Abstract
Alzheimer’s Disease (AD) is a neurodegenerative disorder characterized by the accumulation of dense plaques in the brain, resulting in progressive dementia. A major plaque component is the β-amyloid peptide, which is a cleavage product of the amyloid precursor protein (APP). Studies of dominant inheritable familial AD support the hypothesis that APP is critical for AD development. On the other hand, the pathogenesis of amyloid plaque deposition in AD is thought to be the result of age-related changes with unknown mechanisms. Here we show that the Caenorhabditis elegans homolog of APP, APP-like-1 (apl-1), functions with and is under the control of molecules regulating developmental progression. In C. elegans, the timing of cell fate determination is controlled by the heterochronic genes, including let-7 microRNAs. C. elegans apl-1 shows significant genetic interactions with let-7 family microRNAs and let-7-targeted heterochronic genes, hbl-1, lin-41 and lin-42. apl-1 expression is upregulated during the last larval stage in hypodermal seam cells which is transcriptionally regulated by hbl-1, lin-41 and lin-42. Moreover, the levels of the apl-1 transcription are modulated by the activity of let-7 family microRNAs. Our works places apl-1 in a developmental timing pathway and may provide new insights into the time-dependent progression of AD.
Keywords: Alzheimer’s disease, Amyloid precursor protein, apl-1, Caenorhabditis elegans, developmental timing, heterochronic genes, let-7, microRNA, molting
Introduction
Alzheimer’s Disease (AD) is a neurodegenerative disorder characterized by the accumulation of dense plaques and neurofibrillary tangles in the brain, resulting in progressive dementia. A major plaque component is the β-amyloid peptide (Aβ), which is a cleavage product of the amyloid precursor protein (APP). Studies of dominant inheritable familial AD support the hypothesis that APP is critical for AD development (Hardy and Selkoe, 2002). On the other hand, while the pathogenesis of amyloid plaque deposition in AD is thought to be the result of age-related changes (Goedert and Spillantini, 2006), chronological aspects of amyloid accumulation are largely unknown.
The best understood genes controlling timing during development are the heterochronic genes of the nematode Caenorhabditis elegans. Heterochronic genes control the appropriate temporal execution of stage-specific programs of cell division and differentiation through the four larval stages, L1–L4, of C. elegans (Ambros and Horvitz, 1984; Ambros, 2000; Banerjee and Slack, 2002; Rougvie, 2005). For example, one of the heterochronic genes, let-7, is expressed from the late larval stages and promotes the transition from L4 to adult (Reinhart et al., 2000). Let-7 encodes a microRNA (miRNA) and binds with imperfect complementarity to the 3′UTR of its targets, such as lin-41 and hbl-1, to prevent their translation (Slack et al., 2000; Abrahante et al., 2003; Lin et al., 2003; Rougvie, 2005).
Here we report that the C. elegans APP-like-1 (apl-1) gene functions with and is under the control of the heterochronic genes, including the miRNA let-7. apl-1 shows significant genetic interactions with let-7 family miRNAs and their developmental timing target genes including hbl-1, lin-41 and lin-42. Moreover, apl-1 expression shows a temporal change in hypodermal cells that is controlled by these developmental timing regulators. To our knowledge, this study provides the first indication that APP-related genes could be under the control of molecules regulating timed events.
Materials and Methods
Nematode strains and culture
C. elegans strains were grown under standard conditions. Strains were grown at 20°C for all experiments in this study. The mutant strains used were as follows: wild-type N2 Bristol, hbl-1(ve18) (Abrahante et al., 2003), hbl-1(mg285) (Lin et al., 2003), lin-29(n546) (Ambros and Horvitz, 1984), lin-41(ma104) (Slack et al., 2000), let-7(n2853) (Reinhart et al., 2000), mir-48(n4097);mir-84(n4037) (Abbott et al., 2005). YnIs79 is a strain overproducing apl-1 (Hornsten et al., 2007). To visualize seam cell junctions, we utilized an integrated array (syIs78) carrying ajm-1::gfp (MH27/GFP). To visualize both nuclei and junctions of seam cells, another integrated strain wIs79 containing both ajm-1::gfp and scm-1::gfp was used. MaIs105, a strain expressing the adult stage specific transgene col-19::gfp (Abrahante et al., 1998; Abbott et al., 2005), was kindly provided by C. Hammel and V. Ambros.
The apl-1::gfp::unc-54 reporter constructs were generated via single end overlap extension PCR, fusing the 7.0 kb apl-1 promoter sequence and gfp from vector pPD95.70 (A. Fire). We used animals carrying integrated apl-1::gfp constructs to observe apl-1 expression for all experiments described in this manuscript. The details of the constructions are described in Supplemental Materials.
RNAi experiments
Gene knockdown was achieved through RNAi by feeding as described (Timmons and Fire, 1998; Fraser et al., 2000; Kamath et al., 2003). Except for the experiment to obtain the data shown in Figs. 1E and 1F, synchronized populations of L1 larvae were fed bacteria expressing dsRNA corresponding to the target genes. In the experiment shown in Figs. 1E and 1F, synchronized L1 larvae of mir-48(n4097);mir-84(n4037) were grown on NGM plates containing E. coli OP50 bacterial lawns until 36-hours after hatching at 20°C. Then, these early L4 animals were put on RNAi plates. In mock RNAi experiments, bacteria carrying a control empty vector were used. RNAi vectors used in this study are described in Supplemental Materials.
