Abstract
Endocytosis of the Flaviviridae viruses, hepatitis C virus, GB virus C/hepatitis G virus, and bovine viral diarrheal virus (BVDV) was shown to be mediated by low density lipoprotein (LDL) receptors on cultured cells by several lines of evidence: by the demonstration that endocytosis of these virus correlated with LDL receptor activity, by complete inhibition of detectable endocytosis by anti-LDL receptor antibody, by inhibition with anti-apolipoprotein E and -apolipoprotein B antibodies, by chemical methods abrogating lipoprotein/LDL receptor interactions, and by inhibition with the endocytosis inhibitor phenylarsine oxide. Confirmatory evidence was provided by the lack of detectable LDL receptor on cells known to be resistant to BVDV infection. Endocytosis via the LDL receptor was shown to be mediated by complexing of the virus to very low density lipoprotein or LDL but not high density lipoprotein. Studies using LDL receptor-deficient cells or a cytolytic BVDV system indicated that the LDL receptor may be the main but not exclusive means of cell entry of these viruses. Studies on other types of viruses indicated that this mechanism may not be exclusive to Flaviviridae but may be used by viruses that associate with lipoprotein in the blood. These findings provide evidence that the family of LDL receptors may serve as viral receptors.
Hepatitis C virus (HCV), the principal viral cause of chronic hepatitis, is not readily replicated in cell culture systems, and, as yet, no information on cell receptors for the virus is available. However, several observations from studies on the role of HCV in mixed cryoglobulinemia (1–3) have provided some insights to HCV entry into cells.
Mixed cryoglobulinemia is a systemic vasculitis associated with cold-precipitable immunoglobulins in the blood. During the past 5 years, a strong association of HCV infection with mixed cryoglobulins has been established (4), and the specific concentration of HCV in type II mixed cryoglobulins that consist of polyclonal IgG and monoclonal IgM has been demonstrated (1). It also was shown that very low density lipoprotein (VLDL) is selectively associated with HCV in type II cryoglobulins (2). In studies on the cutaneous vasculitic lesions in type II cryoglobulinemia using in situ hybridization (ISH), the HCV RNA virion form (positive strand) but not the putative replicative form (negative strand) of the virus was detected in keratinocytes in lesions but not normal skin of the same patients (3). Furthermore, it was demonstrated that LDL receptors were up-regulated on keratinocytes in cutaneous vasculitis lesions compared with normal skin (3). These observations and the finding that anti-β lipoprotein precipitates HCV from infected serum (5) suggested that the low density lipoprotein (LDL) receptor also may be a receptor for HCV complexed to VLDL or LDL. This hypothesis led to this study to determine whether the LDL receptor is also a receptor for HCV and other members of the family of viruses Flaviviridae.
Materials and Methods
Reagents, Antisera Virus Stocks, and Cell Lines.
Cyclohexanedione, phenylarsine oxide (PAO), heparin sulfate, and EGTA were purchased from Sigma; 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine iodine (DiI) was purchased from Molecular Probes. Purified IgG 2a mouse monoclonal anti-LDL receptor antibody (C7 clone) was obtained from Oncogene Scientific Products (Cambridge, MA). Anti-bovine viral diarrhea virus (BVDV) envelope antibody bovine serum, α49, was provided by Marc S. Collett (Viro Pharma, Malvern, PA). Mouse monoclonal IgG 2a anti-CD-16, anti-CD-19, and anti-transferrin (CD71) were purchased from Immunotech (Hialeah, FL). Anti-μ was purchased from Jackson ImmunoResearch. Anti-apolipoprotein (αapo) E and αapo A-I were purchased from Cortex Pharmaceuticals (San Leandro, CA); αapo B was purchased from Sigma. Purified mouse monoclonal IgG αapo E (1D7), αapo A-I (3G10), and αapo B (4G3) were purchased from the University of Ottawa Heart Institute. F(ab′)2, an antibody fragment with two antigen-combining sites, preparations of mouse IgG were prepared by treating the mouse monoclonal antibodies from 30 minutes to 10 hours with 3% pepsin (Sigma) (pH 3.5) at 37°C. The F(ab′)2 fragments were isolated by column chromatography using a HR 10/30 Superose 12 column (Amersham Pharmacia). Bovine viral diarrhea virus-free donor calf serum was purchased from Boyt Veterinary Laboratory (Neosho, MO). Potassium bromide density gradient ultracentrifugation was used for preparation of VLDL, LDL, and high density lipoprotein (HDL) from normal sera, and these lipoproteins complexed to HCV from infected sera. The VLDL band [density (d) = 0.95–1.006 g/ml], the LDL band (d = 1.019–1.063), the HDL band (d = 1.063–1.21 g/ml), and HCV free of lipoproteins (d > 1.21) were isolated by aspiration and then dialyzed against Hanks’ balanced salt solution (Sigma) containing 0.01% EDTA. Isolated HCV-VLDL was dissociated to HCV and VLDL by treatment with deoxycholate and fractionated by sucrose density gradient ultracentrifugation as described (6). The high density HCV fraction, free of lipoproteins, was further fractionated by column chromatography on a lecithin pretreated Superose 6 (Amersham Pharmacia). The peak of HCV present in the void volume was contaminated with small amounts of immunoglobulins that were removed by using immobilized rProtein A (Repligen). Immoblotting (dot blots) to detect small amounts of protein was performed as described (7). Sensitivity of the assay was 100 pg for IgG and IgM and 200 pg for apolipoproteins B and E. Lipoproteins were quantitated by Lowry assay using commercial kits (Sigma). Highly purified VLDL, LDL, and HDL were purchased from Cortex. Labeling of LDL with DiI was performed as described (8).
