Abstract
Two mouse monoclonal anti-anti-idiotopic antibodies (anti-anti-Id, Ab3), AF14 and AF52, were prepared by immunizing BALB/c mice with rabbit polyclonal anti-idiotypic antibodies (anti-Id, Ab2) raised against antibody D1.3 (Ab1) specific for the antigen hen egg lysozyme. AF14 and AF52 react with an “internal image” monoclonal mouse anti-Id antibody E5.2 (Ab2), previously raised against D1.3, with affinity constants (1.0 × 109 M−1 and 2.4 × 107 M−1, respectively) usually observed in secondary responses against protein antigens. They also react with the antigen but with lower affinity (1.8 × 106 M−1 and 3.8 × 106 M−1). This pattern of affinities for the anti-Id and for the antigen also was displayed by the sera of the immunized mice. The amino acid sequences of AF14 and AF52 are very close to that of D1.3. In particular, the amino acid side chains that contribute to contacts with both antigen and anti-Id are largely conserved in AF14 and AF52 compared with D1.3. Therapeutic immunizations against different pathogenic antigens using anti-Id antibodies have been proposed. Our experiments show that a response to an anti-Id immunogen elicits anti-anti-Id antibodies that are optimized for binding the anti-Id antibodies rather than the antigen.
Keywords: antigen mimicry, three-dimensional structure
The complementarity determining regions (CDR) of Ab display great structural diversity and constitute a vast array of potential antigenic determinants. Under appropriate experimental conditions, these determinants give rise to immune responses and are called idiotypic (1, 2). Idiotypes of Ab are the sum of idiotopes (Id) or antigenic determinants characterized experimentally by the reaction of an anti-idiotopic (anti-Id) antibody (Ab2) with the Id (Ab1). In most cases, idiotypes have been shown to be associated, partially or entirely, with the CDR of Ab molecules (3).
The observation that external antigens and anti-Id Ab can competitively bind to specific Ab has led to proposals that anti-Id Ab may carry an “internal image” (2) of the external antigen. Functional mimicry of ligands of biological receptors by anti-Id Ab has been described in several systems (4). The potential for mimicking external antigens has led to proposals (5, 6) to use anti-idiotypic Ab as surrogate antigens. Recent reviews (7–9) have discussed this topic from a structural viewpoint. Indeed, anti-Id Ab have been used in exploring therapeutic immunizations against different pathogenic antigens.
A report from this laboratory compared the three-dimensional structures of the complexes between an Ab1-antigen and an Ab1-anti-Id and provided a concrete example of how an anti-Id Ab can structurally mimic an external antigen (10, 11). In this system, the Ab1 was the mAb D1.3 specific for the antigen, hen egg lysozyme (HEL). The three-dimensional crystal structure of the complex between the Fv fragment of D1.3 with HEL has been determined at the resolution of 1.8 Å (12). Subsequently, the three-dimensional structure of a complex between the Fv from D1.3 and the anti-Id Fv from mAb E5.2 (the Ab2; BALB/c IgG1, κ) was determined (10, 11) at the nominal resolution of 1.9 Å. With these high resolution structures, it was possible to assess antigen mimicry by the anti-Id. In this system, the affinity of the anti-Id for the Ab1 (1.4 × 109 M−1) was close to that of the Ab1 for HEL (2.7 × 108 M−1).
A comparison of the three-dimensional structures of the two complexes showed that, of the 18 D1.3 residues that make chemical contacts with the anti-Id and the 17 that contact the antigen, 13 contact both. These 13 residues of D1.3 contribute 75% (687 Å2) of the contacting area with the anti-Id and 87% (675 Å2) of the contacting area with the antigen. Six of the 12 interface hydrogen bonds in the D1.3–antigen complex are superimposable with hydrogen bonds in the D1.3–anti-Id complex. Furthermore, the positions of the atoms by which the antigen contacts D1.3 (Ab1) are close to those of the anti-Id that contact D1.3. It is by this bonding pattern that it can be said that the anti-Id (E5.2) mimics the antigen (HEL) although it does not provide an exact “topological image” of the antigen (10, 11).
