little signal. These results indicate that β-defensins are induced in response to infection by M. paratuberculosis.
Figure 2.
Expression of β-defensins in ileum of paratuberculosis-infected cattle. (A–C) Serial sections of a portion of the ileum of a representative paratuberculosis-free animal. (D–F) Similar sections from a representative paratuberculosis-infected animal. (A and D) Hematoxylin and eosin-stained sections. (B and E) In situ hybridization with sense LAP probe. (C and F) In situ hybridization with antisense LAP probe. Uniform hybridization is seen overlying the lumenal epithelium. (Magnification ×30.)
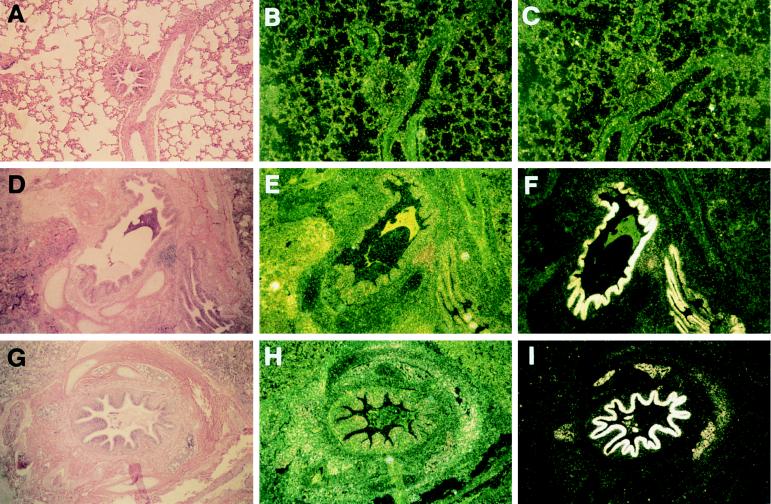
Finally, we investigated β-defensin expression in bovine lung after experimental acute infection with P. haemolytica (Fig. 3). A calf was infected with P. haemolytica by fiberoptic bronchoscopy in one lung and was similarly infused with sterile saline in the other lung. Lung tissue was collected 4 h postinfection (18). Sections were collected from the distal airway and were composed primarily of small bronchioles and alveoli. In situ hybridization of these samples with LAP antisense probe revealed extraordinarily high levels of β-defensin RNA in the bronchiolar epithelium (Fig. 3 D–F). The observed increase in signal was localized to the infected area and was especially evident in regions of tissue that displayed significant pathology. In contrast, tissue collected from the saline-inoculated lung appeared normal and yielded no signal (Fig. 3 A–C). We also investigated β-defensin expression in cattle with bovine leukocyte adhesion deficiency (20, 21). The results of these experiments are shown in Fig. 3, G–I. Tissue from a bovine leukocyte adhesion deficiency cow that was inoculated with P. haemolytica displayed an increase in β-defensin message in the bronchial epithelium and submucosal glands indistinguishable from that observed in normal tissue.
Figure 3.
Expression of β-defensins in respiratory tract of P. haemolytica-inoculated cattle. Lung biopsies were obtained 4 h after introduction of bacteria, and all tissue samples were handled and prepared identically. (A–C) Serial sections of saline-inoculated distal airway. (D–F) Serial sections of P. haemolytica-infected distal airway. (G–I) Serial sections of distal airway from a bovine leukocyte adhesion deficiency-affected animal. (A, D, and G) Hematoxylin and eosin-stained sections. (B, E, and H) In situ hybridization with sense LAP probe. C, F, and I) In situ hybridization with antisense LAP probe. Intense hybridization of the defensin probe is seen overlying the airway lumenal epithelium. (Magnification ×30.)
DISCUSSION
In this study, we have demonstrated widespread epithelial expression, in vivo, of β-defensins in the mammalian body plan. These results extend previous reports that described such expression in the bovine tongue and proximal airway. We have observed expression, localized to the epithelium by in situ hyridization, of β-defensins throughout the digestive tract from the palate to the rectum, throughout the upper airway, in the cells covering the cornea of the eye, and in the skin. In addition to these varied exposed epithelia, we also observed expresson in tissues such as the choroid plexus, the meninges, and the placenta, suggesting a role for β-defensins in host defense at sites other than primary interfaces with the microbial environment. The surprising result that these molecules are expressed throughout the cortex of the brain and in Purkinje cells, although consistent with a host defense function, suggests the possibility of other novel activities in these contexts.