Infected human sera were used as stocks for HCV (3 × 108 genomic equivalents per millimeter [gE/ml]), GB virus C/hepatitis G virus (HGV) (2 × 109 gE/ml), and herpes simplex virus (HSV). BVDV strains NY-1 and National Animal Disease Laboratory (NADL) and vesicular stomatitis virus (VSV), Indiana strain, and respiratory syncytial virus were obtained from American Type Culture Collection (Rockville, MD). Bovine turbinate (BT) and kidney (MDBK) cell lines, Hep G2, a hepatoma cell line that is biochemically similar to hepatocytes (9), Daudi, a B cell lymphoblastoid cell line, the Molt-4 T cell line, HEp 2, a squamous carcinoma cell line, and normal fibroblasts (MRC-5) were obtained from the American Type Culture Collection. The B lymphocyte lines G4 and E11 were generated from fusion of F3B6 human-mouse heterohybridoma with peripheral B cells from patients with type II cryoglobulinemia and rheumatoid arthritis, respectively. Development of the 35G6 peripheral B cell line, cloned from a normal patient, was previously described (10). Four LDL receptor negative cell lines, GM00488C, GM02000G, GM00701C, and GMO3040B, were obtained from the National Institute of General Medical Sciences, Human Genetics Mutant Cell Repository, Coriell Institute for Medical Research (Camden, NJ). Cells resistant to infection with BVDV (CRIB) were provided by R. O. Donis (University of Nebraska, Lincoln, NE).
LDL Receptor Assays.
Cells were cultured in RPMI medium 1640 supplemented with 10% BVDV-free bovine calf serum or expression of the LDL receptor was up-regulated by culturing for 24 hours in RPMI medium 1640 supplemented with 10% lipoprotein-deficient BVDV-free medium. The cells then were washed twice with PBS (pH 7.2). Cytospin preparations were made, fixed with acetone, and blocked with 5% normal mouse serum, and the LDL receptor was visualized by incubating the slides with 5 μg/ml purified IgG 2a monoclonal anti-LDL receptor antibody followed by a 1:50 dilution of FITC-labeled goat anti-mouse [F(ab′)2] second antibody (Jackson ImmunoResearch). The demonstration of LDL receptors on adherent cells, MDBK, CRIB, fibroblasts, Hep G2, and HEp 2 was performed in the same manner except monolayers of cells were cultured and fixed on slides.
Demonstration of endocytosis of DiI-LDL by cells was performed by incubation of 2 × 105 cells for 2 hours at 37°C in 5% CO2 with 20 μg/ml DiI-LDL as described (11). The cells were washed twice with cold PBS and were fixed with 1% buffered paraformaldehyde, and cytospin preparations were made for fluorescent microscopic studies or cells in suspension were analyzed by flow cytometry. Flow cytometric analysis was performed by using the Epic XL-MCL cytometer (Coulter) using a 575 BP filter. Nonspecific binding of DiI-LDL was determined by using DiI-LDL treated with cyclohexanedione and was subtracted from the DiI-LDL binding to give specific DiI-LDL binding to cultured cells.
HCV RNA and Endocytosis Assays.
HCV RNA, reverse transcriptase (RT)–PCR, and ISH assays were performed as described (12). Specificity of the ISH method for HCV was determined by comparing monolayers of human fibroblasts inoculated with either 3 × 107 gE/ml HCV or dilutions of adenovirus or respiratory syncytial virus that produced pathologic changes in cells at 24 hours. After incubation for 24 hours at 37°C, the cultures were assayed for HCV RNA by ISH. The endocytosis assay for HCV was performed as described (12). Daudi cells (5 × 105 cells) were inoculated with 3 × 107 gE HCV or GB virus C/HGV, were incubated for 3 hours at 37°C, were washed three times, and were assayed for intracytoplasmic HCV RNA or GB virus C/HGV RNA by ISH. RT-PCR and ISH assays for GB virus C/HGV RNA were performed as described (13). The same methodology also was used for studies with HCV-lipoprotein recombinants. One hundred micrograms each of normal VLDL, LDL, HDL or cyclohexanedione-treated VLDL, or LDL were incubated with 106 gE HCV free of lipoproteins and immunoglobulins for 30 minutes at 37°C and then were added to the Daudi cells.
For cytolytic viruses, BVDV, NADL, VSV, and HSV, various dilutions of the respective viruses were incubated with monolayers of cells at 4°C for 1 hour, were washed three times with cold PBS, and were incubated with fresh medium. Virus dilutions that produced complete cytolysis at 72 hours for BVDV and VSV and 48 hours for HSV were selected. Immunofluorescent detection of intracytoplasmic BVDV was performed on acetone-fixed slides by using 1:50 dilutions of anti-BVDV serum and a FITC-labeled anti-bovine second antibody. The presence of BVDV in cells was confirmed by RT-PCR using BVDV specific primers (14).
Inhibition Studies.