The results outlined in the preceding paragraphs were obtained by a purely structural approach. To test how well the anti-Id E5.2 mimics the antigen (HEL) physiologically, mice were immunized with E5.2. In the immune sera of some mice (BALB/c and C57BL), anti-HEL Ab were detected. By this test, the anti-Id (E5.2) behaved immunologically like a typical “internal image” Ab, validating the observations made on its structural mimicking of the antigen, HEL (10).
We present results of a study of an idiotypic chain involving D1.3, E5.2, and two newly produced anti-anti-Id mAbs, AF14 and AF52. These anti-anti-Id mAbs were obtained using rabbit polyclonal Ab against the Ab1 D1.3 (Fig. 1). AF14 and AF52 mimic the Ab1 D1.3 in their ability to bind both the antigen and the anti-Id E5.2. To understand how well anti-Id Ab can act as antigen surrogates, we analyze those binding reactions and present structural data on AF14 and AF52.
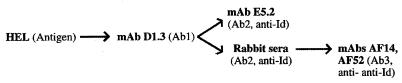
Figure 1.
Generation of anti-HEL, anti-Id, and anti-anti-Id Ab. Arrows indicate immunizations. Immunization with HEL generated mAb D1.3 (Ab1); immunization with the Ab1 D1.3 generated the mAb E5.2 (Ab2, anti-Id), and, in rabbits, the polyclonal Ab2, anti-Id; immunization with the rabbit anti-Id generated the anti-anti-Id mAbs AF14 and AF52 (Ab3). mAb (D1.3, E5.2, AF14, and AF52) were raised in BALB/c mice. Anti-Id sera were raised in New Zealand Black rabbits (see Materials and Methods). AF14 and AF52 mimic D1.3 in their capacity to react with the antigen HEL and with the anti-Id E5.2. mAb E5.2 (anti-Id, Ab2) mimics the antigen HEL as described (10, 11).
MATERIALS AND METHODS
Preparation of Polyclonal Anti-Id Ab.
Two New Zealand Black rabbits were immunized with purified monoclonal D1.3 Ab (IgG1, κ). Four inoculations at 20-day intervals were made, one using complete Freund’s adjuvant and three additional ones with incomplete Freund’s adjuvant. Sera from the two animals were pooled and precipitated with ammonium sulfate (50% saturation). The IgG fraction was further purified by chromatography in a DEAE cellulose column followed by affinity chromatography purification using IgG D1.3 conjugated to a Sepharose column. The purified Ab were extensively absorbed with preimmune mouse IgG coupled to agarose (Sigma) to eliminate anti-isotypic and anti-allotypic Ab. These polyclonal anti-Id Ab accounted for 25% of the mass of the total anti-D1.3 rabbit Ab. They reacted with IgG D1.3 as monitored by an ELISA and did not show reactivity against several unrelated IgG1,κ Ab.
Preparation of Anti-Anti-Id mAbs.
Six- or 7-week-old BALB/c mice were immunized i.p. (four times, once every 20 days) with 50 μg of the purified polyclonal rabbit anti-Id Ab using aluminum hydroxyde as adjuvant. The reactivity of the mice sera against Fab E5.2 and HEL was tested (Fig. 2) by ELISA as described (10). After a final boost with soluble polyclonal rabbit IgG, mice with the strongest anti-HEL response were killed and their spleens were used to obtain hybridomas as described (13). Two clones secreting IgG1,κ mAbs (AF14 and AF52) thus were obtained in two independent fusions; both reacted with E5.2 and HEL.
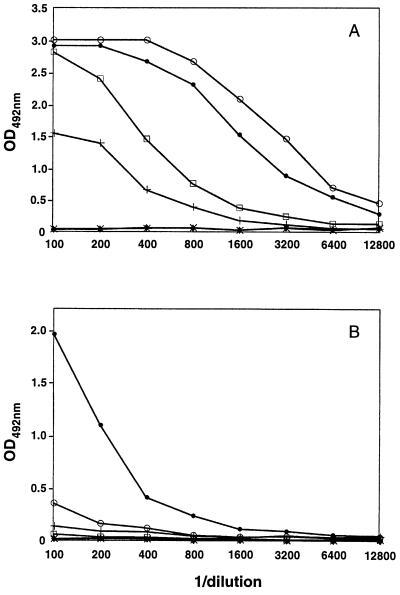
Figure 2.
