Abstract
Theoretical models suggest that overlapping generations, in combination with a temporally fluctuating environment, may allow the persistence of competitors that otherwise would not coexist. Despite extensive theoretical development, this “storage effect” hypothesis has received little empirical attention. Herein I present the first explicit mathematical analysis of the contribution of the storage effect to the dynamics of competing natural populations. In Oneida Lake, NY, data collected over the past 30 years show a striking negative correlation between the water-column densities of two species of suspension-feeding zooplankton, Daphnia galeata mendotae and Daphnia pulicaria. I have demonstrated competition between these two species and have shown that both possess long-lived eggs that establish overlapping generations. Moreover, recruitment to this long-lived stage varies annually, so that both daphnids have years in which they are favored (for recruitment) relative to their competitor. When the long-term population growth rates are calculated both with and without the effects of a variable environment, I show that D. galeata mendotae clearly cannot persist without the environmental variation and prolonged dormancy (i.e., storage effect) whereas D. pulicaria persists through consistently high per capita recruitment to the long-lived stage.
The long-term persistence of competitors without competitive exclusion has led to numerous explanations for the maintenance of species diversity (1–3). Included among these hypotheses are those that posit resource partitioning and spatial or temporal separation of potential competitors at equilibrium (4–6). Alternatively, nonequilibrium theory suggests that a fluctuating environment may promote the coexistence of competitors (2, 7–9). Although variation alone cannot prevent the inevitable fixation of a single genotype or species (10, 11), Chesson and colleagues (2, 12–18) have proposed a way in which temporal recruitment fluctuations among species can lead to the stable coexistence of competitors. Their mechanism called the “storage effect,” may occur as long as each competitor can increase from low densities. One way in which this criterion is met is when each taxon has a long-lived stage that is immune to the effects of competition and is able to carry the population through periods of poor recruitment. In an environment where recruitment is occasionally low, this long-lived stage buffers competitors against exclusion in a way that is not possible in models with nonoverlapping generations.
In its original form, the storage effect results from three components: interspecific competition, a persistent long-lived stage, and temporal variation in recruitment to this long-lived stage. More recently, the theory has been expanded and can now be expressed as the outcome of three ingredients: species-specific responses to the environment, covariance between environment and competition, and subadditive growth rates (16–18). Comprehensive reviews of the general theory are available elsewhere (12–19). Herein, I focus only on the original theory because it provides quantitative methods that are applicable to field data.
The original prerequisites for maintenance of diversity via the storage effect (interspecific competition, a persistent long-lived stage, and temporal variation in recruitment to this long-lived stage) are found in a wide variety of both terrestrial and aquatic communities (19). In some systems (e.g., perennial plants, fish, and marine invertebrates), the overlapping generations are established by long-lived adults and the competition is among juveniles (12, 20, 21). In others (e.g., annual plants, insects, zooplankton, and phytoplankton), competition exists among short-lived active individuals, and the long-lived stage is a dormant seed, egg, or cyst (8, 9, 22, 23). In these systems, overlapping generations result from the repeated germination (or hatching) over a number of seasons of the seeds (or eggs) produced in any single year.
To date, nearly all attention to the storage effect hypothesis has been either strictly theoretical or, when applied to natural communities, merely anecdotal. One notable exception is the work of Pake and Venable (8, 9) on Sonoran Desert annuals. These investigators have demonstrated that all the necessary prerequisites of the model are met and suggested that coexistence of these competitors is mediated by environmental fluctuations and a between-year seed bank. However, they do not analyze quantitatively the contribution of the storage effect to the persistence of each population.