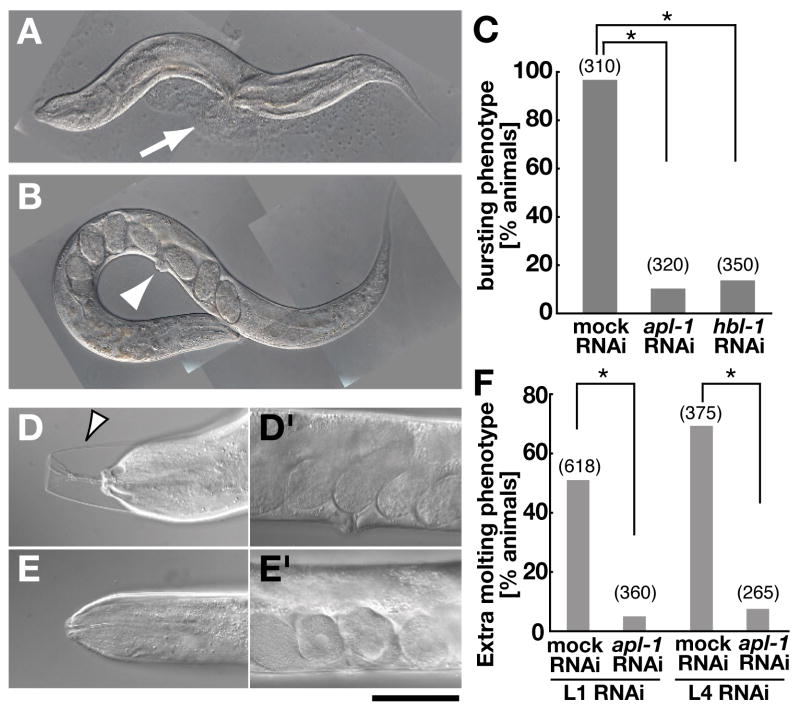
Fig. 1.
Loss of apl-1 function suppresses phenotypes of let-7 family member mutants. (A) let-7(n2853) mutants on mock RNAi died at the L4 molt by bursting though the vulva (arrow) at the restrictive temperature (20 C). (B) In contrast, let-7(n2853) mutants on apl-1 RNAi survived into adulthood, as indicated by the presence of oocytes and a functional vulva (arrowhead). (C) Percentage of let-7(n2853) animals with the bursting vulval phenotype. The vulval bursting phenotype of let-7(n2853) were scored at 6- to 12-hours post-L4 molt. Hbl-1(RNAi) was used as a positive control. (D, E) DIC images of mir-48(n4097);mir-84(n4037) with embryos visible inside the animals (D′, E′) to show that the animals are in the adult stage. (D) Unshed cuticle surrounding the anterior region of the animal (arrowhead) was observed on mock RNAi. (E) The cuticle phenotype of mir-48(n4097);mir-84(n4037) was suppressed treating with apl-1 RNAi from the L4 stage. (F) Percentage of mir-48(n4097);mir-84(n4037) exhibiting the extra molting phenotype in adults. Synchronized populations of L1 or L4 larvae were used (see Materials and Methods).
Observation of worms
Staging of L4 animals was by relative positions of their gonadal distal tip cells to the vulva: early, mid and late L4 animals were defined as animals showing 0–1/4, 1/4–1/2 and >1/2 gonadal turns, respectively. Microscopy images were acquired using a Axioplan II microscope (Carl Zeiss) equipped with a AxioCam MRm CCD camera (Carl Zeiss). We used Image J software (Abramoff et al., 2004) to quantify a mean level of GFP signals inside each nucleus of a seam cells. To observe GFP signals in seam cells of the apl-1::gfp transgenic lines, we observed all seam cells except the few cells surrounding the head and pharyngeal regions, because the non-seam expression of apl-1::gfp in these regions is strong (Supplemental Fig. S3) and interferes with evaluation of the seam cell GFP expression. Therefore, “GFP in all seam cells” described in Figs. 3 and 4 means the apl-1::gfp animals showing GFP expression in all seam cells except the cells in the head region.
Fig. 3.
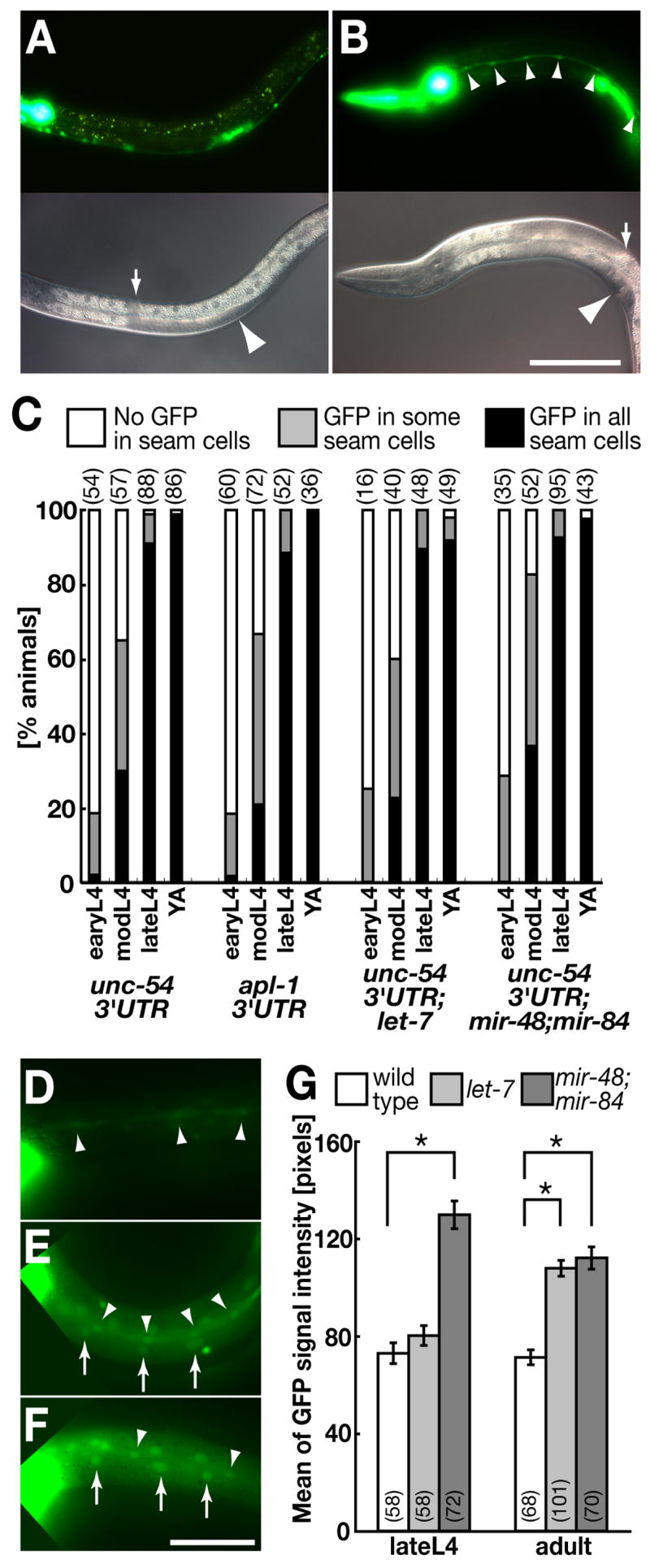