Anti-E5.2 and anti-HEL activity of mouse sera. Four BALB/c mice immune sera raised against polyclonal rabbit anti-Id (anti-D1.3) Ab were measured by ELISA for binding to (A) Fab E5.2 (mAb anti-Id, Ab2) and (B) the antigen HEL. Values obtained for the sera of immunized mice are given by: ○, •, □, +; controls from unimmunized animals: x, ⧫.
Cloning and Sequencing of VH and VL.
mRNA was extracted and purified from ≈5 × 107 hybridoma cells using a Fast-track kit from Invitrogen and an oligo(dT) primer. cDNA was synthesized using The Copykit from Invitrogen. The cDNA was utilized in PCR cycles as a template with degenerate primers designed for amplification of mouse VH and VL. The PCR products were cloned using the TA cloning kit (Invitrogen). Several clones for each chain were sequenced to ascertain the absence of artifacts arising from PCR amplification. The sequencing was done in an Applied Biosystems PRISM automated sequencer by means of dye-primer and dye-terminator reactions using the Applied Biosystems Taq dyedeoxy terminator cycle sequencing kit (Applied Biosystems).
Determination of Association Constants.
The association constants of AF14 and AF52 were determined using the BIAcore technique (Fig. 3). D1.3 was included in these determinations as a control. HEL and FabE5.2 (in solutions of 30 μg/ml and 100 μg/ml, respectively, in 10 mM Na acetate, pH 4.8) were coupled to the solid phase. Fab AF14, AF52, and D1.3 were bound to HEL or E5.2 surfaces and deabsorbed by washing as described (14). Data were analyzed using kinetic methods for high affinity interactions (108–109 M−1 range) (14) or equilibrium Scatchard plots for lower affinities (106–108 M−1) (15). For the AF14–HEL reaction, the association constant was verified by ultracentrifugal equilibrium sedimentation analysis (14).
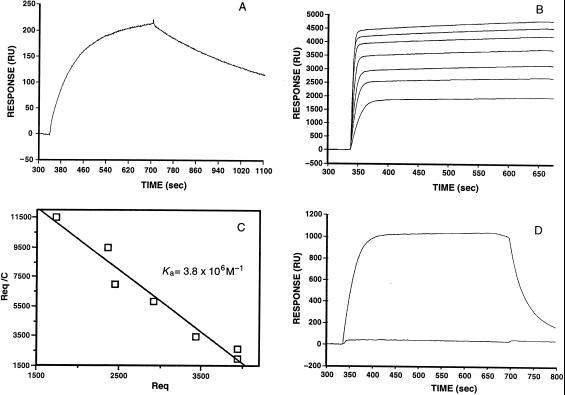
Figure 3.
Determination of binding constants by surface plasmon resonance techniques. (A) Kinetic analysis of the binding of Fab AF14 (liquid phase) to FabE5.2 (solid phase). The association and dissociation phases are clearly visible. From the kon (1.8 × 104 M−1⋅s−1) and koff values (1.8 × 10−5⋅s−1) an equilibrium association constant Ka value (1.0 × 109 M−1) was calculated. (B) Equilibrium analysis of the binding of FabAF52 (liquid phase) to HEL (solid phase). FabAF52 concentrations ranging from 100 nM to 5 μM were allowed to reach equilibrium. (C) Scatchard analysis of the data shown in B. (D) The upper curve gives the association and dissociation phases of the binding of FabAF52 (liquid phase) to HEL. In the lower curve, FabAF52 was injected with a stoichiometric amount of FabE5.2.
RESULTS
To maximize the probability of obtaining a wide anti-Id response and of improving the immunogenicity of this preparation to generate anti-anti-Id mAbs, xenogeneic anti-Id were obtained. The sera of BALB/c mice immunized with the rabbit polyclonal anti-Id preparation were assayed for anti-E5.2 and anti-HEL activity. The results are shown in Fig. 2 (these results are representative of those obtained with six other immunized animals; data not shown). The anti-E5.2 activity was stronger than that against HEL. A similar observation was made before (10) using anti-Id mAb E5.2 as immunogen. The spleens of animals showing a specific anti-E5.2 and anti-HEL activity in ELISA were used for hybridoma production. From two independent fusion experiments, two mAbs, AF14 and AF52 (both IgG1,κ), were obtained. These mAbs were found to have an anti-E5.2 as well as an anti-HEL activity. Binding of HEL to the anti-anti-Id was inhibited by the anti-Id, E5.2 (Fig. 3).
