Abstract
Paraquat (PQ) is a well described pneumotoxicant that produces toxicity by redox cycling with cellular diaphorases, thereby elevating intracellular levels of superoxide (O2⨪). NO synthase (NOS) has been shown to participate in PQ-induced lung injury. Current theory holds that NO reacts with O2⨪ generated by PQ to produce the toxin peroxynitrite. We asked whether NOS might alternatively function as a PQ diaphorase and reexamined the question of whether NO/O2⨪ reactions were toxic or protective. Here, we show that: (i) neuronal NOS has PQ diaphorase activity that inversely correlates with NO formation; (ii) PQ-induced endothelial cell toxicity is attenuated by inhibitors of NOS that prevent NADPH oxidation, but is not attenuated by those that do not; (iii) PQ inhibits endothelium-derived, but not NO-induced, relaxations of aortic rings; and (iv) PQ-induced cytotoxicity is potentiated in cytokine-activated macrophages in a manner that correlates with its ability to block NO formation. These data indicate that NOS is a PQ diaphorase and that toxicity of such redox-active compounds involves a loss of NO-related activity.
Paraquat (PQ) is a well characterized pneumotoxicant (1). It produces oxidative stress by redox cycling with a variety of cellular diaphorases and oxygen to produce superoxide radicals (O2⨪) (2). A diaphorase is a class of enzymes that transfer electrons from NAD(P)H to small molecules, such as nitroblue tetrazolium, quinones, and PQ. PQ diaphorases are usually oxidoreductase enzymes that contain flavins and use either NADH or NADPH as electron donors (3–5). A common cellular diaphorase that can redox cycle with PQ is cytochrome P450 reductase (6). The role of O2⨪ in PQ-mediated cytotoxicity has been suggested from studies where overexpression of superoxide dismutases (SOD) or treatment with SOD mimetics is protective (7–12).
NO is generated by a family of enzymes known as NO synthases (NOS). NOS generates NO and related molecules by catalyzing oxygen- and NADPH-dependent oxidation of l-arginine (13). The three isoforms of NOS (14) fall into the cytochrome P450 reductase-like gene family (15); two of them are typically expressed constitutively [NOS1 (16) and NOS3 (17)], and the third is an inducible form that was first identified in macrophages (NOS2) (18, 19). NOS also generates O2⨪; that is, it has NADPH oxidase activity. Production of O2⨪ can be inhibited by the substrate analog NG-nitro-l-arginine methyl ester (l-NAME) but not by NG-monomethyl-l-arginine (l-NMMA) (20). NOS play important roles in neurotransmission, smooth muscle relaxation, inflammation, host defense, and regulation of cell death, depending in part on the localization of the specific isoform in tissue.
In particular, NO has been implicated in PQ-induced lung injury (21). The mechanism by which NO does so is not known, but is thought to be due to its rapid reaction with O2⨪ to form the strong oxidant peroxynitrite (22). We considered an alternative hypothesis, namely, that PQ uses NOS to generate O2⨪ at the expense of NO. More specifically, we considered the possibility that NOS is a PQ diaphorase of the cytochrome P450 reductase class. We tested this hypothesis with rat recombinant neuronal NOS, in cultured endothelial cells, in aortic ring bioassays, and in RAW 264.7 macrophages. The data reported in these studies support the concept that NOS is, indeed, a PQ diaphorase, and suggest that toxicity associated with such redox-active compounds involves a loss of NO formation, coupled with increased O2⨪ generation.
Methods
Measurement of PQ Diaphorase Activity.
Recombinant rat neuronal NOS (NOS1; Oxis, Portland, OR) was used to assay NADPH oxidation with or without PQ, in the presence or absence of the NOS inhibitors, l-NAME or l-NMMA. Reactions (0.2 ml) were performed in a Bistris propane buffer (40 mM) containing l-arginine (1 mM), CaCl2 (1.2 mM), EDTA (0.9 mM), NADPH (0.35 mM), DTT (3 mM), tetrahydrobiopterin (4 μM), catalase (1 μg/ml), and calmodulin (200 units/ml) at pH 7.4. Reactions were initiated by the addition of 1.5 units of NOS1, and the oxidation of NADPH was monitored at 340 nm for 1 min at 37°C in a plate reader. The rate of NOS-mediated NADPH oxidation was determined by using an extinction coefficient of 6.22 mM−1⋅cm−1 after subtracting the baseline rate of NADPH oxidation. Anaerobic reduction of PQ by NOS1 to PQ⨥ was followed at 600 nm spectrophotometrically and converted to a concentration by using the extinction coefficient ɛ600 = 13,700 M−1⋅cm−1 (23). Reactions (1 ml) were performed in the above NADPH oxidation assay buffer in the absence of l-arginine, DTT, and tetrahydrobiopterin. Reactions were initiated by the addition of 2 units of NOS1 and followed for 3 min at 37°C.