Level of apl-1 expression in seam cells is indirectly regulated by let-7 family microRNAs. (A, B) Strong GFP expression of apl-1::gfp::unc-54 was observed in seam cells in late (B) but not early (A) L4 stages. Neuronal, pharyngeal and uterine cells showed constant GFP expression throughout development. Images A and B were taken for the same exposure time and processed identically. Arrows and arrowheads in lower DIC panels point to distal gonad tips and vulvas, respectively. Scale bar: 100 μm for A and B. (C) Temporal expression profiles in seam cells of apl-1::gfp constructs fused with 3′UTRs of either unc-54 or apl-1 in wild type, let-7(n2853) or mir-48(n4097);mir-84(n4037) backgrounds. Staging of L4 animals were by relative positions of gonadal tips to vulva: early, mid and late L4 animals were defined as animals showing 0–1/4, 1/4–1/2 and >1/2 gonadal turns, respectively. Each number in parentheses represents n of each sample. (D–F) GFP expression of apl-1::gfp::unc-54 in wild type (D), let-7(n2873) (E) and mir-48(n4097);mir-84(n4037) (F) young adults. Arrows and arrowheads indicate GFP-expressing seam cells and hyp7 cells, respectively. Images D–F were taken for the same exposure time and processed identically. Scale bar: 50 μm for D–F. (G) Quantification of GFP levels (mean ± SEM) in seam cell nuclei of apl-1::gfp::unc-54 in seam cells in wild type and let-7 family mutants. *p < 0.001 Student’s t-test. Each number in parentheses indicates n of observed seam cells of each sample.
Fig. 4.
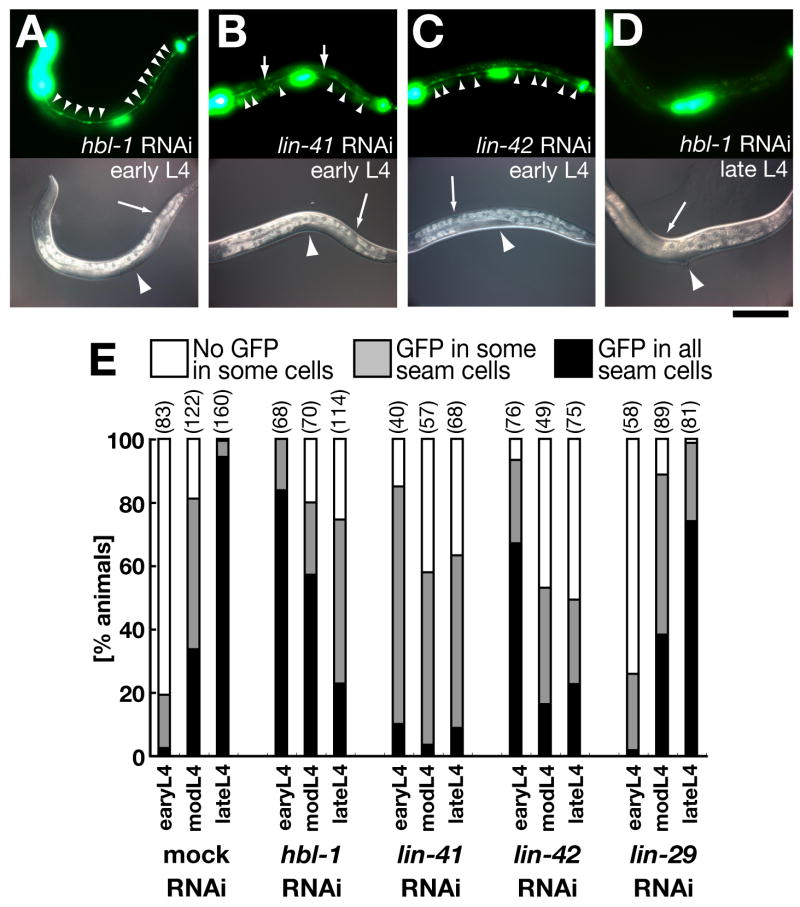
The dynamic expression of apl-1 in seam cells is regulated by heterochronic genes. (A–D) Animals carrying integrated apl-1::gfp::unc-54 constructs on RNAi plates of hbl-1 (A, D), lin-41 (B) and lin-42 (C). (A–C) In the early L4 stage, all of these RNAi animals showed precocious GFP expression in seam cells (arrowheads). Note that seam cells without GFP were frequently observed in lin-41(RNAi) animals (Upper panel in B, arrowheads; also see E). (D) In contrast to the early L4 stage, the GFP signals in the late L4 stage were significantly repressed in the hbl-1(RNAi) animals. Arrows and arrowheads in lower DIC panels point to gonadal distal tip cells and vulvas, respectively. Images A–D were taken for the same exposure time and processed identically. Scale bar: 100 μm. (E) Temporal expression profiles of the apl-1::gfp::unc-54 strain in the L4 stage with RNAi for several heterochronic genes. We used the same strain as animals shown in Fig. 3A and B. Note that lin-41(RNAi) showed precocious GFP expression in seam cells, but the fraction of animals showing GFP expression in all seam cells was reduced as compared with hbl-1(RNAi) and lin-42(RNAi). Each number in parentheses indicates n of observed animals of each sample.