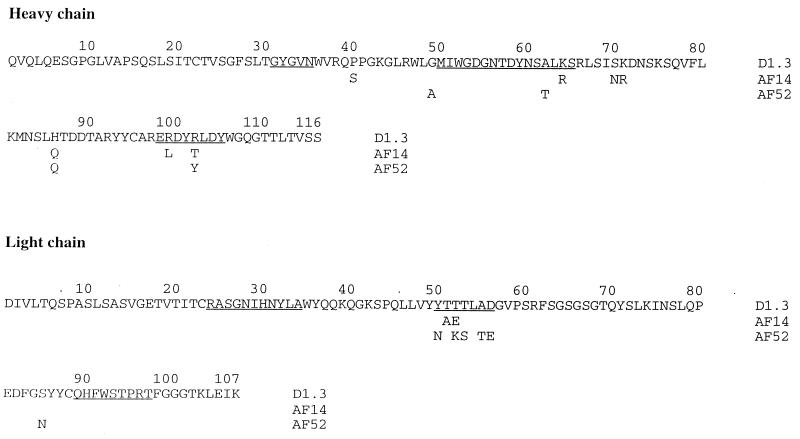
The amino acid sequences of the VH and VL segments of AF14 and AF52, compared with that of D1.3 (16), are shown in Fig. 4. The three sequences are very close, indicating that they probably derive from the same germ-line genes and the same type of rearrangement with D, JH, and JL segments. AF14 differs from D1.3 at nine positions, seven in the VH domain (one in FR2, one in CDR2, three in FR3, and two in CDR3) and two in the VL domain (both in CDR2). AF52 differs from D1.3 at 10 positions: four in VH (one in FR2, one in CDR2, one in FR3, and one CDR3) and six in VL (five in CDR2 and one in FR3). Notably, the VL-JL rearrangement and the resulting VL CDR sequences are identical for the three Ab. The amino acids by which D1.3 contacts the anti-Id E5.2 are the same in the three Ab, except for positions VH99 in AF14 and VL50 in AF52. The amino acids by which D1.3 contacts the antigen HEL are also nearly identical in the three Ab, except for changes at positions VH99 and 102 in AF14 and at positions VL50, 53 and VH102 in AF52 (Fig. 4).
Figure 4.
Amino acid sequences of the VH and VL domains of D1.3, AF14, and AF52. The complete sequence of D1.3 (16) is shown; those of AF14 and AF52 are given only at positions at which they differ from D1.3. CDR positions are underlined.
Table 1 shows the binding constants of mAbs D1.3, AF14, and AF52 toward E5.2 and HEL, measured by surface plasmon resonance (Fig. 3). Both anti-anti-Id Ab bind the anti-Id E5.2 with Ka constants, 1.0 × 109 M−1 and 2.4 × 107 M−1, respectively, which compare in value to those observed in secondary responses against a protein antigen such as HEL in BALB/c mice (17–19). The affinity for HEL is lower (1.0 × 106 M−1 and 3.8 × 106 M−1). The value obtained for the D1.3-HEL Ka constant (Table 1) is similar to values obtained by different techniques (18). Also, the value obtained for the AF14-HEL association constant by sedimentation equilibrium is close to that obtained by plasmon resonance (Table 1). The data presented in Table 1 could explain the titration curves shown in Fig. 2 if it is assumed that AF14 and AF52 are representative of all of the anti-anti-Id Ab present in the sera because, under the conditions of the experiments, the higher affinity Ab would dominate the reactions.
Table 1.
Equilibrium association constants of the reactions among D1.3, AF14, and AF52 with the anti-Id E5.2 and the antigen HEL
| mAb, Fab | Ka, FabE5.2, M−1 | Ka, HEL, M−1 |
|---|---|---|
| D1.3 | 1.4 × 109 | 1.0 × 108 |
| AF14 | 1.0 × 109 | 1.8 × 106 (1.6 × 106) |
| AF52 | 2.4 × 107 | 3.8 × 106 |
All values were obtained by plasmon resonance techniques except the one in parentheses, which was obtained by ultracentrifugal sedimentation equilibrium analysis. See Materials and Methods.
