Abstract
Although most ecologists agree that both top-down and bottom-up forces (predation and resource limitation, respectively) act in concert to influence populations of herbivores, it has proven difficult to estimate the relative contributions of such forces in terrestrial systems. Using a combination of time–series analysis of population counts recorded over 16 years and experimental data, we present the first estimates of the relative roles of top-down and bottom-up forces on the population dynamics of two terrestrial insect herbivores on the English oak (Quercus robur). Data suggest that temporal variation in winter moth, Operophtera brumata, density is dominated by time-lagged effects of pupal predators. By comparison, spatial variation in O. brumata density is dominated by host–plant quality. Overall, top-down forces explain 34.2% of population variance, bottom-up forces explain 17.2% of population variance, and 48.6% remains unexplained. In contrast, populations of the green oak tortrix, Tortrix viridana, appear dominated by bottom-up forces. Resource limitation, expressed as intraspecific competition among larvae for oak leaves, explains 29.4% of population variance. Host quality effects explain an additional 5.7% of population variance. We detected no major top-down effects on T. viridana populations. An unknown factor causing a linear decline in T. viridana populations over the 16-year study period accounts for most of the remaining unexplained variance. We discuss the observed differences between the insect species and the utility of time–series analysis as a tool in assessing the relative importance of top-down and bottom-up forces on herbivore populations.
A long-standing debate exists on the relative importance of top-down and bottom-up forces on the population dynamics of middle trophic-level species such as herbivores. This debate was stimulated in large part by the proposition that the “world is green” because natural enemies regulate the populations of most herbivores below densities at which significant defoliation would occur (1). Conversely, other authors have stressed the unpalatability of most plant tissue to most herbivores, most of the time, and have argued that variation in plant quality limits the populations of many herbivores (2). More recently, a balanced view has emerged in which both natural enemies and plant quality or quantity are seen to interact to influence the population ecology of herbivores (3–13). Few ecologists now champion either bottom-up or top-down forces as dominant, but rather wish to explore the environmental conditions that favor one or the other (14–17).
However, putting real numbers on the relative importance of top-down and bottom-up forces in any single system remains elusive and, at best, broad generalizations emerge. For example, about 50% of the variation in productivity in lakes is determined from below by nutrient input, turnover time of the water, and vertical mixing, whereas the other 50% is thought to result from a “trophic cascade” from higher to lower trophic levels (3, 4, 6, 7, 18). It is difficult to attach numerical values to the relative importance of predation and plant quality for a number of reasons. First, they can act at different temporal scales. The experimental addition of a predator into a system can have a rapid effect on a prey population, whereas addition of nutrients to the same system may take longer to have an effect (14). The reverse can also be true: nutrient additions in aquatic systems may result in more rapid responses by mid-trophic level species than time-lagged responses by top predators (6, 7). In addition, data are often not collected at an appropriate scale to estimate the effects of plant quality on herbivore populations. Pheromone or light-trap data for insect herbivores, for example, cannot be used to estimate the effects of variation among individual plants on the spatial distribution of herbivores because the data are not collected at the level of the plant (19).
Here, we present the first numerical estimates of the relative importance of top-down and bottom-up forces in a terrestrial insect–plant system. We have re-analyzed data from a classic study of the population dynamics of the winter moth, Operophtera brumata (Lepidoptera: Geometridae) (20, 21), including for the first time information on the spatial distribution of larvae among individual oak trees. In addition, we present a companion data set on the green oak leaf roller moth, Tortrix viridana (Lepidoptera: Tortricidae), collected by Varley and Gradwell during their studies of the winter moth, but never published. We argue that long-term sampling data can be used in combination with experimental data to estimate the relative impacts of predators and plant quality on herbivore populations, and discuss differences in the relative importance of top-down and bottom-up forces between two oak herbivores.
System of Study
O. brumata is a polyphagous, univoltine, insect herbivore. Census data were collected in Wytham Woods, near Oxford, England, where O. brumata populations can reach high densities on its preferred host, the pedunculate oak, Quercus robur. Adult O. brumata emerge from November to January in Wytham, and females lay their eggs toward the tops of trees. Larvae emerge in April, in approximate synchrony with bud expansion and spring leaf flush of Q. robur. Larvae feed for about 6 weeks, and diapause as pupae in the soil until adults emerge in winter (21). T. viridana larvae are univoltine and monophagous. They feed concurrently with O. brumata on new oak leaves, again for about 6 weeks. The pupal period of T. viridana is about 3 weeks, and females lay their eggs on oak twigs in July. They overwinter in the egg stage, and larvae emerge the following spring. Like O. brumata, T. viridana can reach high densities on Q. robur, and combined defoliation levels from these two herbivores average 40% leaf area removed in Wytham Woods (22).