In addition, we have shown that β-defensins are induced in states of disease. Examination of β-defensin expression in gastrointestinal epithelium revealed significantly higher message levels in infected vs. noninfected animals. For these studies, we focused on bovine paratuberculosis, a chronic inflammatory disease of the intestinal mucosa (for reviews, see refs. 19 and 22). Paratuberculosis is caused by M. paratuberculosis, an intracellular, acid-fast bacterium affecting mononuclear phagocytes. Infection leads to granuloma formation and a chronic enteritis and is manifested clinically with symptoms of diarrhea, malabsorption, decreased milk production, and eventual death. Numerous epithelial cells and giant cells, often filled with mycobacteria, are shed into the intestinal lumen, especially during later stages of the disease. Similar to LPS, components of the mycobacterial cell wall, such as lipoarabinomannan, might directly stimulate epithelial cells to produce β-defensins. Alternatively, ingested mycobacteria could indirectly influence epithelial β-defensin expression through the production of inflammatory cytokines. Mycobacteria and their cell wall components have been demonstrated to induce the production of cytokines and inflammatory mediators, including tumor necrosis factor α (TNF-α), in bovine monocytes (23, 24). TNF-α stimulates the production of LAP in cultured tracheal epithelial cells (25).
β-defensins were rapidly induced after acute respiratory infection by P. haemolytica. P. haemolytica is a Gram-negative bacterium responsible for pneumonic pasteurellosis in cattle (for review, see ref. 26). Respiratory manifestations of this disease include inflammation and multifocal necrosis along with the production of a suppurative exudate. Our studies showed a marked up-regulation of β-defensin message in respiratory epithelium 4 h after P. haemolytica aspiration. Because this increase correlated with tissue pathology, the induction could represent a direct response to infiltrating bacteria or an indirect response to inflammatory cells. Previous evidence has implicated both mechanisms. TAP RNA is induced in cultured tracheal epithelial cells after exposure to LPS. Increased signal was evident by 6 h, and a further increase was seen by 16 h (16). It is also similar to induction of TNF-α in cultured bovine alveolar macrophages after exposure to P. haemolytica LPS. Appearance of TNF-α RNA occurred within 0.5 h and reached maximal levels at 1–2 h; levels of secreted TNF-α peaked at 4 h (27). TNF is a known mediator of inflammation and is associated with induction of a wide variety of immune-related genes (28). Exposure of tracheal epithelial cells to TNF-α leads to a 3- to 4-fold increase in LAP and TAP RNA (25). It is conceivable that expression of β-defensins after infection by P. haemolytica is stimulated directly by LPS or indirectly by TNF.
Our results also demonstrate that the kinetics and apparent extent of epithelial β-defensin induction is not impaired in an individual with a specific genetic condition associated with impaired neutrophil function. For these studies, we focused on bovine leukocyte adhesion deficiency, a granulocytopathy syndrome of cattle characterized by frequent and severe bacterial infection (20). The results suggest that, in the cow, the epithelial defensin system must be complemented by functional neutrophils for optimal defense of mucosal barriers.
These results support a role for the β-defensins as important host defense effector molecules that are rapidly mobilized by the epithelium upon insult. This response is extraordinarily local in nature and may occur independently of circulating defensive cells in some contexts. Finally, our results have important implications for the diagnosis and treatment of disease. Antimicrobial peptides are induced during infection, so their presence might be used to diagnose and localize sites of infection. Additionally, regulating the expression of these molecules pharmacologically may be useful in the prevention and treatment of disease.