Miscellaneous
Methods of reverse transcription-PCR and X-gal staining are described in Supplemental Materials.
Results
Loss of apl-1 function partially suppresses phenotypes associated with loss of let-7 family miRNAs
Loss-of-function (lf) mutants of let-7 reiterate earlier seam cell fates and die by bursting through the vulva at adults (Figs. 1A, C) (Reinhart et al., 2000). The let-7 mutant phenotype appears to be caused by over-expression of several target genes, as knocking down expression of let-7 targets partially suppresses the let-7 bursting phenotype (Reinhart et al., 2000; Slack et al., 2000; Abrahante et al., 2003; Lin et al., 2003; Grosshans et al., 2005; Lall et al., 2006). During the course of an RNA interference (RNAi)-based screen to identify suppressors of let-7, we found that the let-7 vulval bursting phenotype was suppressed by postembryonic reduction of the C. elegans APP-like gene, apl-1 (Daigle and Li, 1993) (Figs. 1B, C). This suppression is specific to apl-1, as RNAi constructs targeting different regions of the apl-1 gene suppressed let-7 bursting, but mock RNAi did not (Fig. 1C and Supplemental Fig. S1A). let-7(lf) causes retarded development of specialized hypodermal cells called seam cells, which results in additional seam cell divisions and a defect in secretion of adult-specific cuticular structures (alae) (Reinhart et al., 2000). As shown in Table 1, apl-1(RNAi) partially rescued these seam cell defects in young let-7(lf) adults.
Table 1.
apl-1 (RNAi) suppresses the retarded seam cell phenotype of let-7
| Number of seam cell nuclei per sidea
|
||
| let-7(n2853);wIs79; mock RNAi | 20.1 ± 3.7 (n = 67) | |
| let-7(n2853);wIs79; apl-1(RNAi) | 17.8 ± 2.6 (n = 50) * | |
| Adult alae formation per sideb
|
Any alae
|
>50% alae
|
| let-7(n2853); mock RNAi (n = 33) | 45% | 12% |
| let-7(n2853); apl-1(RNAi)(n = 35) | 95%** | 61% ** |
Number of seam cell nuclei and the presence of alae in let-7(n2853) were scored in young adult animals at a point where let-7(n2853) animals had not yet burst at 20 °C.
p < 0.001 as judged from Mann-Whitney’s U test;
p < 0.01 as judged from chi-square test.
This suppression was not just a consequence of not having a normal vulva through which to burst, as the vulval development in the apl-1(RNAi) animals appears normal (data not shown). Apl-1(RNAi) adult animals were small (Supplemental Fig. S2B), but the suppression of the let-7 bursting phenotype by apl-1(RNAi) was not caused by the smaller body size, as the let-7 bursting phenotype was not suppressed by sma-1(RNAi) or dpy-7(RNAi) (83%, n = 408, and 89%, n = 441, showing the bursting phenotype, respectively), which also caused smaller body shape (Johnstone et al., 1992; McKeown et al., 1998). In addition, we did not observe a significant suppression of the let-7 bursting phenotype by RNAi against sel-12, the C. elegans homolog of presenilin (Levitan and Greenwald, 1995), and feh-1, the C. elegans homolog of Fe65 (Zambrano et al., 2002) (92%, n = 312 and 94%, n = 253 showing the bursting phenotype, respectively). This demonstrates the specificity of the let-7 suppression by apl-1, and suggests that the suppression might not be mediated by the presenilin-dependent proteolytic processing of apl-1 or the Fe65-dependent intracellular regulation of apl-1.
It should be noted that postembryonic RNAi of apl-1 by feeding synchronized L1 wild-type animals exhibited a ~40% reduction of apl-1 mRNA (Supplemental Fig. S2A) and therefore created a partial loss of apl-1 function, whereas complete or strong loss of apl-1 function caused early larval lethality and a molting defect (Zambrano et al., 2002; Hornsten et al., 2007). Since the early lethality in strong apl-1 mutants hampers an examination of apl-1 function after the first molt, this partial loss of apl-1 function by RNAi proved to be crucial to identifying novel, non-lethal roles for apl-1.