Herein I report results from, to my knowledge, the first explicit mathematical analysis of the storage effect’s contribution to the maintenance of species diversity. Twenty-one years of recruitment data are presented for two species of Daphnia in Oneida Lake, NY. In addition, evidence for the duration of the long-lived stage (diapausing egg) in the sediment egg bank and interspecific competition is summarized herein and presented in detail elsewhere (24). Because spatial structure of the eggs in the sediment may be important in the analyses of the long-term population dynamics, the egg bank is analyzed first as a homogeneous and then as a layered system. For Daphnia galeata mendotae, persistence in Oneida Lake, NY, is unambiguously dependent upon the storage effect, whereas its competitor, Daphnia pulicaria, persists through consistently high per capita recruitment.
STUDY SITE AND METHODS
In Oneida Lake, NY, zooplankton data collected over the past 30 years (25) show a striking negative correlation between the water-column densities of two zooplankton species, D. galeata mendotae and D. pulicaria [Spearman Rank rs = −0.259; P < 0.001 (24)]. For at least the past three decades, these two species have alternated as the numerically dominant daphnid species. D. pulicaria dominates in some years, D. galeata mendotae dominates in others, and some years involve a seasonal replacement. This negative correlation results at least in part from temporal variation in the intensity of size-selective fish predation (25) and interspecific competition (24). For Daphnia, like many species of zooplankton, the persistent life-history stage is a diapausing egg. In Oneida Lake, both species produce diapausing eggs from late May to early June, and in some years again in the late fall (24). These eggs have accumulated to sediment densities reaching tens of thousands per m2 and have the potential to remain viable for more than 125 years (24). Hatching of these eggs back to the water column occurs each spring after ice-out but does not continue throughout the summer (24). That these two species are competitors and that both species posses a long-lived life history stage satisfies two of the three necessary conditions of the storage effect model. Evidence that the third criterion, temporal fluctuations in recruitment, also exists for the two Daphnia species in Oneida Lake is presented below.
When the long-lived life history stage is an egg bank, the number of eggs (Xi) in each species’ (i) bank changes through time (t) as:
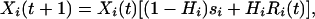 |
1 |
where Hi is the hatching fraction, si is the probability of survival in the egg bank, and Ri is the annual per capita recruitment to the egg bank from hatched eggs (which may vary temporally) (19). Eq. 1 reflects only the dynamics of the egg bank and does not explicitly include the dynamics of the Daphnia in the water column. Consequently, “per capita recruitment” is based on the number of dormant eggs that hatch and produce new dormant eggs, irrespective of the number of Daphnia in the plankton. However, the number of Daphnia in the water in any given year has the potential to affect recruitment through competition.
The amount of generational overlap [(1 − Hi)si] dictates how long an egg remains in the egg bank. Although hatching from the egg bank can result in the production of new diapausing eggs, it also represents a loss term from the long-lived stage. The survivorship term for the dormant eggs reflects three additional loss processes: predation, senescence, and deep burial. Because dormant eggs need to be near the sediment surface to hatch, if an egg is buried below the top few centimeters, it is effectively lost unless sediment mixing returns it to the surface.
Coexistence of competitors occurs when each species is able to increase from low numbers (22, 26), that is, when each population’s theoretical mean boundary growth rate (population growth rate at low density) is positive:
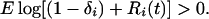 |
2 |
In Eq. 2, the amount of generational overlap in the egg bank [(1 − Hi)si] has been replaced by the general term (1 − δi), where δ represents the sum of all four loss processes from the egg bank. In addition, recruitment remains dependent on the hatching fraction but is expressed simply as R. Alternatively, Warner and Chesson (14) express Eq. 2 as:
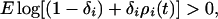 |
3 |
where ρi(t) = Ri(t)/δi. If ρi(t) > 1, then recruitment exceeds loss and species i increases and persists, but if ρi(t) < 1, species i will decrease.