DISCUSSION
To define an idiotope, to assess its relation to an Ab combining site and to test the “internal image” postulate, requires, at least, the three-dimensional structures of the antigen-Ab1 and of the Ab1-Ab2 (Id-anti-Id) complexes. These complexes will define: (i) the antigenic determinant; (ii) the functional Ab combining site and the idiotope; and (iii) a possible mimicry of the antigen by the anti-Id. Several anti-Id Ab and Id-anti-Id complexes have been studied by x-ray crystallographic techniques (20–24). In these systems (9), the structure of the external antigen (21, 23) or of the Id-anti-Id complex (21, 22, 24) or of the Ab1-antigen complex (21–23) was not known. Thus, these systems cannot answer the questions outlined above. The studies that come closer to the one presented here are those of Garcia et al. (21, 22) in which the three-dimensional structure of an anti-anti-Id Ab complexed to the ligand angiotensin was determined. The anti-anti-Id mAb had a sequence similarity with the Ab1, which is comparable to that shown here among D1.3 (Ab1), AF14, and AF52.
A structural approach (10, 11) has revealed how an anti-Id Ab may mimic an external antigen, a postulate first envisaged by Ehrlich (25) and further elaborated in more recent times (2, 5–9). This approach showed that the mimicry is functional because the anti-Id Ab makes chemical contacts with, mostly, the same residues of a specific Ab1 that contact antigen. In other words, the Id and the combining site of the Ab1 are closely related and overlap extensively. This fact, however, does not imply that the anti-Id closely resembles the antigen. The phenomenon by which two different molecules, or two different parts of a molecule, bind the same combining site has been reported for other systems, for example, for the specific binding of the human growth hormone to its receptor, in which two different areas of one hormone molecule bind the combining sites of two identical receptor molecules (26).
In this work, we sought to expand the structural and immunological aspects of this idiotypic system using a xenogeneic preparation of anti-Id, anti-D1.3. Because of their diversity, these rabbit polyclonal Ab are more apt to carry an “internal image” of the antigen. They are not limited by the same constraints that may be imposed by limitations in the germ-line genes or their expression in a syngeneic strain of animals or by the fact that the syngeneic system used before was conceived to prepare mAbs suitable for structural studies. Thus, polyclonal rabbit Ab were used as immunogens to prepare anti-anti-Id Ab in BALB/c mice.
From two independent fusion experiments, two hybridoma cell lines and the corresponding anti-anti-Id mAbs were obtained, AF14 and AF52, which exhibited anti-HEL activity. AF14 and AF52 are also reactive with the anti-Id E5.2, previously obtained by syngeneic immunizations with the Ab1, D1.3. It is striking that the binding to E5.2 displays affinities compatible with those of a secondary Ab response to a protein antigen such as HEL (17–19). Presumably, the rabbit anti-Id Ab contained components that were very close, or perhaps identical, in combining site structure to the mouse monoclonal anti-Id, E5.2. This is remarkable if one considers that the genetic mechanisms of Ab diversity are very different in rabbits and mice. Although anti-D1.3 anti-Ids could have elicited Ab close to D1.3, only those that interact with D1.3 as E5.2 does could assure that the D1.3 combining site would be sufficiently well preserved to bind HEL [for example, the anti-Id E225 does not give the same result (10, 21)]. Thus, the mAbs D1.3, anti-Id E5.2, and anti-anti-Id AF14 and AF52 are linked by Id-anti-Id interactions, and the conclusions obtained before by a study (10, 11) of the crystal structure of the Id-anti-Id complex are relevant to antigen mimicry.