Measurement of Nitrite and Nitrate.
Nitrite and nitrate were assayed by using a nonenzymatic colorimetric kit (NB-88; Oxford Biomedical Research, Oxford, MI). NADPH oxidation was stopped after 5 hr by the addition of 25 μl of zinc sulfate (30% wt/vol) to precipitate the NOS protein. Nitrate was nonenzymatically converted to nitrite by overnight incubation of 150 μl of reaction mixture from the NADPH oxidation assay with cadmium beads. Nitrite concentration was determined from a standard curve using 100 μl sample or standard.
Saturated NO Solution.
A saturated NO solution was made and quantified using an oxymyoglobin method as previously described (24).
Cell Culture Conditions.
The CPA-47 endothelial cell line (CRL-1733) was purchased from the American Type Culture Collection. Endothelial cells were grown in T-75 flasks containing Ham’s F-12K medium supplemented with 1 mM l-glutamate and 10% equine serum (GIBCO/BRL). Endothelial cells were plated at 3.5 × 104 cells per well in 24-well plates, and experiments were performed 48 hr later when cells were near confluence. Medium was changed to MEM without serum supplement at the start of each experiment. Endothelial cells were from passages 30 to 36. The RAW-264.7 monocyte–macrophage cell line (TIB-71) was purchased from the American Type Culture Collection. The cells were grown in T-75 flasks containing DMEM (GIBCO/BRL) supplemented with 1 mM pyruvate, 4.5 g/liter glucose and 10% FBS. Macrophages were plated at 2.0 × 104 cells per well in 24-well plates, and experiments were performed when cells were near confluence. Macrophages were immunostimulated with 50 ng/ml lipopolysaccharide (LPS) from Escherichia coli serotype O111:B4 (Sigma) and 50 units/ml recombinant mouse IFN-γ (R & D Systems). Macrophages were used between passages 2 and 8. Cell cultures were kept in a 37°C incubator with air and 5% carbon dioxide.
Assessment of Cell Injury.
The percent release of cytosolic lactate dehydrogenase (LDH) into the culture medium was used to assess the integrity of the cell membrane. LDH activity was measured by following the loss of NADH at 340 nm as previously described (8).
Aconitase and Fumarase Measurement.
Aconitase and fumarase were measured as previously described (12). Specifically, aconitase activity was determined spectrophotometrically by monitoring the formation of cis-aconitate from isocitrate at 240 nm. Fumarase activity was measured by monitoring the increased absorbance at 240 nm after the formation of fumarate from l-malate. Aconitase activity was expressed as a ratio of aconitase activity to fumarase activity to correct for differences in cell numbers.
Western Blot Analysis.
Cells were lysed in ice-cold 50 mM Tris buffer (pH 7.4) containing 1% SDS and protease inhibitor mixture (Boehringer Mannheim). Protein (10 μg) was separated on a 4–20% gradient SDS/PAGE at 250 V for 30 min and transferred onto a nitrocellulose membrane at 30 V for 12 hr. Proteins were detected by using polyclonal Abs against human NOS2 and NOS3 (Transduction Laboratories, Lexington, Kentucky; 1/1000) and nitrated (forming nitrotyrosine) keyhole limpet hemocyanin (Upstate Biotechnology, Lake Placid, NY; 1/5000). Primary Abs were detected with a goat anti-rabbit-horseradish peroxidase Ab (Jackson ImmunoResearch; 1/15,000) and developed using an enhanced chemiluminescence kit (Amersham Pharmacia).
Rabbit Aortic Ring Bioassay.
New Zealand White male rabbits weighing 3–4 kg were anesthetized with pentobarbital (30 mg/kg, i.v.). The descending thoracic aorta was isolated and placed in Krebs–Henseleit buffer (pH 7.4) and stored at 4°C until used. The vessels were cut into 5-mm rings and submerged in 25-ml double-jacketed tissue baths containing Krebs–Henseleit buffer bubbled with 95% oxygen and 5% carbon dioxide at 37°C. Rings were hung on stainless steel stirrups connected to calibrated force transducers, equilibrated with 2 g resting tension for 1 hr before use, and contracted with phenylephrine (1 μM). Vessels were incubated with PQ for 15 min before acetylcholine-induced or exogenous NO relaxations were performed.