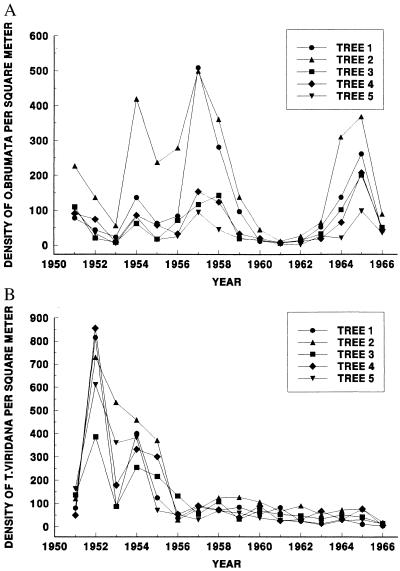
Census data were collected according to the methods of Varley et al. (21). For the purposes of the analyses presented here, we use only the yearly larval density estimates. Briefly, counts were made from each of five mature Q. robur trees from 1950 to 1968. The original (by-tree) data are missing for 1950, 1967, and 1968, and only mean counts, averaged among trees, are available for those years. We have therefore used only the data for the remaining 16 years (1951–1966) in the analyses presented here. The densities of O. brumata larvae were estimated using two 0.5 m2 water-filled trays under each tree. Larvae were captured in the trays as they spun down to pupate in the soil. Data were pooled for the two traps to give an estimate of larval density per m2 of ground per tree. The densities of T. viridana larvae were estimated by direct larval counts in the canopy of each tree: many T. viridana larvae pupate in leaf rolls, and so the tray method would have underestimated density. Two branch samples from each tree were collected, the larvae counted, and the volume of branch sample calculated. Using estimates of total canopy area and volume (21), the density estimates for T. viridana were converted to m2 of ground area, comparable with the estimates of O. brumata density. Original data for both insect species are presented in Fig. 1.
Figure 1.
The densities of (A) O. brumata and (B) T. viridana larvae on five Q. robur trees, sampled in Wytham Woods, Oxfordshire, from 1951 to 1966.
Methods of Analyses
We have used time–series analysis (23, 24) to examine feedback processes (density dependence) in the time–series for O. brumata and T. viridana. First, densities were log-transformed (Fig. 2) and examined to see if the time–series were stationary (fluctuate around a constant mean). The T. viridana time–series is not stationary, exhibiting a linear decline over time (Fig. 2B). We therefore detrended the data using the methods of Berryman (25) in which data are transformed to fluctuate around a line of slope = 0. Briefly, a regression line was fitted through the data in Fig. 2B such that:
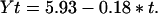 |
Data were then transformed such that Nt = Xt − Yt + Ñ, where Nt is the new time–series, Xt is the untransformed time–series, and Ñ is the mean of the untransformed series. The detrended data are shown in Fig. 3. Using mean densities from the five trees, we then generated partial autocorrelation functions (24) for both time–series in which log-transformed mean densities at time t are correlated with log-transformed densities at time t − 1, t − 2, … . t − 10 (Fig. 4). Partial autocorrelation functions provide a visual estimate of the dominant time lag at which a negative feedback process (density dependence) is operating and, although population dynamics are inherently nonlinear (26), the Box–Jenkins approach is useful for indicating broad patterns in the data (27). Subsequently, we calculated the per capita rate of increase, r, of each population for each pair of years in the time–series from the equation rt = ln (Nt/Nt − 1), where Nt is the population density at time t. Finally, we correlated the ranges of r for each time–series with population density at time t − 1, t − 2, … . t − 10 to assess the time lag at which any density dependence in the system was operating. Significant regressions are given in Fig. 5. Although time–series of 30–40 time steps are considered ideal for detecting the action of factors that commonly influence insect populations, relatively few such data sets exist (24). Shorter time–series have provided convincing evidence for delayed density dependence in previous analyses of forest insect data (27). Because time–series analysis is based on autocorrelation, the P values presented with the correlation analyses are for indication only, and should not be interpreted as accurate probabilities associated with accepting or rejecting a particular relationship.
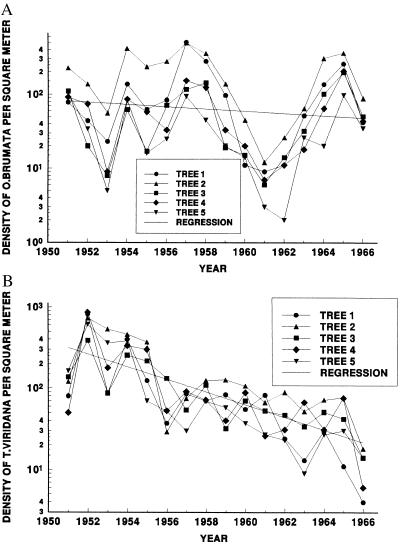
Figure 2.