Both the storage of reproductive potential and recruitment fluctuations are likely to occur in many systems; hence, Warner and Chesson (14) provide an explicit analysis for quantifying the contribution of the storage effect mechanism to persistence. The contribution of the nonstorage component to the long-term population growth rate addresses the question of whether or not the population can increase without the extremely good recruitment years (and their subsequent storage) that result from environmental fluctuations. In other words, if a population can increase without recruitment fluctuation, then something other than the storage effect must permit long-term persistence. To quantify this nonstorage component, ρ̃i is defined as the geometric mean of ρi, which removes the year-to-year variance in Ri. The average over all years (j) gives the population’s mean instantaneous growth rate without environmental variation (Γ):
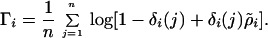 |
4 |
The storage effect’s contribution to the growth rate includes the annual variance in Ri and is the overall mean instantaneous growth rate with the nonstorage component (above) removed:
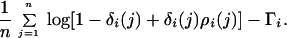 |
5 |
According to Warner and Chesson (14), if competition has been demonstrated and Eq. 4 is negative while Eq. 5 is positive for one or both species, then the storage effect is important in maintaining coexisting species in the system. If Eq. 4 is positive, then that species most likely does not depend on storage for its long-term persistence, because even without environmental fluctuations the population still maintains a positive growth rate. In short, the storage effect maintains diversity because the storage protects a rare species from extinction and the recruitment fluctuations lead to periodic large population increases.
Maintenance of diversity via the storage effect implicitly assumes that the species are competitors and that competition affects recruitment. That competition is occurring must be demonstrated experimentally, independent of a mathematical analysis of the long-lived stage. Consequently, as a conservative recommendation, Warner and Chesson (14) advised that recruitment fluctuations should be negatively correlated between species. Note however, that this negative correlation is not, in fact, required for coexistence by the storage effect. Rather, interspecific competition must limit recruitment in a way that is affected strongly by the environment.
Parameterizing this model for the Oneida Lake daphnid assemblage requires estimates of both the recruitment and loss rate for each species’ egg bank. Annual estimates of recruitment to the egg bank were calculated from 21 years of plankton samples collected by the Cornell Biological Field Station. For each year, the number of ephippia produced per m2 in the water column were estimated from a weekly plankton sample and converted to daily production rates by dividing the number of ephippia per female by the length of the molt cycle (27–29). By integrating daily production rates over each sampling interval, weekly production rates were calculated. I obtained annual production estimates by summing over all sampling dates. Annual estimates of ephippial production were converted to annual diapausing egg production by multiplying by two eggs per case and correcting for the fact that approximately 10% of females shed empty cases (personal observations). In the years 1975 to 1991, one plankton sample from the archived collection of the Cornell Biological Field Station was examined for each week, whereas my own duplicate weekly samples were examined in 1992 to 1995. These numbers were converted to per capita recruitment rates based on the average number of eggs that hatch [10 eggs per m2 for D. pulicaria, 5 eggs per m2 for D. galeata mendotae (24)].
The second parameter in the model is the loss rate from the egg bank. In the theoretical exploration of this model, Warner and Chesson (14) assumed that loss rates were small and constant across years, because as the per capita death rate approaches 1, the long-lived stage is eliminated and there is no storage. The actual loss rate from the Oneida Lake egg bank results from four processes, hatching, deep burial, predation, and senescence, of which only hatching has been quantified (see above). The rate at which eggs are lost due to deep burial depends on the depth to which the hatching cue penetrates. The limited data that exist suggest that eggs may only receive the appropriate light and oxygen cues within the top 0.15–0.33 cm of sediment (N. G. Hairston, Jr., personal communication). However, due to the potential for vertical mixing within the sediment, precisely quantifying the loss due to deep burial is not straightforward. Moreover, although I have evidence of egg mortality due to both predation and senescence (24), quantitative estimates are unavailable.