Blocking of LDL receptor with various dilutions of antibodies (anti-LDL receptor, 5–20 μg/ml; control antisera, 5–20 μg/ml) or inhibitors was performed by pretreatment of cells with various concentrations of antisera or inhibitor for 15 minutes at 37°C and inoculating with virus without washing the cells. Additions of antisera during incubation period were made at 45-minute intervals. Treatment of LDL and VLDL with cyclohexanedione was performed as described (15). In experiments with cytopathic virus, cells were pretreated with 50 μg/ml of anti-LDL receptor antibody for 30 minutes at 4°C before inoculation with virus at 4°C.
Inhibition of endocytosis by PAO was assessed by pretreating cells with a range of final PAO concentration of 0.1–100 μM as described (16), and then endocytosis of LDL or HCV was evaluated by the DiI-LDL assay or HCV-ISH, respectively, as described earlier.
Results
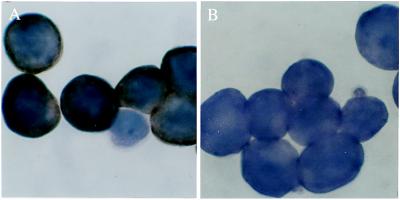
Endocytosis of HCV via the LDL Receptor. We previously demonstrated that endocytosis of HCV in vitro correlates with the titer of HCV in the inoculum. The percentage of cells positive for HCV RNA by ISH correlated directly with the number of gE of HCV per cell as determined by RT-PCR (12). There was also a crude correlation between intensity of ISH staining for HCV RNA and gE HCV per cell by RT-PCR. The specificity of this ISH assay for HCV is shown in Fig. 1. Cell cultures infected with viruses other than HCV were negative using this assay.
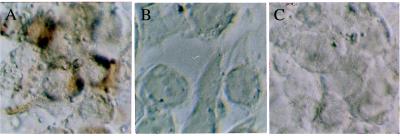
Figure 1.
Demonstration of the specificity of the ISH method for HCV. (A) HEp 2 cells 24 hours after inoculation with HCV. The brown staining indicates the presence of positive-strand HCV. (B and C) HEp 2 cells incubated with respiratory syncytial virus or adenovirus, respectively, show no staining for HCV using the same ISH method as in A. Note the cytopathic effect of these viruses on the Hep 2 monolayers. Original magnification, ×500.
For a further investigation of endocytosis of HCV by cells in vitro, a variety of human cell cultures (see Materials and Methods) were demonstrated to have LDL receptors with the use of anti-LDL receptor antibody or DiI-LDL uptake. These cell lines then were inoculated with a high titer HCV-positive human serum. Intracellular HCV RNA then was detected by using ISH. To determine whether endocytosis of HCV correlated with the level of LDL receptor expression on cells, the well known modulatory effect of lipoproteins on the LDL receptor was used to increase the number of LDL receptors (up-regulate) on cells by culturing in lipoprotein-deficient media. Relative differences in endocytosis of LDL by various cultured cell lines could be demonstrated by the specific uptake of DiI-LDL. The specific DiI-LDL uptake of Hep G2 cells as shown in Table 1 was four times greater than of the peripheral B cell line, G4, without up-regulation.
Table 1.
Uptake of LDL by cultured cells
| Cell line | Culture medium | Treatment | Mean fluorescence |
|---|---|---|---|
| G4 | Routine* | None | 0.8 ± 0.4 |
| G4 | Routine | DiI-LDL | 4 ± 2.0 |
| G4 | Lipoprotein deficient† | DiI-LDL | 16 ± 7 |
| Hep G2 | Routine | None | 3 ± 1 |
| Hep G2 | Routine | DiI-LDL | 16 ± 5 |
RPMI with 10% fetal bovine serum.
RPMI with lipoprotein-deficient serum.
Up-regulating these B cells produced a LDL uptake equivalent to that of the Hep G2 cells without up-regulation. These results were confirmed by immunofluorescent studies using anti-LDL receptor antibody staining and DiI-LDL uptake (Fig. 2 A and B).
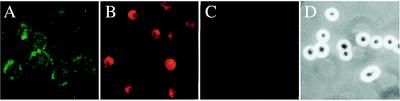
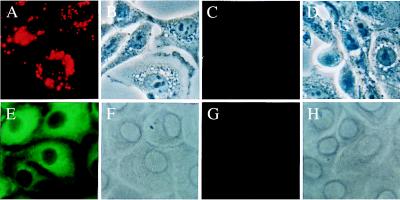
Figure 2.
(A) Demonstration of the LDL receptor on up-regulated G4 cell using anti-LDL receptor antibody (green fluorescent staining). The signal was very weak in ≈30% of G4 cells without up-regulation of the LDL receptor (not shown). (B) Demonstration of the uptake of 5 μg of DiI-LDL by G4 cells that have up-regulated LDL receptors as in A. (C) Inhibition of 5 μg of DiI-LDL endocytosis by 30× excess of unlabeled LDL. (D) Same field as C by phase contrast microscopy. Original magnification, ×500.
Up-regulation of the LDL receptors of G4 cells resulted in 70–80% of cells staining positive for the LDL receptor, a result that was similar to the 70–80% cells positive for the LDL receptor on Hep G2 cells (Fig. 3E) and Daudi cells (Fig. 3G), also known to have higher densities of LDL receptor (11).