Results
PQ Diaphorase Activity of Neuronal NOS.
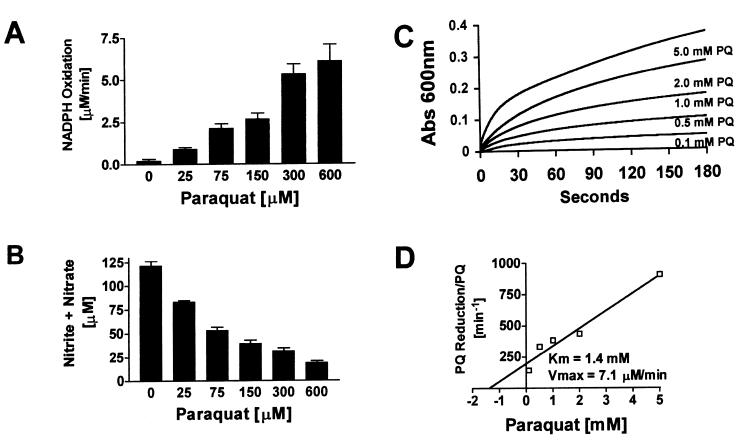
The addition of PQ to a recombinant NOS1 reaction mixture produced a concentration-dependent increase in NADPH oxidation (Fig. 1A). The formation of NO, assessed by the accumulation of nitrite and nitrate, was inhibited by PQ and inversely related to diaphorase activity (Fig. 1B). The calculated concentration of PQ that inhibited NO formation by 50% (IC50) was 62 μM with a 95% confidence interval of 47–82 μM. NOS1 exhibited PQ diaphorase activity, even in the presence of 1 mM l-arginine. PQ diaphorase activity was also confirmed by following the direct reduction of PQ by NOS1 to its cation radical (PQ⨥) under anaerobic conditions (Fig. 1C). The Km and Vmax of NOS1 for the PQ reductase activity, calculated using a Hanes plot (velocity/substrate concentration vs. substrate concentration), were 1.4 mM and 7.1 μM/min, respectively (Fig. 1D).
Figure 1.
NOS1 is a PQ diaphorase. (A) Stimulation of NOS1-dependent NADPH oxidation by increasing concentrations of PQ. NADPH oxidation was continuously monitored spectrophotometrically at 340 nm in a microtiter plate format for 1 min. (B) Inhibition of NO formation by increasing concentrations of PQ. The same microtiter plates used above were left at room temperature for 5 hr, and then an aliquot was assayed for total nitrite and nitrate. (C) Anaerobic reduction of PQ to its cation radical (PQ⨥) by NOS1. Formation of PQ⨥ was followed at 600 nm for 3 min. (D) Hanes plot (Km and Vmax values) for the PQ reductase activity of rat NOS1.
NOS Inhibitors, PQ Diaphorase Activity, and Endothelial Cell Cytotoxicity.
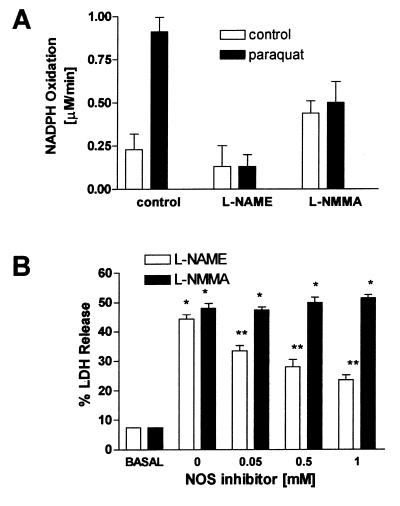
The effect of l-NAME and l-NMMA on PQ’s ability to stimulate NADPH oxidation by NOS1 was examined (Fig. 2A). PQ (25 μM) increased NADPH oxidation about 3-fold over the basal rate. l-NAME (1 mM) had little effect on basal NADPH oxidation, but completely inhibited PQ-stimulated NADPH oxidation. l-NMMA (1 mM) produced a small increase in basal NADPH oxidation and partially blocked PQ-induced NADPH oxidation. The NOS inhibitors thus exhibited differential effects on NOS1’s ability to act as a PQ diaphorase. Importantly, only l-NAME shuts down the NADPH oxidase activity.