Lastly, because there are estimates for both the annual input to the egg bank from the water column and the number of eggs in the sediment (24), Eq. 1 must be balanced through the death rate. Because the estimate of eggs produced often greatly exceeded the number found in the top layer of the sediment, the recruitment values may be consistently overestimated (24). If this is the case, this bias can be corrected in Eq. 1 by either reducing all recruitment rates by a constant fraction or by increasing the death rate. Because the storage effect results from the interaction between recruitment variation and generational overlap, growth rates for each species were calculated for 10 loss rates ranging from 0.01 to 0.90. For each estimate of the death rate, growth rates were calculated twice, once using the calculated recruitment values (high value), and once using values that had been reduced by 50% (low value).
The above calculations assume that within the active egg bank the sediment is homogeneous. No allowances are made for the potential of vertical mixing within the sediment [although more recently, Chesson (17) has provided a theoretical analysis of the effects of spatial heterogeneity]. In Oneida lake, the sediment egg bank extends to a depth of 25 cm and exceeds 2.5 × 104 eggs per m2 (24). Although some of these deeply buried eggs can be induced to hatch in the laboratory, they cannot contribute to the growth rate of the population unless they are mixed up to the surface. To incorporate the effects of sediment mixing, I constructed a matrix model for a three-layered system and computed the boundary growth rates of each population. The first layer included eggs from 0 to 0.15 cm in the sediment, the second encompassed 0.15 cm to 2 cm, and the third compartment included all eggs below 2 cm. The depth of the first layer was based on the depth to which the hatching cue penetrates (N. G. Hairston, Jr., personal communication) and the second layer extends to the typical depth of bioturbation in freshwater systems (30, 31). The generalized transition probabilities for the matrix are:
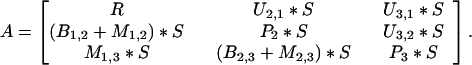 |
The terms in the matrix are all annual per capita rates and represent the recruitment rate to the egg bank (R), the rate of upward mixing between the initial (i) and final (f) layer (Ui,f), the rate of passive burial downward to a lower layer (Bi,f), the rate of active downward mixing (Mi,f), and the proportion of eggs in layers two and three that do not move (P). Six separate matrices were analyzed with survivorship (S) ranging between 0.99 and 0.10. Eggs in the top layer either hatch or move downward, and the hatching and mortality are subsumed into the recruitment term.
On the basis of my field data for recruitment, sedimentation rates, and vertical distributions of eggs and literature numbers for mixing (30), the initial values were set at:
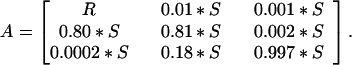 |
The rate of passive downward movement (burial) reflects the sedimentation rate in Oneida Lake (24). Because the rate of downward mixing has been shown to be less than upward transport (30, 32), M is always less than U. On the basis of 1.5 years of sediment core data that showed no substantial upward movement into the top 2 cm (24), these rates of active transport by invertebrates were assumed to be quite small.
To calculate the growth rate without environmental variation (analogous to Eq. 4), I used matlab 4.0 to find the logarithm of the dominant eigenvalue of matrix A with R set as the geometric mean of the 21 annual recruitment values (years 1975–1995). In the variable recruitment case, the number of eggs in each compartment was set to low densities at a ratio that reflected the egg distribution at these three depths in the sediment of Oneida Lake (1:19:230 for D. galeata mendotae and 1:19:307 for D. pulicaria). For each time step, a value of R was selected at random from the 21 years of field recruitment data. Simulations of Xi(t + 1) = A × Xi(t) were run for as many generations as necessary for the long-term growth rate to stabilize (up to 1000). Stabilization of the growth rate was confirmed by calculating two intermediate growth rates within each simulation (e.g., for a 1000-generation simulation, growth rates were calculated between the 500th and 750th generations and between the 750th and 1000th generations). For each species, I ran 10 simulations and found the final growth rate by difference from the population over the longest interval (e.g., for a 1000-generation run, between population sizes at 500 generations and 1000 generations). These 10 growth rates were averaged to estimate the boundary growth rate. The value obtained from the constant recruitment case (analogous to Eq. 4) was subtracted from this number to give a result analogous to that of Eq. 5.