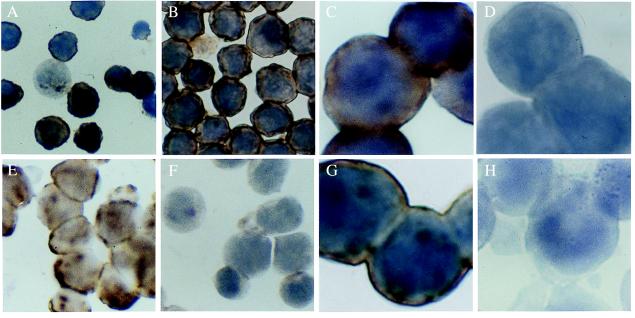
Figure 3.
(A) HCV ISH of HCV-inoculated G4 cells that were not up-regulated. Dark brown cytoplasmic staining indicates the presence of the virion form of HCV; only ≈30% of the cells are weakly positive. (B) By contrast, HCV was present in most cells in cultures in which the LDL receptor was up-regulated before HCV exposure. Original magnification for A and B, ×500. (C) High-power view of HCV-infected G4 cells with up-regulated LDL receptor as in B. (D) G4 cells prepared as in B and C were pretreated with anti-LDL receptor antibody. Blocking of the receptor by the antibodies decreased the uptake of the virus below the detection limit of ISH. Control antisera (see text) did not have such blocking effect (not shown). Original magnification for C and D, ×1,250. (E) Uptake of HCV by Hep G2 hepatoma cell line is shown by ISH. (F) Blocking the LDL receptor with its specific antibody completely prevented the endocytosis of the virus, as demonstrated by ISH in E. Original magnification for E and F, ×500. (G) Incubation of Daudi cells with HCV-positive serum resulted in positive staining by ISH. (H) Pretreatment of Daudi cells with 2 μM PAO completely inhibited the endocytosis of HCV, as illustrated in G. Original magnification for G and H, ×1250.
The percentage of cells positive for HCV by ISH was shown to correlate with the percentage of cells positive for LDL receptor by immunofluorescence using anti-LDL receptor antibody or DiI-labeled LDL. Endocytosis of HCV by peripheral B cells that showed 5–30% weakly positive cells in routine culture (Fig. 3A) when up-regulated showed a percentage of positive cells and an intensity of staining (Fig. 3B) comparable to Hep G2 cells (Fig. 3E) and Daudi cells (Fig. 3G) that were each 70–80% positive.
Direct evidence that the LDL receptor-mediated endocytosis of HCV was obtained by inhibiting endocytosis with anti-LDL receptor antibody. The endocytosis of HCV could be inhibited in a dose-dependent manner by preincubating the cells with anti-LDL receptor antibody. At sufficient concentrations of anti-LDL receptor antibody, complete inhibition of endocytosis of the virus could be demonstrated for both G4 and Hep G2 cells (Fig. 3 C–F). Similar results were obtained by using infected serum or VLDL-HCV complexes isolated from type II cryoglobulins as the inoculum in these experiments. No inhibition was observed with control mouse IgG 2a or antisera to specific cell surface antigens at a concentration up to 20 times the lowest inhibiting concentration of anti-LDL receptor: antisera to μ heavy chain, CD-19, and CD-16 surface antigens on peripheral B cells and Daudi cells and antiserum to transferrin receptor on Hep G2 cells did not inhibit the endocytosis of HCV by these cells. Moreover, treatment of Daudi cells with the endocytosis inhibitor PAO at 2 μM concentration completely inhibited the endocytosis of HCV (Fig. 3 G and H).
The role of the LDL receptor in the endocytosis of HCV was confirmed by demonstrating competitive inhibition with LDL and VLDL but not HDL, which is known not to bind to the LDL receptor. With use of hepatoma cells (Hep G2) or B cells (G4 and E11), 25–100 μg/ml of LDL or VLDL completely inhibited endocytosis of HCV whereas concentrations of HDL up to 200 μg/ml (a 5- to 20- and 10- to 40-fold molar excess over LDL and VLDL, respectively) did not inhibit. Treatment of LDL or VLDL with cyclohexanedione, which is known to alter a critical arginine residue in the LDL receptor binding site of apolipoproteins E and B (15), the main apolipoproteins found in VLDL and LDL, respectively, eliminated the inhibition by LDL or VLDL. Moreover, 25 units/ml of heparin sulfate or 2 μM EGTA inhibited endocytosis of HCV. Both are known inhibitors of LDL receptor endocytosis of lipoprotein (17).
To determine whether lipoproteins were involved in the endocytosis of HCV, we performed inhibition studies using various previously characterized antisera to apolipoproteins [αapo E, ID7 (18); αapo B, 4G3 (19); and αapo A-I, 3G10 (20)]. F(ab′)2 fragments were prepared and were used for all of the studies; inhibitory activities of the preparations were tested against DiI-labeled VLDL, LDL, and HDL isolated from a normal serum. Optimum F(ab′)2 antibody concentrations and conditions for inhibition of endocytosis were determined. Optimum conditions required addition of F(ab′)2s after pretreatment during the incubation period, and both αapo E and αapo B were required for maximal inhibition of VLDL endocytosis, whereas αapo B was sufficient for maximal inhibition of LDL endocytosis. Under these conditions, the maximum inhibition of HCV endocytosis achieved was 65%, with the remaining positive cells showing only trace staining. Pretreatment with αapo A-I gave 10% inhibition, with the remaining positive cells showing no decrease of staining compared with the control. The addition of αapo A-I during incubation did not increase inhibition. The finding that both αapo E and αapo B were required and that additional F(ab′)2s during the incubation increased inhibition was most likely attributable to the complexity of VLDL metabolism and dissociation of F(ab′)2s binding at 37°C. Hence, it could not be determined whether VLDL alone or both VLDL and LDL mediated endocytosis of HCV. Moreover, because complete inhibition could not be achieved, direct endocytosis of HCV by the LDL receptor could not be excluded.