Figure 2.
(A) NOS inhibitors l-NAME and l-NMMA have differential effects on NOS1-dependent NADPH oxidation. NADPH oxidation was continuously monitored spectrophotometrically at 340 nm in a microtiter plate format for 1 min in the presence (solid bars) or absence (open bars) of PQ (25 μM). NOS inhibitors were tested at a concentration of 1 mM. (B) Attenuation of PQ-induced endothelial cell injury by l-NAME, but not by l-NMMA. Endothelial cells were grown in 24-well plates and treated with increasing concentrations of either l-NAME or l-NMMA in the presence of 2 mM PQ. The percent release of LDH into the culture medium 24 hr after PQ treatment was used to assess cell injury. ∗ indicates a significant difference from control group, and ∗∗ indicates significant differences from the PQ group, P < 0.05.
To test whether the above effect was present in intact cells, endothelial cell cultures constitutively expressing NOS3 protein as determined by Western blot analysis (data not shown) were treated with 2 mM PQ for 24 hr in the presence of increasing concentrations of either l-NAME or l-NMMA (Fig. 2B). Cytotoxicity was measured as the percent release of LDH and compared with vehicle-treated cells (basal). PQ produced a 3-fold increase in LDH release. l-NAME, but not l-NMMA, produced a dose-dependent protection from PQ toxicity. These data are consistent with the differential effects of the two inhibitors on the NADPH oxidase activity of NOS and suggest that part of the toxicity exerted by PQ in endothelial cells is a result of NOS3 acting as a PQ diaphorase.
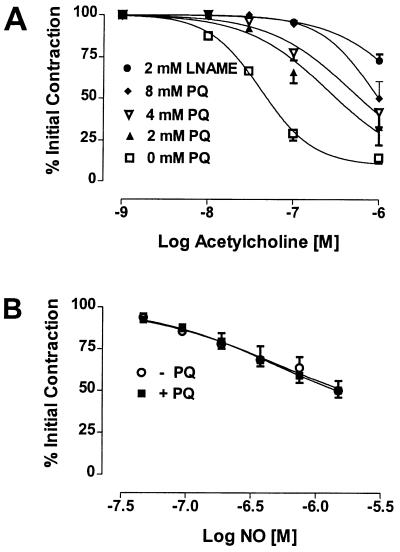
PQ, Endothelium-Derived Relaxation Factor (EDRF), and NO-Mediated Relaxations.
Rabbit aortic rings were treated with increasing concentrations of PQ prior to initiating a constriction response with phenylephrine. EDRF responses were then initiated by the addition of acetylcholine. PQ produced a dose-dependent inhibition of EDRF-induced relaxations (Fig. 3A). Greater than 90% of the relaxation response in this bioassay could be inhibited by l-NAME. In contrast, PQ did not inhibit exogenously added NO-induced relaxation (Fig. 3B). These data are consistent with the concept that NOS3 is acting as a PQ diaphorase at the expense of NO synthesis.
Figure 3.
(A) PQ inhibits EDRF activity. Rabbit aortic rings were constricted with 0.1 μM phenylephrine (initial contraction) and relaxed with increasing concentrations of acetylcholine in the presence or absence of increasing concentrations of PQ or l-NAME (2 mM). (B) PQ does not affect exogenous NO-induced aortic ring relaxation. Rabbit aortic rings were constricted with 1 μM phenylephyrine (initial contraction) and relaxed with increasing concentrations of NO in the presence or absence of 2 mM PQ. Relaxations are expressed as the percent of the initial phenylephrine-induced contraction.
PQ-Induced Cytotoxicity in Cytokine-Activated Macrophages.