RESULTS AND DISCUSSION
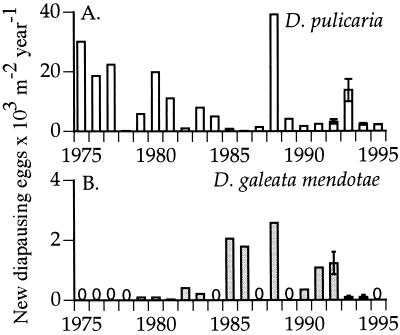
Annual recruitment to the egg bank varied for both D. pulicaria and D. galeata mendotae (Fig. 1). Although each species experienced years in which it had higher recruitment than its competitor, D. pulicaria was favored more often. Over the 21 years investigated, D. pulicaria produced more diapausing eggs than D. galeata mendotae in 19 years, whereas the reverse was true only in 2 years. Moreover, D. galeata mendotae experienced 8 years of complete recruitment failure, whereas D. pulicaria produced at least a few hundred new diapausing eggs per m2 each year. This temporal fluctuation in recruitment satisfies the third necessary condition of the storage effect hypothesis.
Figure 1.
Estimates for the number of new diapausing eggs produced in each year by D. pulicaria (A) and D. galeata mendotae (B) in Oneida Lake, NY. Error bars on the estimates for 1992 to 1995 represent 1 SEM. Note that the scale on the graph of D. pulicaria is 10 times higher than that of the D. galeata mendotae graph.
This variation in recruitment to the egg bank is explained in part by the density of Daphnia in the water column. For example, for D. pulicaria, which produced the majority of its dormant eggs in the spring, more than 50% of the variation in the springtime production estimates is explained by the average spring D. pulicaria density (regression, logarithm transformed, r2 = 0.52, P = 0.0003). The more D. pulicaria that are present in the plankton during dormant egg production, the higher the recruitment to the egg bank. I have shown elsewhere (24) that the production of immediately hatching eggs by D. pulicaria can be reduced by the presence of D. galeata mendotae and vice versa. When fewer immediately hatching eggs are produced by a population, there are fewer individuals in the water column to produce the dormant eggs.
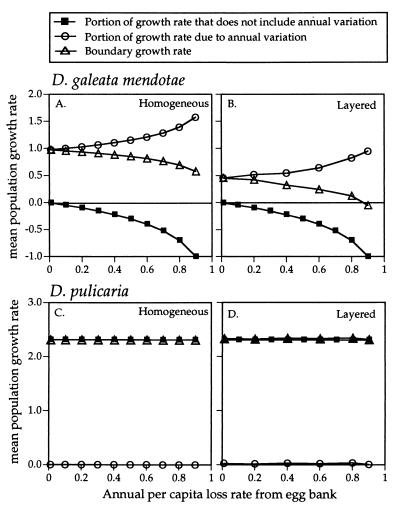
The Warner and Chesson calculations for D. galeata mendotae, using the “low” estimates of recruitment, suggest that without a fluctuating environment (Eq. 4), this species would not persist in Oneida Lake (Fig. 2A). Even with very low loss rates (i.e., high generational overlap), the portion of the growth rate based solely on the mean recruitment is negative. The qualitative results of the analysis did not differ when the “high” estimates of recruitment were used (data not plotted). Without environmental fluctuation, the species cannot persist. The primary reason for this result is that this species often experienced years of complete recruitment failure, eight times for the 21-year period studied herein. Moreover, D. galeata mendotae suffered at least four consecutive years of zero recruitment to the egg bank (Fig. 1B). Without a multiple-year dormant stage and occasional years in which it is strongly favored, this species would have been eliminated from the community. In contrast, the D. pulicaria population does not rely on the storage effect for persistence (Fig. 2C). That is, the geometric mean recruitment rate is always greater than the per capita loss rate. This qualitative result is found by using a range (1–103) of estimates for the number of eggs hatching (and, therefore, values of per capita recruitment).