Endocytosis experiments of isolated HCV-lipoprotein complexes and recombination experiments with HCV and lipoproteins provided more definitive data on the role of lipoproteins in endocytosis of HCV via the LDL receptor. Isolation of HCV by dissociation of HCV-VLDL complexes was unsuccessful; however, density gradient fractionation of a serum containing a high concentration of HCV produced not only HCV lipoprotein fractions but also a high density HCV fraction free of lipoprotein. Immunoglobulins contaminating the latter fraction were removed, providing a “free” HCV fraction for recombinant studies. Comparison of endocytosis of the various fractions is shown in Table 2. The HCV-VLDL and HCV-LDL, but not the HCV-HDL or high density HCV, fractions were endocytosed. Addition of VLDL or LDL but not HDL, isolated from normal serum, to the free HCV resulted in restoration of endocytosis. Cyclohexanedione treatment of the VLDL or LDL abrogated the rescue (data not shown).
Table 2.
Comparison of endocytosis of HCV in lipoprotein fractions and the high density HCV fraction
| Fraction | HCV, gE/ml | Lipoprotein, concentration, mg/ml | Endocytosis of 5 μg DiI-labeled fraction mean fluorescence, log scale | Endocytosis of 1 × 106gEHCV from each fraction, percent cell positive, intensity of staining |
|---|---|---|---|---|
| VLDL | 2.5 × 106 | 0.48 | 8.49 | 90%, ++ |
| LDL | 2.9 × 106 | 1.47 | 9.98 | 75%, + |
| HDL | 8.3 × 106 | 2.56 | 1.88 | 0 |
| d > 1.21 | 3.9 × 106 | − | − | 0 |
++, Moderately positive; +, weakly positive.
Further studies were performed by using the LDL receptor-deficient fibroblast cells (21). Inoculation of these cells with HCV showed only weak endocytosis that could not be increased with preincubation of cells in lipoprotein deficient medium nor inhibited by anti-LDL receptor antibody (data not shown). Furthermore, this low level endocytosis could not be competitively inhibited with excess VLDL.
Replication of Endocytosed HCV.
Replication of HCV has been reported in Hep G2 and Daudi cell cultures. Extended cultures of Hep G2, Daudi, and G4 cells were tested serially by ISH for evidence of replication. In the Hep G2 cells, only positive-strand HCV was detected in the cells up to 1 week, but, at 3 weeks, 85% of the cells contained positive-strand HCV and 65% contained negative-strand HCV (data not shown). At 4 weeks, the cells were negative for HCV. In Daudi cells, only positive strand was detected through day 10, but on days 15 and 20, both positive- and negative-strand genome sequences were present in 80% cells. The cells died in the fourth week of culture. Only the positive strand of HCV was detected in G4 cells up to 1 week; the cells died after 1 week.
Endocytosis of Other Flaviviridae Viruses.
We investigated commercial bovine sera that were known to be contaminated with the pestivirus BVDV (22, 23). Human cell lines routinely cultured in media containing bovine serum were found to be positive for intracytoplasmic BVDV by immunofluorescence using anti-BVDV antibody. The presence of BVDV was confirmed by RT-PCR using BVDV-specific primers. Negative-strand BVDV was not detected in cells nonpermissive to infection. BVDV-positive human nonpermissive cells became negative over a 4-week culture period in noncontaminated media. Endocytosis of BVDV by nonpermissive cultured cells could be inhibited completely with anti-LDL receptor antibody but not with the control anti-transferrin receptor antibody (data not shown for this set of experiments).
With use of the cytopathic NADL strain of BVDV and permissive cells, BT or bovine kidney (MDBK) cells, anti-LDL receptor antibody but not control antiserum inhibited the cytopathic effect and positive fluorescence at 3 days (Fig. 4 A–D). Five days after infection, there was complete cytolysis of both the inhibited and control cells. Similar studies using VSV and HSV showed no inhibition of infection by anti-LDL receptor antibody for HSV (Fig. 4 E, F, and I) but some inhibition for VSV at 48 hours, which was no longer apparent at 72 hours (Fig. 4 G–I).
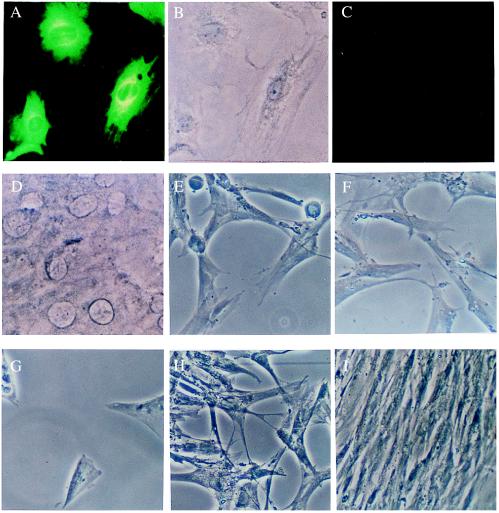
Figure 4.