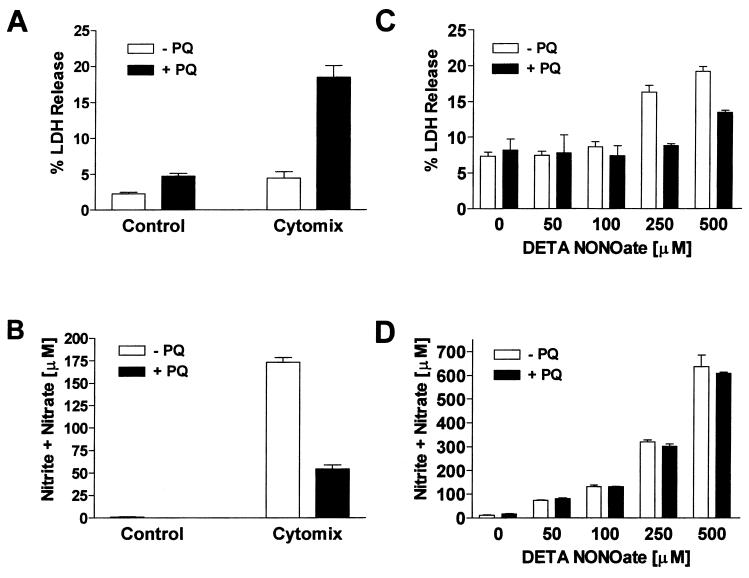
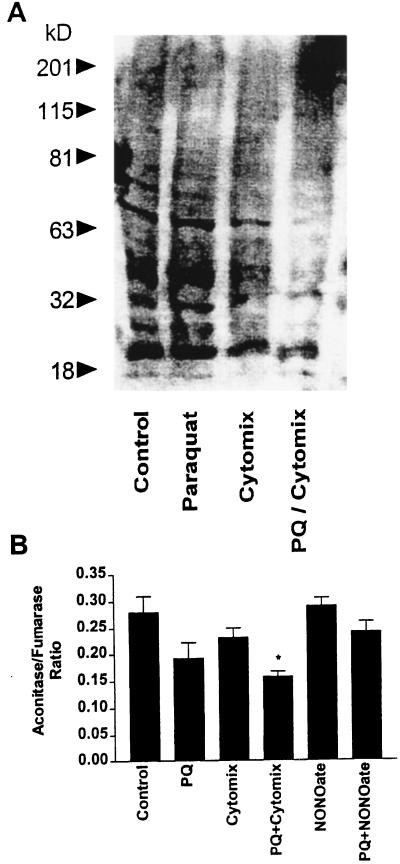
NOS2 was induced in RAW cells by using a mixture of IFN-γ (50 units/ml) and LPS (50 ng/ml), referred to as “cytomix.” Macrophages were treated with 0.1 mM PQ, and cytotoxicity and NO production were assessed at 24 hr. PQ or cytomix treatments alone produced very little cell injury (Fig. 4A). PQ-induced cytotoxicity was potentiated in activated macrophages (Fig. 4A), yet PQ significantly attenuated NO production (Fig. 4B) without affecting NOS2 expression (data not shown). The inhibition of NO production could not be explained by increased cytotoxicity, because the percent decrease in nitrite and nitrate was greater than the percent increase in cell death. A decrease in nitrotyrosine staining was also seen with the combination of PQ and cytomix (Fig. 5A). These data are consistent with the idea that NOS2 in activated macrophages is acting as a PQ diaphorase and that cytotoxicity results from overproduction of O2⨪, not NO or peroxynitrite.
Figure 4.
(A) PQ-induced cell injury is potentiated in immunostimulated macrophages. Cell injury was assessed 24 hr after cytomix and PQ treatment by measuring LDH release. (B) Induction of NO formation by cytomix and its inhibition by PQ. NOS2 activity was assessed 24 hr after cytomix by measuring the accumulation of NO metabolites, nitrite and nitrate. Macrophages were grown in 24-well plates and immunostimulated by treatment with cytomix. Unstimulated (basal) and activated (cytomix) cells were treated with or without PQ (0.1 mM). (C) NO-mediated macrophage injury and its inhibition by PQ. Cell injury was assessed 24 hr after DETA NONOate and PQ treatment by measuring LDH release. (D) PQ does not alter the formation of NO metabolites from DETA NONOate. NO release was assessed 24 hr after DETA NONOate and PQ treatment by measuring the accumulation of nitrite and nitrate. Macrophages were grown in 24-well plates and treated with increasing concentrations of DETA NONOate in the presence or absence of 0.1 mM PQ.
Figure 5.
(A) Decreased nitrotyrosine formation with PQ treatment. Protein nitration was assessed 24 hr after cytomix and PQ treatment by Western blot analysis. Macrophages were grown in 24-well plates and immunostimulated by treatment with cytomix. Unstimulated (basal) and activated (cytomix) cells were treated with or without PQ (0.1 mM). (B) Differential effect on PQ-mediated aconitase inactivation by cytomix and DETA NONOate treatments. Aconitase activity was measured in cell lysates 24 hr after cytomix, DETA NONOate, and PQ treatments. Macrophages were grown in 24-well plates and treated with cytomix or 250 μM DETA NONOate. Control, activated (cytomix), and DETA NONOate groups were treated with or without PQ (0.1 mM). ∗ indicates a significant difference from control group, P < 0.05.