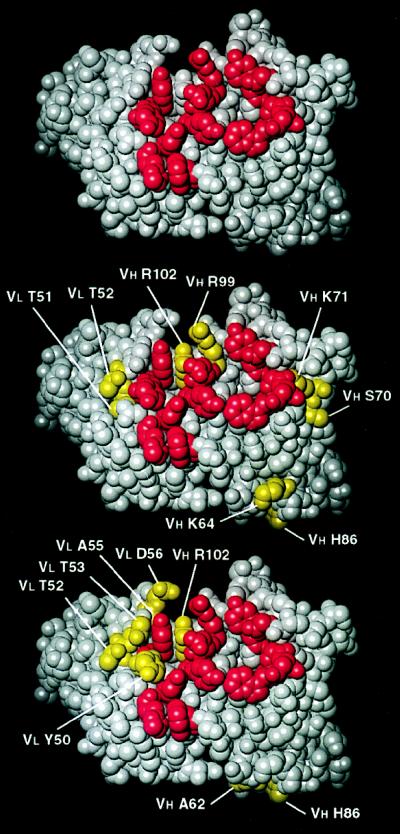
The anti-anti-Id AF14 and AF52 are very close in amino acid sequence to the Ab1 D1.3. They appear to be the expression of the same VH and VL germ-line genes as D1.3 and, more strikingly, to be the product of the same rearrangements of the VH, D, JH, and VL, JL segments. Few of the amino acid replacements (see Fig. 4) affect the CDR or the residues making contacts with the anti-Id or the antigen. This is shown in Fig. 5 in which the 13 residues of D1.3 that contact both the antigen HEL and the anti-Id E5.2 are shown with the corresponding residues of AF14 and AF52. These residues are important for the combining site of D1.3 as well as the idiotope defined by the reaction with E5.2. The models in Fig. 5 show that AF14 and AF52 conserve the residues that in D1.3 conform the central patch of the idiotope and the antigen combining site. In AF14, only one of the central core of contacting amino acids is changed (Arg99 to Leu in VH CDR3); all other changes occur in CDR positions that would not alter contacts with HEL or E5.2 and in “framework” (27) positions. In the case of AF52, only one of the 13 residues that contact both HEL and E5.2 is changed (Tyr50 to Asn in VL CDR2); all other changes in sequence occur at CDR and framework positions that do not participate directly in binding.
Figure 5.
Three-dimensional models of the Ab1 D1.3 (10, 11) (Top), the anti-anti-Id AF14 (Middle), and the AF52 (Bottom). AF14 and AF52 were modeled based on their close sequence homology with D1.3. Residues in red are those that, in D1.3, contact both the antigen HEL and the anti-Id, E5.2. In this front view of the combining site, residues of AF14 and AF52 (in yellow) that differ from those of D1.3 are labeled.
Alanine scanning studies (15) have indicated a number of residues of D1.3 that are important in binding the antigen HEL. Amino acid replacements at only 5 of the 13 positions illustrated in Fig. 3 alter binding significantly (changes in ΔG larger than 1 Kcal/mol). All of these residues are preserved in AF14 and AF52. A different picture emerged from the alanine scanning studies on the complex with E5.2. Here, most of the D1.3 contact residues were found to play a significant role in ligand binding. Of 15 replacements of D1.3 residues in contact with E5.2, those at 13 positions resulted in changes of the free energy of binding that were larger than 1 Kcal/mol. Only one of these residues is changed in AF14 (Arg99 to Leu), and none is changed in AF52. This analysis shows that AF14 and AF52 retain those residues of D1.3 that, from an energetic point of view, dominate the binding to HEL and E5.2.
The differences in the Ka constants for the reactions of the anti-anti-Id AF14 and AF52 with E5.2 and HEL compared with the D1.3-E5.2 and D1.3-HEL reactions can be attributed to the sequence variations. The VH Arg102Thr change in AF14 and Arg102Tyr in AF52 should cause the loss of one hydrogen bond in each of the reactions to HEL compared with the D1.3-HEL reaction. Moreover, a second hydrogen bond is likely lost in the AF14-HEL reaction by the VH Arg99Leu replacement. Likewise, affinity of the AF52-E5.2 reaction is reduced compared with the D1.3-E5.2 reaction by the probable loss of a single hydrogen bond from the VL Tyr50Asn variation. The most surprising reaction is that of AF14 with E5.2. In this case, the Ka association constant is the same as for the D1.3-E5.2 reaction even though there is a nonconservative substitution in VH CDR3 (Arg99Leu) that should result in the loss of a hydrogen bond between AF14 and E5.2. We suspect that a conformational change in VH CDR3 of AF14 leads to contacts that stabilize the reaction with E5.2 in a manner unlike that in the D1.3-E5.2 complex.