(A) Demonstration of infection of BT cell monolayers by cytopathic BVDV (NADL strain) after 72 hours of incubation by immunofluorescence using anti-BVDV (green fluorescent staining). (B) Same field as in A by phase contrast microscopy. (C) Preincubation of BT cell monolayers with anti-LDL receptor antibody has completely prevented the infection (shown in A) of BT cells, as seen after 72 hours of culturing. Same detection method as in A. (D) Phase contrast photomicrograph of the same field as in C. Original magnification for A–D, ×500. (E) Inoculation of MRC-5 fibroblasts with HSV resulted in widespread cytolysis and destruction of the monolayer after 48 hours. (F) Pretreatment of the monolayers with anti-LDL receptor antibody, using the same protocol as in C, did not prevent cytolysis and cell death. (G) Incubation of MRC-5 cells with dilutions of VSV also resulted in cytopathy and deterioration of the monolayer. (H) Pretreatment with anti-LDL receptor showed some inhibition of the destruction of the cells. Pretreatment and incubation conditions were as in C and F. (I) Control MRC-5 cell monolayer treated with anti-LDL receptor antibody as in C, F, and H but not inoculated subsequently with virus. Note the uniformity and healthy appearance of these fibroblasts. Original magnification for E–I, ×250.
Additional evidence for endocytosis of BVDV by LDL receptor was obtained by using a cell line (CRIB) resistant to BVDV that was derived from a permissive bovine kidney cell line (MDBK). As illustrated in Fig. 5, the CRIB cells that do not permit entry of BVDV (24) also do not endocytose LDL. The CRIB cells were negative for LDL receptors by immunofluorescence using anti-LDL receptor antibody (data not shown). However, the absence of DiI-LDL staining (see Fig. 5) is a more sensitive indication of the absence of LDL receptor and LDL receptor endocytosis because accumulation of DiI-LDL occurs from the rapid turnover of LDL by LDL receptor in the course of cholesterol metabolism.
Figure 5.
(A) Intense uptake of DiI-LDL by a monolayer of the MDBK cells. (B) Phase contrast microscopy of the same field as A. (C) The lack of endocytosis of DiI-LDL was demonstrated in the CRIB cell line that is resistant to BVDV infection. (D) Same field as in C with phase contrast. (E) Demonstration of the infection of MDBK cells with the NY-1 noncytopathic strain of BVDV after 72 hours of incubation by immunofluorescence using anti-BVDV (green fluorescent staining). (F) Phase contrast picture of E. (G) No BVDV was demonstrated by immunofluorescence in the BVDV-resistant CRIB cells that have been incubated with the virus. Conditions were as in E–F. (H) Phase contrast for G. Original magnification for A–H, ×500.
A third member of the Flaviviridae family, GB virus C/HGV, was reported to associate with lipoproteins in the blood (25). Evidence also was obtained for LDL receptor-mediated endocytosis of this virus, as illustrated in Fig. 6.
Figure 6.
(A) Daudi cells inoculated with HGV show the presence of HGV virion in the cytoplasm using ISH specific for this virus. (B) Preincubation of the cells with anti-LDL receptor antibody decreased the uptake of this virus below the detection limit of ISH. Original magnification for A and B, ×1,250.
Discussion
Several lines of evidence were presented supporting the hypothesis that HCV and other members of Flaviviridae are endocytosed via the LDL receptor. Endocytosis of HCV was shown to correlate with cell LDL receptor activity by experimental modulation of LDL receptor activity and with the striking demonstration that the CRIB cell line, resistant to infection with BVDV for unknown reasons (24), lacked detectable LDL receptor activity. Direct evidence for the hypothesis was provided by inhibiting endocytosis of HCV, BVDV, and GB virus C/HCV with anti-LDL receptor antibody. Moreover, known biochemical inhibitors of LDL endocytosis and the endocytosis inhibitor, PAO, inhibited LDL receptor-mediated endocytosis of these viruses. The reports that up-regulation of LDL receptors facilitated persistent HCV infection in vitro (26) and the induction of HCV binding with transfection of the LDL receptor gene in COS-7 cells that were unable to bind HCV (27) are consistent with these findings.
Evidence also was presented that endocytosis of HCV via the LDL receptor was mediated by the complexing of HCV to VLDL or LDL. Although, HCV also appeared to complex with HDL in the serum to the same extent as VLDL and LDL, there was no evidence of HDL-mediated endocytosis of HCV. It could not be determined from the studies performed whether the low level of endocytosis of HDL compared with those of VLDL and LDL, as shown by cytometric studies with DiI-labeled lipoproteins, was responsible for the absence of detectable levels of HDL-mediated endocytosis of HCV.
The complete inhibition by anti-LDL receptor antibody of HCV endocytosis by a variety of cells in vitro suggests that the LDL receptor may be the main mechanism for entry of HCV into cells. However, the detection of small amounts of intracellular HCV in LDL-deficient fibroblasts inoculated with HCV that could not be inhibited by anti-LDL receptor antibody suggested that receptors not related to the LDL receptor mediate viral entry in these cells. Furthermore, the finding that there was only a delaying effect of anti-LDL receptor antibody on the cytopathic effect of the NADL strain of BVDV indicates that receptors for BVDV other than LDL receptors or low affinity LDL receptors were present. This finding is consistent with the observations of Flores and Donis (24) that CRIB cells could be infected with high concentrations of BVDV. Hence, the LDL receptor may be the main but not exclusive means of entry of these viruses into these cells.