To further exclude the role of peroxynitrite in toxicity, macrophages were treated with increasing concentrations of the NO-donor, diethylenetriamine (DETA) NONOate, in the presence of 0.1 mM PQ. Macrophage cytotoxicity and NO release were assessed 24 hr after treatments (Fig. 4C). PQ toxicity was not potentiated by DETA NONOate at concentrations that generated similar amounts of NO to those produced by activated macrophages. In fact, DETA NONOate-induced cell injury was attenuated by PQ (Fig. 4D). These data are also consistent with a decrease in aconitase activity (a sensitive marker of O2⨪ and peroxynitrite formation) seen in the PQ/cytomix group but not seen in the PQ/DETA NONOate group (Fig. 5B). Taken together, our results challenge the notion that the potentiation of PQ-induced cell injury in activated macrophages is because of a direct interaction between O2⨪ and NO.
Discussion
We report that NOS potentiates PQ toxicity in several in vitro and cellular models. A common view in the literature is that such toxicity results from the NO/O2⨪ interaction. In contrast, we show that PQ is actually using NOS as an electron source to generate O2⨪ and does so at the expense of NO formation (i.e., NOS switches from an oxygenase to a PQ reductase). The implications and corollaries are: (i) PQ blocks neuronal, endothelial, and macrophage NOS activity; (ii) PQ-induced endothelial cell toxicity is blocked by inhibitors of NOS that prevent NADPH oxidation, but is not attenuated by those that do not; (iii) PQ inhibits endothelium-derived but not NO-induced relaxations; and (iv) PQ-induced cytotoxicity in cytokine-activated macrophages correlates with its ability to block endogenous NO formation.
The concept that NOS can produce O2⨪ is not new. NADPH oxidase activity is an intrinsic property of all NOS isoforms and is potentiated by l-arginine or biopterin depletion (20, 25). NOS is also known to exhibit diaphorase activity with nitroblue tetrazolium (26, 27), quinones (28, 29) and with the chemotherapeutic agent adriamycin (30). Our studies suggest that PQ functions in much the same way, i.e., it acts as an acceptor of NOS electrons. PQ then forms the PQ cation radical (PQ⨥), which rapidly reacts with oxygen to form O2⨪, and, as a consequence, NO production is inhibited. These factors will likely impact on the pulmonary pathophysiology of PQ poisoning (31).
NOS2 has been implicated in modulating PQ toxicity in mice. Hollinger (32) showed that treating mice with LPS could potentiate PQ lethality. In these studies, mice that were treated with LPS 24 hr before receiving PQ had a 2-fold increase in 7-day cumulative mortality. Hollinger’s observation is consistent with our finding that LPS induction of NOS2 potentiated PQ-mediated cell-injury responses in culture. In contrast, PQ attenuated NO-induced cell injury, indicating that O2⨪ may even be protective in models of nitrosative stress.
The main target organs involved in PQ toxicity are the lung and, to a lesser extent, the kidneys (33). The lungs are preferentially targeted because of PQ’s rapid uptake and accumulation in this organ through polyamine transporters (34, 35). Severe PQ poisoning produces adult respiratory distress syndrome, pulmonary hypertension, edema, and progressive lung fibrosis. The fact that PQ produces increased pulmonary artery and airway pressures emphasizes the importance of NO deficiency in the toxicological response and may explain why patients suffering from PQ poisoning improve when treated with inhaled NO (36–38).
Lung endothelium is particularly sensitive to PQ-mediated injury (1, 7). PQ-mediated endothelial cell dysfunction was also demonstrated in rat aorta (39). Because PQ poisoning could be reversed by pretreatment with SOD, the authors concluded that PQ was blocking EDRF responses through the reaction of O2⨪ with NO. However, PQ did not inhibit nitroprusside-mediated relaxations in these studies, in agreement with our findings that PQ inhibited EDRF responses, but not those of an NO donor. PQ’s effect thus resulted from the combination of increased O2⨪ generation and inhibition of NO synthesis. Evidently, NO-related activity in the vessels is resistant to O2⨪, perhaps due to in vivo formation of S-nitrosothiol. Other studies (21, 40) may benefit from reinterpretation in this light.