ABBREVIATIONS
- TAP
tracheal antimicrobial peptide
- LAP
lingual antimicrobial peptide
- LPS
lipopolysaccharide
- TNF
tumor necrosis factor
References
- 1.Zasloff M. Curr Opin Immunol. 1992;4:3–7. doi: 10.1016/0952-7915(92)90115-u. [DOI] [PubMed] [Google Scholar]
- 2.Lehrer R I, Lichtenstein A K, Ganz T. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 3.Hill C P, Yee J, Selsted M E, Eisenberg D. Science. 1991;251:1481–1485. doi: 10.1126/science.2006422. [DOI] [PubMed] [Google Scholar]
- 4.White S H, Wimley W C, Selsted M E. Curr Opin Struct Biol. 1995;5:521–527. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 5.Lehrer R I, Barton A, Daher K A, Harwig S S L, Ganz T, Selsted M E. J Clin Immunol. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kagan B L, Selsted M E, Ganz T, Lehrer R I. Proc Natl Acad Sci USA. 1990;87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouellette A J, Greco R M, James M, Frederick D, Naftilan J, Fallon J T. J Cell Biol. 1989;108:1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones D E, Bevins C L. J Biol Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 9.Selsted M E, Miller S I, Henschen A H, Ouellette A J. J Cell Biol. 1992;118:929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond G, Jones D E, Bevins C L. Proc Natl Acad Sci USA. 1993;90:4596–4600. doi: 10.1073/pnas.90.10.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schonwetter B S, Stolzenberg E D, Zasloff M. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 13.Selsted M E, Tang Y, Morris W L, McGuire P A, Novotny M J, Smith W, Henschen A H, Cullor J S. J Biol Chem. 1993;268:6641–6651. [PubMed] [Google Scholar]
- 14.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, Wang I, Lehrer R I. FEBS Lett. 1996;396:319–321. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 16.Diamond G, Russell J P, Bevins C L. Proc Natl Acad Sci USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitlock R H. In: Proceedings of the Third International Colloquium on Paratuberculosis. Chiodini R J, Kreeger J M, editors. Providence, RI: International Association for Paratuberculosis; 1991. pp. 1–11. [Google Scholar]
- 18.Ackermann M R, Kehrli M E, Jr, Brogden K A. Vet Pathol. 1996;33:639–646. doi: 10.1177/030098589603300602. [DOI] [PubMed] [Google Scholar]
- 19.Cocito C, Gilot P, Coene M, DeKesel M, Poupart P, Vannuffel P. Clin Microbiol Rev. 1994;7:328–345. doi: 10.1128/cmr.7.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kehrli M A, Jr, Ackermann M R, Schuster D E, van der Maaten M J, Schmalstieg F C, Anderson D C, Hughes B J. Am J Pathol. 1992;140:1489–1492. [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster D E, Kehrli M E, Ackermann M R, Gilbert R O. Proc Natl Acad Sci USA. 1992;89:9225–9229. doi: 10.1073/pnas.89.19.9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitlock R H, Buergelt C. Vet Clin North Am Food Anim Pract. 1996;12:345–356. doi: 10.1016/s0749-0720(15)30410-2. [DOI] [PubMed] [Google Scholar]
- 23.Adams J L, Czuprynski C J. Microb Pathog. 1994;16:401–411. doi: 10.1006/mpat.1994.1040. [DOI] [PubMed] [Google Scholar]
- 24.Adams J L, Czuprynski C J. Microb Pathog. 1995;19:19–29. doi: 10.1006/mpat.1995.0041. [DOI] [PubMed] [Google Scholar]
- 25.Russell J P, Diamond G, Tarver A P, Scanlin T F, Bevins C L. Infect Immun. 1996;64:1565–1568. doi: 10.1128/iai.64.5.1565-1568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank G H. In: Pasteurella and Pasteurellosis. Adlam C, Rutter J M, editors. New York: Academic; 1989. pp. 197–223. [Google Scholar]
- 27.Yoo H S, Maheswaran S K, Lin G, Townsend E L, Ames T R. Infect Immun. 1995;63:381–388. doi: 10.1128/iai.63.2.381-388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinarello C A. In: Inflammation: Basic Principles and Clinical Correlates. Gallin J I, Goldstein I M, Snyderman R, editors. New York: Raven; 1992. pp. 211–232. [Google Scholar]