Figure 2.
Mean population growth rates of D. pulicaria and D. galeata mendotae calculated by assuming a homogeneous egg bank (A and C) (Eqs. 4 and 5) and layered egg bank (B and D) using the “low” estimates for recruitment (see text for explanation). In each case, egg death rates ranged between 0.01 (long-lived egg bank) and 0.90. (A–D) ▪, That portion of the population growth rate that does not include recruitment variation; ○, that portion of the growth rate due to annual variation in recruitment; ▵, boundary growth rate (the sum of each circle/square pair). Squares that fall below the zero line indicate the death rate at which the population can no longer persist without variable recruitment. For D. galeata mendotae (A and B), all the squares fall below this line, indicating that this species can never persist without variable recruitment and reestablishment from the egg bank. Furthermore, at very high values of the egg death rate, the simulated boundary growth rate is negative (B), indicating that the population would not persist. For D. pulicaria (C and D), the storage effect is not necessary for long-term persistence.
As pointed out by Warner and Chesson (14), it is essential when using Eqs. 4 and 5 that ρ be measured only at low population densities, when the effects of competition are weak. Because competition occurs among the active individuals in the water column, I found the median springtime daphnid densities and again calculated all values for Eqs. 4 and 5 by using only those years that fell below the median population size (1977–1981, 1983, 1985, 1986, 1993, and 1994). For both D. galeata mendotae and D. pulicaria, the qualitative results do not change.
The estimates of each population’s boundary growth rate obtained from the layered egg-bank model support the qualitative results obtained with the unlayered model (Fig. 2 B and D). For D. galeata mendotae, the boundary growth rate is negative without variation. In contrast, D. pulicaria persists through consistently high recruitment.
CONCLUSIONS
The results of these analyses indicate that even though both daphnid species exhibit the necessary prerequisites for persistence via the storage effect, this mechanism may only be necessary in maintaining the weaker competitor in Oneida Lake. However, even though the D. pulicaria population does not rely on the storage effect for persistence, dormant eggs are nevertheless an important stage in its short-term dynamics. This population is often completely eliminated from the plankton by midsummer and not seen again until the following spring (25). Therefore, without the reliable colonization source provided by dormant eggs, D. pulicaria would not have persisted in Oneida Lake. Moreover, long-lived diapausing eggs are likely to be present in many other systems where competing zooplankton are found to coexist (33, 34). However, the role of this dormant stage and the overlapping generations that it creates are almost never considered when the community dynamics are examined. This oversight limits our understanding of community dynamics because diapause may prove to be a common mechanism promoting species coexistence in aquatic systems.