Two other let-7-related miRNAs, mir-48 and mir-84, are similar in sequence (Lim et al., 2003). A mir-48(lf);mir-84(lf) mutant displays a penetrant extra molting phenotype that results in a double cuticle in the adult (Figs. 1D, F) (Abbott et al., 2005; Hayes et al., 2006). Apl-1 RNAi suppressed the molting phenotype in the mir-48(lf);mir-84(lf) mutant (Figs. 1E, F). Interestingly, apl-1(RNAi) suppressed the cuticle defect when applied in either the first (L1) or fourth (L4) larval stage (Fig. 1F). These results suggest that phenotypes caused by loss of let-7 family miRNAs are at least partially due to excessive activity of apl-1 in the late larval and/or adult stages.
apl-1 itself may not be a canonical heterochronic gene
While apl-1(RNAi) greatly suppressed the let-7 mutant phenotypes, apl-1(RNAi) animals alone exhibited no obvious heterochronic phenotype on alae formation (Supplemental Fig. S2C) and seam cell fusion (Supplemental Fig. S2D). We did not find any other major abnormality up to the L4 stage when the animals took on a small appearance (Supplemental Fig. S2B). In addition to the small body size of the apl-1(RNAi) adults described above, we observed that a small percentage of apl-1(RNAi) animals failed to shed their larval cuticle at the L4 molt (Fig. 2B and Supplemental Fig. S1B). In addition, an apl-1 overexpressor strain ynIs79 (Hornsten et al., 2007) did not show any heterochronic defect in alae formation either (0% of L4 animals showing any precocious alae, n = 37; 99% of adult animals showing complete alae, n = 65), suggesting that apl-1 itself should not be classified as a canonical heterochoronic gene.
Fig. 2.
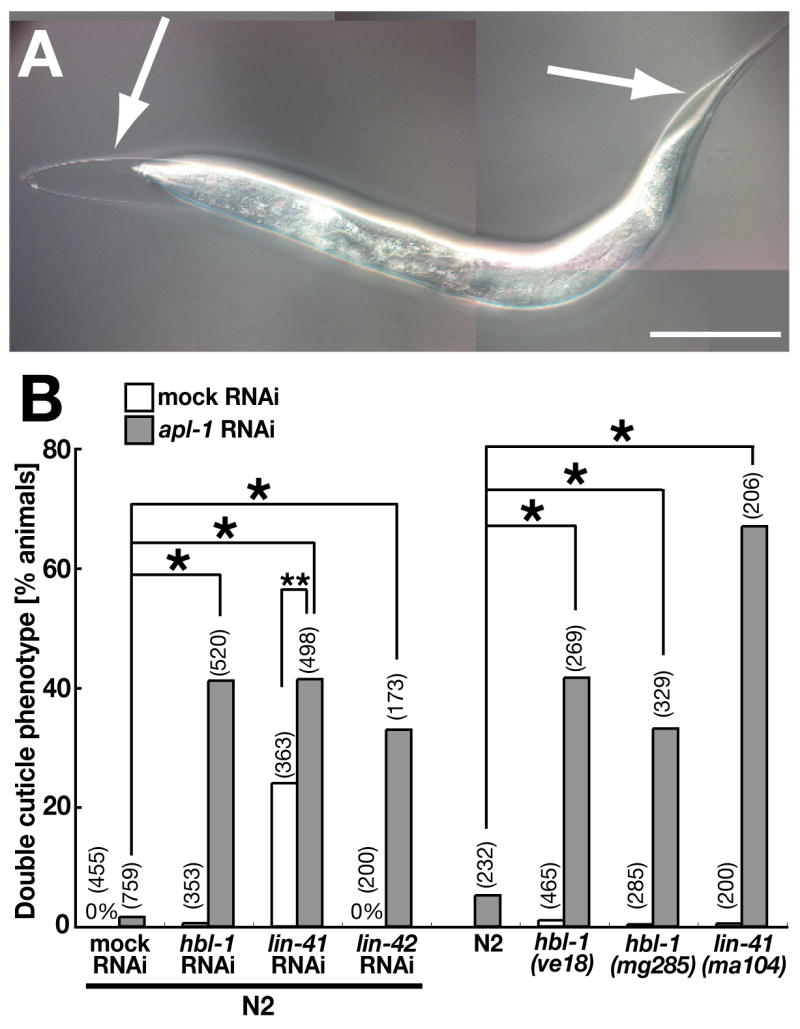
apl-1 genetically interacts with heterochronic genes downstream of let-7 in the regulation of molting. (A) N2 larvae were fed both apl-1 and hbl-1 RNAi bacteria from the L1 stage. Unshed cuticle was observed at the L4 molt (arrows). (B) Quantification of the molting defect of double mutants of apl-1 and other heterochronic mutants at the L4 molt. Synchronized L1 animals were used for the RNAi experiments. Data shown in the left half of the graph were obtained using two different RNAi clones. Each number in parentheses indicates n of observed animals in each sample. *p < 0.001 and **p < 0.01 chi-square test. Scale bar: 100 μm for A and B, 50 μm for D and E, and 117 μm for G.
apl-1 genetically interacts with hbl-1, lin-41 and lin-42, which are downstream of let-7
We examined whether apl-1 also acts with other heterochronic genes that are epistatic to let-7, including hbl-1, lin-41, and lin-42, which promote larval development and prevent precocious adult differentiation (Rougvie, 2005). Indeed, apl-1(RNAi);hbl-1(lf), apl-1(RNAi);lin-41(lf) and apl-l(RNAi);lin-42(lf) double mutants showed a growth defect during the L4 stage and typically could not shed their larval cuticle at the L4 molt (Figs. 2A, B, and Supplemental Fig. S1B), while each of the single mutants only rarely showed the phenotype (Fig. 2B and Supplemental Fig. S1B). Besides the synthetic effect of apl-1 and the heterochronic genes on molting, apl-1(lf) did not appear to modify the penetrance of precocious seam cell differentiation in these mutants (Supplemental Figs. S2D–F). This suggests that apl-1 acts with these heterochronic genes specifically in the regulation of molting at the larval-to-adult switch or that the partial loss of apl-1 function was not sufficient to overcome these cell fate defects.