NO has paradoxical effects in oxidative stress models, where it can either be protective (41, 42) or injurious (43). Freeman and coworkers (44) have shown that NO is protective to both pulmonary cells and animals in various models of oxidative stress. Our data suggest that PQ is actually using NOS as an electron source to generate O2⨪ and, in the process, decreasing the generation of NO. These data may help explain some of the paradoxical responses reported in the literature and give insight as to why inhaled NO may be beneficial in people suffering from PQ poisoning.
Acknowledgments
We thank Mike Nicks and Kathy Evans for technical assistance, and Drs. James D. Crapo and Bruce Freeman for their helpful suggestions. This work was supported in part by Grant RO1 HL59602 from the National Institutes of Health (to B.J.D.) and by a grant from the American Lung Association (to B.J.D.).
Abbreviations
- PQ
paraquat
- NOS
NO synthase
- l-NAME
NG-nitro-l-arginine methyl ester
- l-NMMA
NG-monomethyl-l-arginine
- EDRF
endothelium-derived relaxation factor
- SOD
superoxide dismutase
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Brooks R E. Lab Invest. 1971;25:536–545. [PubMed] [Google Scholar]
- 2.Bus J S, Aust S D, Gibson J E. Biochem Biophys Res Commun. 1974;58:749–755. doi: 10.1016/s0006-291x(74)80481-x. [DOI] [PubMed] [Google Scholar]
- 3.Dicker E, Cederbaum A I. Biochem Pharmacol. 1991;42:529–535. doi: 10.1016/0006-2952(91)90315-v. [DOI] [PubMed] [Google Scholar]
- 4.Liochev S I, Fridovich I. Free Radical Biol Med. 1994;16:555–559. doi: 10.1016/0891-5849(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 5.Shimada H, Hirai K, Simamura E, Pan J. Arch Biochem Biophys. 1998;351:75–81. doi: 10.1006/abbi.1997.0557. [DOI] [PubMed] [Google Scholar]
- 6.Clejan L, Cederbaum A I. Biochem Pharmacol. 1989;38:1779–1786. doi: 10.1016/0006-2952(89)90412-7. [DOI] [PubMed] [Google Scholar]
- 7.Day B J, Crapo J D. Toxicol Appl Pharmacol. 1996;140:94–100. doi: 10.1006/taap.1996.0201. [DOI] [PubMed] [Google Scholar]
- 8.Day B J, Shawen S, Liochev S I, Crapo J D. J Pharmacol Exp Ther. 1995;275:1227–1232. [PubMed] [Google Scholar]
- 9.St. Clair D K, Oberley T D, Ho Y S. FEBS Lett. 1991;293:199–203. doi: 10.1016/0014-5793(91)81186-c. [DOI] [PubMed] [Google Scholar]
- 10.Darr D J, Yanni S, Pinnell S R. Free Radical Biol Med. 1988;4:357–363. doi: 10.1016/0891-5849(88)90087-1. [DOI] [PubMed] [Google Scholar]
- 11.Krall J, Bagley A C, Mullenbach G T, Hallewell R A, Lynch R E. J Biol Chem. 1988;263:1910–1914. [PubMed] [Google Scholar]
- 12.Patel M, Day B J, Crapo J D, Fridovich I, McNamara J O. Neuron. 1996;16:345–355. doi: 10.1016/s0896-6273(00)80052-5. [DOI] [PubMed] [Google Scholar]
- 13.Palmer R M, Ferrige A G, Moncada S. Nature (London) 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 14.Nathan C. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 15.Bredt D S, Hwang P M, Glatt C E, Lowenstein C, Reed R R, Snyder S H. Nature (London) 1991;351:714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- 16.Bredt D S, Snyder S H. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- 17.Lamas S, Marsden P A, Li G K, Tempst P, Michel T. Proc Natl Acad Sci USA. 1992;89:6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hevel J M, White K A, Marletta M A. J Biol Chem. 1991;266:22789–22791. [PubMed] [Google Scholar]