Acknowledgments
I thank S. Ellner for informative conversations regarding both the storage effect and the layered egg bank models and E. L. Mills for allowing me to use his archived plankton samples. Earlier drafts of the manuscript were improved by the comments of A. J. Bohonak, P. L. Chesson, C. R. Freeman-Gallant, M. A. Geber, N. G. Hairston, Jr., C. D. Harvell, E. L. Mills, and an anonymous reviewer. This work was supported by National Science Foundation Doctoral Dissertation Improvement Grant DEB-9410966 and by a National Science Foundation predoctoral fellowship.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Connell J H. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- 2.Chesson P L. In: Community Ecology. Diamond J, Case T J, editors. New York: Harper & Row; 1986. pp. 240–256. [Google Scholar]
- 3.Rothhaupt K O. Arch Hydrobiol. 1990;118:1–29. [Google Scholar]
- 4.MacArthur R H. Ecology. 1958;39:599–619. [Google Scholar]
- 5.MacArthur R H, Levins R. Am Nat. 1967;101:377–399. [Google Scholar]
- 6.Schoener T W. Science. 1974;185:27–29. doi: 10.1126/science.185.4145.27. [DOI] [PubMed] [Google Scholar]
- 7.Hutchinson G E. Am Nat. 1961;95:137–146. [Google Scholar]
- 8.Pake C E, Venable D L. Ecology. 1995;76:246–261. [Google Scholar]
- 9.Pake C E, Venable D L. Ecology. 1996;77:1427–1436. [Google Scholar]
- 10.May R M. Am Nat. 1973;107:621–650. [Google Scholar]
- 11.Pamilo P. Ann Zool Fenn. 1988;25:99–106. [Google Scholar]
- 12.Chesson P L, Warner R R. Am Nat. 1981;117:923–943. [Google Scholar]
- 13.Chesson P L. In: Population Biology, Lecture Notes in Biomathematics 52. Freeman H I, Strobeck C, editors. New York: Springer; 1983. pp. 188–198. [Google Scholar]
- 14.Warner R R, Chesson P L. Am Nat. 1985;125:769–787. [Google Scholar]
- 15.Chesson P L, Huntly N. Ann Zool Fenn. 1988;25:5–16. [Google Scholar]
- 16.Chesson P L, Huntly N. Trends Ecol Evol. 1989;4:293–298. doi: 10.1016/0169-5347(89)90024-4. [DOI] [PubMed] [Google Scholar]
- 17.Chesson P L. Philos Trans R Soc London B. 1990;330:43–51. [Google Scholar]
- 18.Chesson P L. Theor Popul Biol. 1994;45:227–276. [Google Scholar]
- 19.Hairston N G, Jr, Ellner S, Kearns C M. In: Spatial and Temporal Aspects of Population Processes. Rhodes O E Jr, Chesser R K, Smith M H, editors. Chicago: Univ. of Chicago Press; 1996. pp. 109–145. [Google Scholar]
- 20.Leigh E G., Jr . In: The Ecology of a Tropical Forest. Leigh E G Jr, editor. Washington, DC: Smithsonian Inst. Press; 1982. pp. 63–66. [Google Scholar]
- 21.Buttler A J, Chesson P L. Aust J Ecol. 1990;15:521–531. [Google Scholar]
- 22.Ellner S. Vegetatio. 1987;69:199–208. [Google Scholar]
- 23.Hairston N G., Jr Limnol Oceanogr. 1996;41:1087–1092. [Google Scholar]
- 24.Cáceres, C. E. (1997) Ph.D. dissertation (Cornell Univ., Ithaca, NY).
- 25.Mills E L, Forney J L. In: Complex Interactions in Lake Communities. Carpenter S R, editor. New York: Springer; 1987. pp. 11–30. [Google Scholar]
- 26.Turelli M. Proc Natl Acad Sci USA. 1978;75:5085–5089. doi: 10.1073/pnas.75.10.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edmondson W T. Oecologia. 1968;1:1–37. doi: 10.1007/BF00377252. [DOI] [PubMed] [Google Scholar]
- 28.Paloheimo J E. Limnol Oceanogr. 1974;19:692–694. [Google Scholar]
- 29.Braner, M. (1988) Ph.D. dissertation (Cornell University, Ithaca, NY).
- 30.Kearns C M, Hairston N G, Jr, Kesler D H. Hydrobiologia. 1996;332:63–70. [Google Scholar]
- 31.McCall P L, Tevesz J S. In: Animal-Sediment Relations: The Biogenic Alteration of Sediments. McCall P L, Tevesz J S, editors. New York: Plenum; 1982. pp. 105–176. [Google Scholar]
- 32.Davis R B. Limnol Oceanogr. 1974;19:466–488. [Google Scholar]
- 33.Leibold M A. Oecologia. 1991;86:510–520. doi: 10.1007/BF00318317. [DOI] [PubMed] [Google Scholar]
- 34.Hu S S, Tessier A J. Ecology. 1995;76:2278–2294. [Google Scholar]