In contrast, apl-1 did not genetically interact with lin-29, one known gene downstream of hbl-1, lin-41 and lin-42 in adult seam cell differentiation (Ambros and Horvitz, 1984; Ambros, 2000; Rougvie, 2005). Apl-1(RNAi) did not modify retarded alae formation of the lin-29 mutant (0% alae formation of lin-29 mutants on either mock RNAi (n = 23) or apl-1 RNAi (n = 27). The extra molting phenotype of lin-29 (Ambros and Horvitz, 1984) was also not affected by apl-1 RNAi (data not shown).
apl-1 expression is temporally regulated in seam cells
The spatial and temporal expression pattern of apl-1 was evaluated by following the expression of an apl-1 promoter-driven gfp construct fused with the heterologous unc-54 3′UTR (apl-1::gfp::unc-54). Apl-1 was expressed in several types of cells throughout development (Supplemental Figs. S3A, B), which is consistent with data from in situ RNA hybridization (Supplemental Figs. S3C–E) and a previously reported GFP-reporter assay with a shorter apl-1 promoter (Hornsten et al., 2007). Notably, we observed strong GFP expression in seam cells (Fig. 3B), whose development is well known to be regulated by the heterochronic genes let-7, hbl-1, lin-41 and lin-42 (Rougvie, 2005) and which have an essential role in the C. elegans molting process (Frand et al., 2005). Furthermore, apl-1 expression in seam cells was temporally regulated (Figs. 3A–C). The GFP signal in seam cells was not detected (or in rare cases, weakly detected) from L1 to early L4 stages (Figs. 3A, C), frequently detected from the mid L4 stage, and almost always detected with high-level expression at late L4 and adult stages (Figs. 3B, C). This temporal expression profile of apl-1 was observed in two independent integrated lines of apl-1::gfp::unc-54 (Supplemental Fig. S4). No temporal change of apl-1 expression was apparent in any other cells (data not shown).
apl-1 expression in seam cells is indirectly repressed by let-7 family miRNAs
One of the simplest interpretations of available genetic data is that the heterochronic genes that genetically interact with apl-1 regulate apl-1 expression in seam cells. We first examined whether apl-1 was a direct target of let-7 family miRNAs. Although weak potential binding sites for let-7 family miRNAs were predicted in the apl-1 3′UTR (Supplemental Fig. S5)(Enright et al., 2003; John et al., 2004; Lall et al., 2006), we did not observe any difference in the temporal expression profile between apl-1::gfp constructs fused with either the native apl-1 3′UTR or the unregulated unc-54 3′UTR in seam cells (Fig. 3C). Additionally, a lacZ reporter expressed in seam cells detected no significant temporal down-regulation through the apl-1 3′UTR, as 35% (n = 1,421) and 29% (n = 1,344) of animals showing X-gal staining at L3 animals and young adults carrying col-10::lacZ constructs, respectively. These results suggest that apl-1 is not likely to be a direct target of the let-7 family miRNAs in seam cells.
Rather, we found that apl-1 was transcriptionally up-regulated in hypodermal cells of let-7 family mutants. Apl-1::gfp::unc-54 GFP signals in seam cells, as well as other hypodermal cells (hyp7), were significantly elevated in the background of let-7 family mutants (Figs. 3E–G) as compared to a wild type background (Figs. 3D, G), whereas timing of expression was not altered (Fig. 3C). Specifically, the level of GFP signal was elevated in the adult stage in the let-7(lf) background, while mir-48(lf);mir-84(lf) showed an elevation both in the late L4 and adult stages (Fig. 3G). This trend is consistent with the difference in expression profiles between let-7 miRNA and miR-48; miR-84. Transcription of let-7 miRNA begins in the L3 stage and is progressively upregulated during the L4 stage, while miR-48 and miR-84 reach half-maximal expression at the L3 stage (Abbott et al., 2005; Esquela-Kerscher et al., 2005). The effect of the let-7 family miRNAs was specific for the apl-1 promoter, as a GFP signal from a seam cell marker promoter (scm-1::gfp) was not elevated in either let-7(lf) or mir-48(lf);mir-84(lf) backgrounds (data not shown). These results lead us to hypothesize that the apl-1 transcription in seam cells is regulated indirectly by let-7 family miRNAs, perhaps through downstream targets of the let-7 miRNAs.
apl-1 transcription is regulated by hbl-1, lin-41 and lin-42
The heterochronic regulators hbl-1, lin-41 and lin-42 are downstream of let-7 (Rougvie, 2005). We found that apl-1 expression was precociously observed at the L3 molt in hbl-1, lin-41 and lin-42 mutant seam cells (Figs. 4A–C, E), suggesting that these heterochronic genes normally repress the precocious expression of apl-1 during the L4 stage. In addition, hbl-1, lin-41 and lin-42 also play roles in temporally maintaining apl-1 expression later in development, as the number of animals showing correct seam cell expression in the late L4 stage was significantly reduced in hbl-1, lin-41 or lin-42 mutants (Figs. 4D, E; data not shown). These temporal expression changes of apl-1 by the heterochronic genes was observed in two independent integrated lines of apl-1::gfp::unc-54 (Supplemental Fig. S4C). By contrast, the temporal expression of apl-1 in seam cells was independent of lin-29 (Fig. 4E), which is downstream of hbl-1, lin-41 and lin-42 in adult seam cell differentiation (Ambros, 2000; Rougvie, 2005). These results show that developmental timing regulators hbl-1, lin-41 and lin-42 control apl-1 expression negatively in early larval stages and positively in the late L4 stage (Supplemental Fig. S6), consistent with the known, dual role of HBL-1 in seam cell development (Lin et al., 2003).
Discussion
The heterochronic pathway regulates the temporal expression of apl-1 in seam cells
Hornsten et al. recently reported that apl-1 plays an essential role in C. elegans early larval development, including molting and morphogenesis (Hornsten et al., 2007). Along with the early function of apl-1 in neuronal cells, our study shows a role for apl-1 in hypodermal cells in later development, namely the transition from L4 to adult stages (also see Supplemental Fig. S6). We demonstrate that the heterochronic pathway temporally regulates apl-1 expression in seam cells and propose that this modulates their developmental timing, although our data indicate that apl-1 itself may not be a canonical heterochronic gene.