The evidence of HCV replication in Hep G2 and Daudi cells, although transient, suggests that endocytosis of HCV mediated by the LDL receptor can result in infection. Whether this route of entry can result in a productive infection in vivo and whether all members of the Flaviviridae family of viruses use this mechanism of cell entry remain to be determined. The evidence presented in this study that VSV also may be endocytosed by the LDL receptor appears to be contrary to an earlier study (28) that found interferon-α induces a soluble LDL receptor fragment that inhibits VSV infection but concluded that the LDL receptor was not a receptor for VSV. The latter conclusion was reached because VSV was found to infect LDL receptor-deficient fibroblasts. From our finding that entry of HCV into LDL receptor-deficient fibroblast was independent of the LDL receptors, it is apparent that Fischer et al. (28) did not present definitive evidence that VSV was not endocytosed by means of the LDL receptor because receptors for VSV other than LDL receptors may have been present on those cells.
LDL receptor-mediated endocytosis may not be exclusive to Flaviviridae. Rather, this mechanism may be used by all viruses that can associate with VLDL or LDL in the blood. The reported association of VSV with lipoproteins in the blood, particularly VLDL (29), is consistent with this notion.
Thus far, there only has been indirect evidence of in vivo endocytosis of HCV mediated by the LDL receptor, that is, the apparent endocytosis of HCV by keratinocytes in the inflammatory skin lesions of patients with type II cryoglobulinemia (3). In this study, evidence that BVDV contaminated media from bovine serum is endocytosed by a wide variety of human cells nonpermissive to BVDV infection suggests that this mechanism may explain the presence of positive-strand HCV that has been widely reported in human nonhepatic cells, particularly peripheral blood mononuclear cells. This mechanism must be considered before assigning HCV tropism for tissues other than the liver where clear evidence for HCV replication has been demonstrated.
The potential involvement of the LDL receptor in HCV infection has implications for the current therapy of HCV infection and also provides the rationale for a new approach to therapy—specifically, administration of anti-LDL receptor antibody or analogues of the LDL receptor binding site of apolipoproteins B and E. The effect of IFNα, the current therapy for HCV infection, may be mediated in part by the down-regulation of LDL receptors. IFNα is known to induce IL-1 receptor antagonist (30), which blocks the IL-1 receptor-mediated stimulation by IL-1. Because IL-1 is known to increase LDL receptor activity (31), IFNα would indirectly cause a down-regulation of LDL receptor activity by stimulating IL-1 receptor antagonist production, thereby decreasing IL-1 receptor-mediated stimulation by IL-1.
It has been reported that a minor group of human rhinovirus (HRV2) (32) and Rous sarcoma virus A (33) enters cells via the LDL receptor-related protein. In the latter report, evidence was presented that a viral envelope moiety binds to LDL receptor-related protein. Our study provides evidence that the family of LDL receptors may serve as viral receptors.
Acknowledgments
We are grateful to Dr. Marc S. Collett for providing BVDV reagents and advice, Dr. Ruben O. Donis for providing the CRIB cell line, Dr. Charles A. Dinarello for helpful discussions, and Polly Zorolow for editing. This study was supported in part by the Robert E. Wise, M.D., Research and Education Institute, Lahey Clinic Medical Center, Burlington, MA, and National Institutes of Health Grant 1R21AI40672.
Abbreviations
- αapo
anti-apolipoprotein
- BT
bovine turbinate
- BVDV
bovine viral diarrheal virus
- CRIB
cells resistant to infection with BVDV
- d
density
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine iodine
- gE
genomic equivalents
- HDL
high density lipoprotein
- HCV
hepatitis C virus
- HGV
hepatitis G virus
- HSV
herpes simplex virus
- ISH
in situ hybridization
- LDL
low density lipoprotein
- NADL
National Animal Disease Laboratory
- PAO
phenylarsine oxide
- RT
reverse transcriptase
- VLDL
very low density lipoprotein
- VSV
vesicular stomatitis virus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
This work was presented in part at the Keystone Symposium on Molecular and Cellular Biology: Hepatitis C and Beyond, Burlington, VT, January 23–29, 1996 (abstr. 007), the Ninth Triennial International Symposium on Viral Hepatitis and Liver Disease, Rome, April 21–26, 1996 (abstr. B122), and the Fifth International Meeting on Hepatitis C Viruses Molecular Virology and Pathogenesis, Venice, Italy, June 25–28, 1998 (abstr. F3).