- 19.Stuehr D J, Cho H J, Kwon N S, Weise M F, Nathan C F. Proc Natl Acad Sci USA. 1991;88:7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pou S, Pou W S, Bredt D S, Snyder S H, Rosen G M. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 21.Berisha H I, Pakbaz H, Absood A, Said S I. Proc Natl Acad Sci USA. 1994;91:7445–7449. doi: 10.1073/pnas.91.16.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemery B, van Klaveren R J. Hum Exp Toxicol. 1995;14:308–309. [PubMed] [Google Scholar]
- 23.Wantanabe T, Honda K. J Phys Chem. 1982;86:2617–2619. [Google Scholar]
- 24.Hogg N, Kalyanaraman B. In: Nitric Oxide Protocols. Titheradge M A, editor. Totowa, NJ: Humana; 1998. pp. 231–236. [Google Scholar]
- 25.Xia Y, Dawson V L, Dawson T M, Snyder S H, Zweier J L. Proc Natl Acad Sci USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson T M, Bredt D S, Fotuhi M, Hwang P M, Snyder S H. Proc Natl Acad Sci USA. 1991;88:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hope B T, Michael G J, Knigge K M, Vincent S R. Proc Natl Acad Sci USA. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasquez-Vivar J, Hogg N, Pritchard K A, Jr, Martasek P, Kalyanaraman B. FEBS Lett. 1997;403:127–130. doi: 10.1016/s0014-5793(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 29.Miller R T, Martasek P, Roman L J, Nishimura J S, Masters B S. Biochemistry. 1997;36:15277–15284. doi: 10.1021/bi972022c. [DOI] [PubMed] [Google Scholar]
- 30.Vasquez-Vivar J, Martasek P, Hogg N, Masters B S, Pritchard K A, Jr, Kalyanaraman B. Biochemistry. 1997;36:11293–7. doi: 10.1021/bi971475e. [DOI] [PubMed] [Google Scholar]
- 31.Kobzik L, Bredt D S, Lowenstein C J, Drazen J, Gaston B, Sugarbaker D, Stamler J S. Am J Respir Cell Mol Biol. 1993;9:371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- 32.Hollinger M A. J Pharmacol Exp Ther. 1984;230:292–294. [PubMed] [Google Scholar]
- 33.Vandenbogaerde J, Schelstraete J, Colardyn F, Heyndrickx A. Forensic Sci Int. 1984;26:103–114. doi: 10.1016/0379-0738(84)90066-5. [DOI] [PubMed] [Google Scholar]
- 34.Smith L L. Arch Toxicol Suppl. 1982;5:1–14. doi: 10.1007/978-3-642-68511-8_1. [DOI] [PubMed] [Google Scholar]
- 35.Forman H J, Aldrich T K, Posner M A, Fisher A B. J Pharmacol Exp Ther. 1982;221:428–433. [PubMed] [Google Scholar]
- 36.Maruyama K, Takeuchi M, Chikusa H, Muneyuki M. Intensive Care Med. 1995;21:778–779. doi: 10.1007/BF01704750. [DOI] [PubMed] [Google Scholar]
- 37.Eisenman A, Armali Z, Raikhlin-Eisenkraft B, Bentur L, Bentur Y, Guralnik L, Enat R. J Toxicol Clin Toxicol. 1998;36:575–584. doi: 10.3109/15563659809028051. [DOI] [PubMed] [Google Scholar]
- 38.Koppel C, von Wissmann C, Barckow D, Rossaint R, Falke K, Stoltenburg-Didinger G, Schnoy N. J Toxicol Clin Toxicol. 1994;32:205–214. doi: 10.3109/15563659409000452. [DOI] [PubMed] [Google Scholar]
- 39.Hsu K S, Lin-Shiau S Y. Eur J Pharmacol. 1995;292:315–320. doi: 10.1016/0926-6917(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 40.Berisha H, Pakbaz H, Absood A, Foda H D, Said S I. Ann NY Acad Sci. 1994;723:422–425. [PubMed] [Google Scholar]
- 41.Wink D A, Hanbauer I, Krishna M C, DeGraff W, Gamson J, Mitchell J B. Proc Natl Acad Sci USA. 1993;90:9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su W Y, Day B J, Kang B H, Crapo J D, Huang Y C, Chang L Y. Am J Physiol. 1996;271:L581–L586. doi: 10.1152/ajplung.1996.271.4.L581. [DOI] [PubMed] [Google Scholar]
- 43.Radi R, Beckman J S, Bush K M, Freeman B A. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez H H, Nieves B, Chumley P, Rivera A, Freeman B A. Free Radical Biol Med. 1996;21:43–52. doi: 10.1016/0891-5849(95)02226-0. [DOI] [PubMed] [Google Scholar]