There are two major possibilities for the induction of anti-anti-Id Ab that resemble the Ab1. One is that a suitably selected anti-Id will mimic the antigen so closely as to produce the equivalent of an Ab response to the original antigen. A second possibility is that the anti-Id is specific for the Id and will thus stimulate lymphocytes displaying anti-anti-Id Ab that should be identical or essentially identical to the Ab1. The results presented here can be interpreted as indicating that the second mechanism is more likely because the anti-anti-Id display higher binding equilibrium constants for the anti-Id than for the antigen HEL. Although only two anti-anti-Id mAbs were obtained (out of five attempted fusions), the experiments illustrated in Fig. 2 support the conclusion that the anti-anti-Id response of different animals has higher affinity for the anti-Id than for the antigen. This conclusion could be tempered by the facts that (i) the affinity constants for the antigen and the anti-Id are not so different in the case of AF52; (ii) only two anti-anti-Id were obtained and analyzed (although the sera of 10 animals were assayed and five fusions were performed to obtain the two anti-anti-Id); and (iii) the idiotope of D1.3 and its combining site are extremely close so that preservation of one of them will nearly result in preservation of the other, thus ensuring that the anti-anti-Id Ab, resembling D1.3 at the 13 positions analyzed above, will be capable of binding the antigen as well as the anti-Id. Anti-anti-Id Ab displaying this conformation should be efficiently selected for by the anti-Id.
The therapeutic potential of anti-idiotypic Ab has been explored in many systems (see refs. 28 and 29 for recent examples). An implication of the findings reported in this paper is that properly selected monoclonal anti-Id Ab may behave as antigen surrogates but with the limitation that they will not induce as vast an array of anti-antigens as the original antigen. They will induce Ab that will be optimal for binding anti-Id Ab rather than the antigen because the structural requirements for optimal binding of anti-Id will be close to, but not identical with, those for binding antigen.
Acknowledgments
We thank Dr. Ellen Goldman for help in the ultracentrifugal experiments and Dr. Alfred Nisonoff for advice and a critical reading of the manuscript. F.A.G. was supported by Fundación Antorchas and Universidad de Buenos Aires (Subsidio FA 111), National Institutes of Health Grant GM52801 (R.A.M.), a W. E. Elkins Professorship at the University of Maryland (R.J.P.), and the Lucille P. Markey Charitable Trust.
Footnotes
Abbreviations Ab1, antibody specific for external antigen; Ab2, anti-idiotypic antibody; Ab3, anti-anti-idiotypic antibody; HEL, hen egg lysozyme; CDR, complementarity determining regions; VH, VL, the variable domains of the heavy (H) and light (L) Ig (Ig) polypeptide chains; Id, idiotype or idiotope.
References
- 1.Oudin J, Michel M. C R Acad Sci Paris. 1963;257:805–808. [PubMed] [Google Scholar]
- 2.Jerne N K. Ann Immunol Inst Pasteur. 1974;125C:373–389. [PubMed] [Google Scholar]
- 3.Nisonoff A. J Immunol. 1991;147:2429–2438. [PubMed] [Google Scholar]
- 4.Gaulton G N, Greene M I. Annu Rev Immunol. 1986;4:253–280. doi: 10.1146/annurev.iy.04.040186.001345. [DOI] [PubMed] [Google Scholar]
- 5.Nisonoff A, Lamoyi E. Clin Immunol Immunopathol. 1981;21:397–406. doi: 10.1016/0090-1229(81)90228-2. [DOI] [PubMed] [Google Scholar]
- 6.Roitt I M, Cooke A, Male D K, Hay F C, Guarnotta G, Lydyard P M, DeCarvalho L P, Thanavala Y, Ivanyi J. Lancet. 1981;i:1041–1045. doi: 10.1016/s0140-6736(81)92199-1. [DOI] [PubMed] [Google Scholar]