It is noteworthy that apl-1 is the first identified gene that is transcriptionally regulated by let-7 targets, including hbl-1 and lin-42 that encode orthologs of Drosophila transcription factors Hunchback and Period, respectively. While lin-29 is a well known gene downstream of let-7 and the let-7 targets and plays an essential roles in promoting adult programs (Ambros, 2000; Rougvie, 2005), it is unlikely that lin-29 is directly controlled by the let-7 target transcription factors because the lin-29 transcript is detected from the L1 stage onwards (Rougvie and Ambros, 1995). Therefore, an elucidation of the molecular mechanisms of the apl-1 expression in seam cells provides a foothold to understand the transcriptional regulation of let-7-dependent terminal differentiation pathway in C. elegans.
Possible function of apl-1 in vivo
apl-1(RNAi) suppresses the extra cell division in seam cells and retarded alae formation in the let-7 adult. Therefore, the down-regulation of the apl-1 expreession is critical for promoting the adult program in seam cells. However, the actual physiological function of apl-1 for adult determination is not revealed from our study nor from previous studies (Zambrano et al., 2002; Hornsten et al., 2007). In mammals, it has been speculated that APP has role in cell proliferation and differentiation. For example, mammalian APP has been proposed to have a receptor-like function on the cell surface (Gralle and Ferreira, 2007). Indeed, some extracellular APP ligands, such as F-spondin and the low-density lipoprotein receptor-related protein, have been identified, _olstering the hypothesis that APP might function as a receptor (Schmitz et al., 2002; Gralle and Ferreira, 2007). An intracellular signaling pathway of APP has also been proposed, especially in axonal outgrowth and vesicle transport in neuronal cells (Gralle and Ferreira, 2007; Grimm et al., 2007). In addition to the receptor-like function, the secretory N-terminal ectodomain of APP (sAPP), which is the product cleaved by secretases, is also proposed to be involved in different physiological processes in mammals. For example, sAPP can promote cell proliferation in several types of cells including epithelial cells (Schmitz et al., 2002; Grimm et al., 2007), which is reminiscent of our genetic data showing that apl-1 is involved in let-7-dependent cell division in seam cells. Specifically, an extracellular domain of APL-1 is sufficient to rescue the apl-1 null mutant lethality (Hornsten et al., 2007). Therefore, it can be speculated that C. elegans APL-1 could also serve as a receptor receiving a ligand to regulate seam cell development and molting, or sAPL-1 could inhibit the proliferation and differentiation in seam cells. On the other hand, it is unlikely that seam cells produce the secretary APL-1 during development, as sel-12(RNAi) does not suppress the let-7 bursting phenotype and sel-12 expression of seam cells has not been reported. Further genetic characteristics of apl-1 and the biochemical properties of APL-1 proteins will need to be investigated.
Our data show that apl-1 has an important function in C. elegans molting. While a genome-wide RNAi-based screen has recently been performed to identify endocrine and enzymatic regulators of molting in C. elegans (Frand et al., 2005), apl-1 was not identified there. The reason might be because the apl-1(RNAi) itself does not create a strong molting phenotype, and the phenotype is only highlighted when apl-1(RNAi) is combined with heterochoronic mutations, such mir-48;mir-84 and hbl-1. The let-7 mutants and some double mutants of let-7 family miRNAs are well known to show an extra molting phenotype in the adult stage (Reinhart et al., 2000; Abbott et al., 2005), indicating that let-7 family miRNAs repress some of the molting machinery in adult. Specifically a recent paper shows that let-7 family miRNAs repress nhr-23 and nhr-25, both of which encode nuclear hormone receptors essential for C. elegans molting (Hayes et al., 2006). Apl-1 is an additional example of let-7-dependent repression in adults required for the molting process. It would be intriguing to examine molecular relationships among apl-1 and other identified molting genes.
Implication for Alzheimer’s disease
Our study provides the first evidence that APP-related genes can be under the transcriptional control of molecules regulating developmentally timed events. Besides the importance of the proteolytic regulation of APP to yield Aβ, it has been speculated that regulation of APP expression is associated with the development of AD, specifically supported by recent evidence that APP locus duplication is found in families with inheritable familial AD (Rovelet-Lecrux et al., 2006). In conjunction with the fact that heterochronic genes are involved in the aging process (Boehm and Slack, 2005) and the strong conservation of many C. elegans heterochronic genes in vertebrates (Rougvie, 2005), this study may provide new insights into the time-dependent progression of AD.