References
- 1.Agnello V, Chung R T, Kaplan L M. N Engl J Med. 1992;327:1490–5. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 2.Agnello V. Springer Semin Immunopathol. 1997;19:111–129. doi: 10.1007/BF00945029. [DOI] [PubMed] [Google Scholar]
- 3.Agnello V, Ábel G. Arthritis Rheum. 1997;40:2007–2015. doi: 10.1002/art.1780401113. [DOI] [PubMed] [Google Scholar]
- 4.Monti G, Galli M, Invernizzi F, Pioltelli P, Saccardo F, Monteverde A, Pietrogrande M, Renoldi P, Bombardieri S, Bordin G. Q J Med. 1995;88:115–26. [PubMed] [Google Scholar]
- 5.Thomssen R, Bonk S, Propfe C, Heermann K H, Kochel H G, Uy A. Med Microbiol Immunol. 1992;181:293–300. doi: 10.1007/BF00198849. [DOI] [PubMed] [Google Scholar]
- 6.Prince A M, Huima-Byron T, Parker T S, Levine D M. J Viral Hepat. 1996;3:11–17. doi: 10.1111/j.1365-2893.1996.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 7.Agnello V, Barnes J L. J Exp Med. 1986;164:1809–1814. doi: 10.1084/jem.164.5.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold K S, Inneratiry T, Pitas R, Mahley R. In: Lipoprotein Analysis: A Practical Approach. Converse C A, Skinner E R, editors. Oxford: IRL; 1992. pp. 145–168. [Google Scholar]
- 9.Knowles B B, Howe C C, Aden D P. Science. 1980;209:497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 10.Knight G B, Agnello V, Bonagura V, Barnes J L, Panka D J, Zhang Q X. J Exp Med. 1993;178:1903–1911. doi: 10.1084/jem.178.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yen C F, Kalunta C I, Chen F S, Kaptein J S, Lin C K, Lad P M. J Immunol Methods. 1994;177:55–67. doi: 10.1016/0022-1759(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 12.Agnello V, Ábel G, Knight G B, Muchmore E. Hepatology. 1998;28:573–584. doi: 10.1002/hep.510280240. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Knight G B, Ábel G, Agnello V. J Virol Methods. 1999;79:149–159. doi: 10.1016/s0166-0934(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 14.Pellerin C, van den Hurk J, Lecomte J, Tussen P. Virology. 1994;203:260–268. doi: 10.1006/viro.1994.1483. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd J, Bicker S, Lorimer A R, Packard C J. J Lipid Res. 1979;20:999–1006. [PubMed] [Google Scholar]
- 16.Kreutz L C, Ackermann M R. Virus Res. 1996;42:137–147. doi: 10.1016/0168-1702(96)01313-5. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian R, Hiroaki H, Kashyp T M. J Lab Clin Med. 1995;125:479–485. [Google Scholar]
- 18.Weisgraber K H, Innerarity T L, Harder K J, Mahley R W, Milne R W, Marcel Y L, Sparrow J T. J Biol Chem. 1983;258:12348–12354. [PubMed] [Google Scholar]
- 19.Pease R J, Milne R W, Jessup W K, Law A, Provost P, Fruchart J-C, Dean R T, Marcel Y L, Scott J. J Biol Chem. 1990;265:553–568. [PubMed] [Google Scholar]
- 20.Marcel Y L, Provost P R, Koa H, Raffai E, Dac N V, Fruchart J-C, Rassart E. J Biol Chem. 1991;266:3644–3653. [PubMed] [Google Scholar]
- 21.Mahley R W, Innerarity T L, Pitas R E, Weisgraber K H, Brown J H, Gross E. J Biol Chem. 1977;252:7279–7287. [PubMed] [Google Scholar]
- 22.Nuttall P A, Luther P D, Stott E J. Nature (London) 1977;266:835–837. doi: 10.1038/266835a0. [DOI] [PubMed] [Google Scholar]
- 23.Yanagi M, Bukh J, Emerson S U, Purcell R H. J Infect Dis. 1996;174:1324–1327. doi: 10.1093/infdis/174.6.1324. [DOI] [PubMed] [Google Scholar]
- 24.Flores E F, Donis R O. Virology. 1995;208:565–575. doi: 10.1006/viro.1995.1187. [DOI] [PubMed] [Google Scholar]
- 25.Sato K, Tanaka T, Okamoto H, Miyakawa Y, Mayumi M. Biochem Biophys Res Commun. 1996;229:719–725. doi: 10.1006/bbrc.1996.1871. [DOI] [PubMed] [Google Scholar]
- 26.Seipp S, Mueller H M, Pfaff E, Stremmel W, Theilmann L, Goeser T. J Gen Virol. 1997;78:2467–2476. doi: 10.1099/0022-1317-78-10-2467. [DOI] [PubMed] [Google Scholar]
- 27.Monazahian M, Bohme I, Bouk S, Koch A, Scholz C, Grethe S, Thomssen R. J Med Virol. 1999;57:223–229. doi: 10.1002/(sici)1096-9071(199903)57:3<223::aid-jmv2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Fischer D G, Tal N, Novick D, Barak S, Rubinstein M. Science. 1993;262:250–253. doi: 10.1126/science.8211145. [DOI] [PubMed] [Google Scholar]
- 29.Mills B J, Beebe D P, Cooper N R. J Immunol. 1979;123:2518–2524. [PubMed] [Google Scholar]
- 30.Tilg H, Mier J W, Vogel W, Aulitzky W E, Wiedermann C J, Vannier E, Huber C, Dinareello C A. J Immunol. 1993;150:4687–4692. [PubMed] [Google Scholar]
- 31.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 32.Hofer F, Gruenberger M, Kowalski H, Machat H, Huettinger M, Kuechler E, Blaas D. Proc Natl Acad Sci USA. 1994;91:1839–1842. doi: 10.1073/pnas.91.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bates P, Young J A, Varmus H E. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]