- 7.Erlanger B F. Int Rev Immunol. 1989;5:131–137. doi: 10.3109/08830188909061979. [DOI] [PubMed] [Google Scholar]
- 8.Bona C A. Proc Soc Exp Biol Med. 1996;213:32–42. doi: 10.3181/00379727-213-44033. [DOI] [PubMed] [Google Scholar]
- 9.Poljak R J. Proc Natl Acad Sci USA. 1994;91:1599–1600. doi: 10.1073/pnas.91.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields B A, Goldbaum F A, Ysern X, Poljak R J, Mariuzza R A. Nature (London) 1995;374:739–742. doi: 10.1038/374739a0. [DOI] [PubMed] [Google Scholar]
- 11.Braden B C, Fields B A, Ysern X, Dall’Acqua W, Goldbaum F A, Poljak R J, Mariuzza R A. J Mol Biol. 1966;265:137–151. doi: 10.1006/jmbi.1996.0629. [DOI] [PubMed] [Google Scholar]
- 12.Bhat T N, Bentley G A, Boulot G, Greene M I, Tello D, Dall’Acqua W, Souchon H, Schwarz F P, Mariuzza R A, Poljak R J. Proc Natl Acad Sci USA. 1994;91:1089–1093. doi: 10.1073/pnas.91.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galfre G A, Milstein C. Methods Enzymol. 1981;73:3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- 14.Goldbaum F A, Schwarz F P, Eisenstein E, Cauerhff A, Mariuzza R A, Poljak R J. J Mol Recogn. 1996;9:6–12. doi: 10.1002/(SICI)1099-1352(199601)9:1%3C6::AID-JMR240%3E3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Dall’Acqua W, Goldman E R, Eisenstein E, Mariuzza R A. Biochemistry. 1996;35:9667–9676. doi: 10.1021/bi960819i. [DOI] [PubMed] [Google Scholar]
- 16.Boulot G, Eiselé J-L, Bentley G A, Bhat T N, Ward S, Winter G, Poljak R J. J Mol Biol. 1990;213:617–619. doi: 10.1016/S0022-2836(05)80248-7. [DOI] [PubMed] [Google Scholar]
- 17.Harper M, Lema F, Boulot G, Poljak R J. Mol Immunol. 1987;2:97–108. doi: 10.1016/0161-5890(87)90081-2. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz F P, Tello D, Goldbaum F A, Mariuzza R A, Poljak R J. Eur J Biochem. 1995;228:388–394. [PubMed] [Google Scholar]
- 19.Smith-Gill S J, Wilson A C, Potter M, Prager E M, Feldmann R J, Mainhart C R. J Immunol. 1982;128:314–322. [PubMed] [Google Scholar]
- 20.Bentley G A, Boulot G, Riottot M-M, Poljak R J. Nature (London) 1990;348:254–257. doi: 10.1038/348254a0. [DOI] [PubMed] [Google Scholar]
- 21.Garcia K C, Ronco P M, Verroust P J, Brünger A T, Amzel L M. Science. 1992;257:502–507. doi: 10.1126/science.1636085. [DOI] [PubMed] [Google Scholar]
- 22.Garcia K C, Desiderio S V, Ronco P M, Verroust P J, Amzel L M. Science. 1992;257:528–531. doi: 10.1126/science.1636087. [DOI] [PubMed] [Google Scholar]
- 23.Ban N, Escobar C, Garcia R, Hasel K, Day J, Greenwood A, McPherson A. Proc Natl Acad Sci USA. 1994;91:1604–1608. doi: 10.1073/pnas.91.5.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans S V, Rose D R, To R, Young N M, Bundle D R. J Mol Biol. 1994;241:691–705. doi: 10.1006/jmbi.1994.1544. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich P. Proc R Soc London. 1900;66:424–448. [Google Scholar]
- 26.De Vos A, Ultsch M, Kossiakoff A A. Science. 1992;255:306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]
- 27.Kabat E A, Wu T T, Perry H M, Gottesman K S, Foeller C. Sequences of Proteins of Immunological Interest. 5th Ed. Washington, DC: U. S. Department of Health and Human Services; 1991. , NIH Publication No. 91–3242. [Google Scholar]
- 28.Magliani W, Conti S, De Bernardis F, Gerloni M, Bertolotti D, Mozzoni P, Cassone A, Polonelli L. Nat Biotechnol. 1997;15:155–158. doi: 10.1038/nbt0297-155. [DOI] [PubMed] [Google Scholar]
- 29.Whittum-Hudson J A, An L-L, Saltzman W M, Prendergast R A, MacDonald A B. Nat Med. 1996;2:1116–1121. doi: 10.1038/nm1096-1116. [DOI] [PubMed] [Google Scholar]