Supplementary Material
Acknowledgments
We thank Yuji Kohara and his colleagues for permitting us to include images from their database; Victor Ambros, Diya Banerjee, Andrew Fire, Christopher Hammel and Caenorhabditis Genetics Center for stocks; and Michelle Boehm and Lena J. Chin for critical reading of this manuscript. R.N. is supported by a fellowship from the Human Frontier Science Program. C.L. was supported by a grant from the Alzheimer’s Association. F.J.S. was supported by grants from the Ellison Medical Foundation and NIH (GM64701).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott AL, Alvarez-Saavedra E, Miska EA, Lau NC, Bartel DP, Horvitz HR, Ambros V. The let-7 MicroRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Dev Cell. 2005;9:403–414. doi: 10.1016/j.devcel.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahante JE, Daul AL, Li M, Volk ML, Tennessen JM, Miller EA, Rougvie AE. The Caenorhabditis elegans hunchback-like gene lin-57/hbl-1 controls developmental time and is regulated by microRNAs. Dev Cell. 2003;4:625–637. doi: 10.1016/s1534-5807(03)00127-8. [DOI] [PubMed] [Google Scholar]
- Abrahante JE, Miller EA, Rougvie AE. Identification of heterochronic mutants in Caenorhabditis elegans. Temporal misexpression of a collagen:green fluorescent protein fusion gene. Genetics. 1998;149:1335–1351. doi: 10.1093/genetics/149.3.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Ambros V. Control of developmental timing in Caenorhabditis elegans. Curr Opin Genet Dev. 2000;10:428–433. doi: 10.1016/s0959-437x(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Slack F. Control of developmental timing by small temporal RNAs: a paradigm for RNA-mediated regulation of gene expression. Bioessays. 2002;24:119–129. doi: 10.1002/bies.10046. [DOI] [PubMed] [Google Scholar]
- Boehm M, Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005;310:1954–1957. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- Daigle I, Li C. apl-1, a Caenorhabditis elegans gene encoding a protein related to the human beta-amyloid protein precursor. Proc Natl Acad Sci USA. 1993;90:12045–12049. doi: 10.1073/pnas.90.24.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Johnson SM, Bai L, Saito K, Partridge J, Reinert KL, Slack FJ. Post-embryonic expression of C. elegans microRNAs belonging to the lin-4 and let-7 families in the hypodermis and the reproductive system. Dev Dyn. 2005;234:868–877. doi: 10.1002/dvdy.20572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand AR, Russel S, Ruvkun G. Functional genomic analysis of C. elegans molting. PLoS Biol. 2005;3:e312. doi: 10.1371/journal.pbio.0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Gralle M, Ferreira ST. Structure and functions of the human amyloid precursor protein: the whole is more than the sum of its parts. Prog Neurobiol. 2007;82:11–32. doi: 10.1016/j.pneurobio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Hartmann T. Amyloid beta as a regulator of lipid homeostasis. Trends Mol Med. 2007;13:337–344. doi: 10.1016/j.molmed.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Grosshans H, Johnson T, Reinert KL, Gerstein M, Slack FJ. The temporal patterning microRNA let-7 regulates several transcription factors at the larval to adult transition in C. elegans. Dev Cell. 2005;8:321–330. doi: 10.1016/j.devcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hayes GD, Frand AR, Ruvkun G. The mir-84 and let-7 paralogous microRNA genes of Caenorhabditis elegans direct the cessation of molting via the conserved nuclear hormone receptors NHR-23 and NHR-25. Development. 2006;133:4631–4641. doi: 10.1242/dev.02655. [DOI] [PubMed] [Google Scholar]
- Hornsten A, Lieberthal J, Fadia S, Malins R, Ha L, Xu X, Daigle I, Markowitz M, O’Connor G, Plasterk R, Li C. APL-1, a Caenorhabditis elegans protein related to the human beta-amyloid precursor protein, is essential for viability. Proc Natl Acad Sci USA. 2007;104:1971–1976. doi: 10.1073/pnas.0603997104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone IL, Shafi Y, Barry JD. Molecular analysis of mutations in the Caenorhabditis elegans collagen gene dpy-7. EMBO J. 1992;11:3857–3863. doi: 10.1002/j.1460-2075.1992.tb05478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Lall S, Grun D, Krek A, Chen K, Wang YL, Dewey CN, Sood P, Colombo T, Bray N, Macmenamin P, Kao HL, Gunsalus KC, Pachter L, Piano F, Rajewsky N. A genome-wide map of conserved microRNA targets in C. elegans. Curr Biol. 2006;16:460–471. doi: 10.1016/j.cub.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Levitan D, Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer’s disease gene. Nature. 1995;377:351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Weinstein EG, Abdelhakim A, Yekta S, Rhoades MW, Burge CB, Bartel DP. The microRNAs of Caenorhabditis elegans. Genes Dev. 2003;17:991–1008. doi: 10.1101/gad.1074403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Johnson SM, Abraham M, Vella MC, Pasquinelli A, Gamberi C, Gottlieb E, Slack FJ. The C. elegans hunchback homolog, hbl-1, controls temporal patterning and is a probable microRNA target. Dev Cell. 2003;4:639–650. doi: 10.1016/s1534-5807(03)00124-2. [DOI] [PubMed] [Google Scholar]
- McKeown C, Praitis V, Austin J. sma-1 encodes a betaH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development. 1998;125:2087–2098. doi: 10.1242/dev.125.11.2087. [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Rougvie AE. Intrinsic and extrinsic regulators of developmental timing: from miRNAs to nutritional cues. Development. 2005;132:3787–3798. doi: 10.1242/dev.01972. [DOI] [PubMed] [Google Scholar]
- Rougviea E, Ambros V. The heterochronic gene lin-29 encodes a zinc finger protein that controls a terminal differentiation event in Caenorhabditis elegans. Development. 1995;121:2491–2500. doi: 10.1242/dev.121.8.2491. [DOI] [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38:24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Tikkanen R, Kirfel G, Herzog V. The biological role of the Alzheimer amyloid precursor protein in epithelial cells. Histochem Cell Biol. 2002;117:171–180. doi: 10.1007/s00418-001-0351-5. [DOI] [PubMed] [Google Scholar]
- Slack FJ, Basson M, Liu Z, Ambros V, Horvitz HR, Ruvkun G. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol Cell. 2000;5:659–669. doi: 10.1016/s1097-2765(00)80245-2. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Zambrano N, Bimonte M, Arbucci S, Gianni D, Russo T, Bazzicalupo P. feh-1 and apl-1, the Caenorhabditis elegans orthologues of mammalian Fe65 and beta-amyloid precursor protein genes, are involved in the same pathway that controls nematode pharyngeal pumping. J Cell Sci. 2002;115:1411–1422. doi: 10.1242/jcs.115.7.1411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.