Log-transformed densities of (A) O. brumata and (B) T. viridana larvae on five Q. robur trees, sampled in Wytham Woods, Oxfordshire, from 1951 to 1966. The time–series for O. brumata is stationary, whereas the time–series for T. viridana exhibits a declining trend.
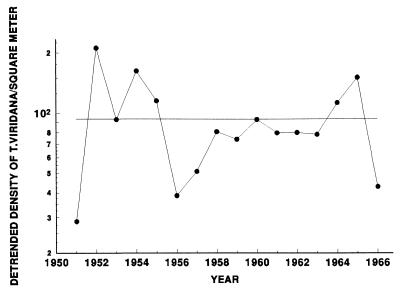
Figure 3.
Mean log-transformed densities of T. viridana larvae on five Q. robur trees, sampled in Wytham Woods, Oxfordshire, from 1951 to 1966. The data have had the declining trend removed by transformation (see text for details) and points represent the mean of five trees.
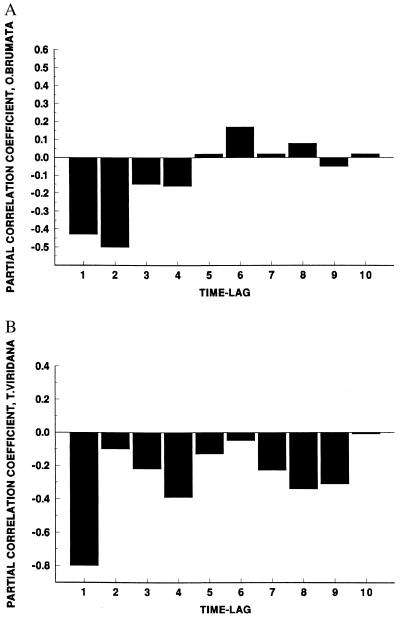
Figure 4.
Partial autocorrelation functions describing correlations between (A) O. brumata and (B) T. viridana larval densities at time t and densities at time t − 1, t − 2, … . t − 10. Note the dominant lag at t − 2 for O. brumata and t − 1 for T. viridana.
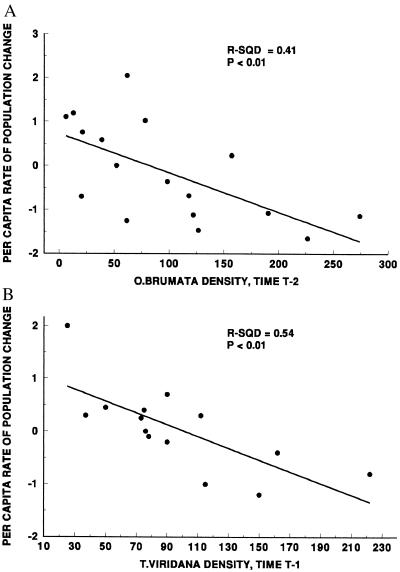
Figure 5.
Regressions between the per capita rate of change of (A) O. brumata and larval density at time t − 2 and (B) T. viridana and larval density at t − 1. Because there is inherent autocorrelation in the regressions, probability estimates are presented for illustrative purposes only.
Methods for Estimating the Relative Roles of Top-Down and Bottom-Up Forces
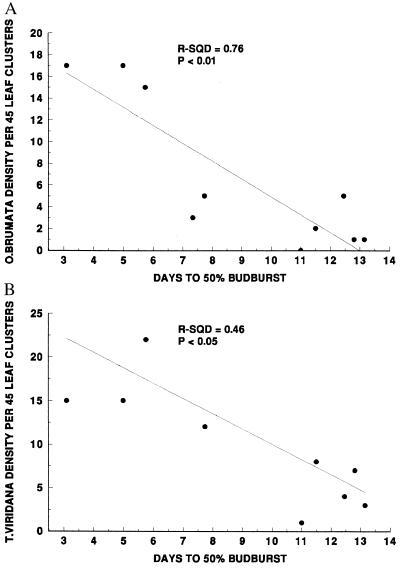
We used two-way analysis of variance with “tree” and “year” as main effects to calculate the total variance in the census data (original data for O. brumata and detrended data for T. viridana), and to partition that variance among year effects, tree effects, and “error” (Table 1). Note that these analyses were used only to calculate variance estimates: because the data represent repeated measures from the same trees over time, the degrees of freedom and probability estimates associated with the main effects cannot be used to assess actual probabilities of accepting or rejecting inferences from the analyses. Nonetheless, the analyses are valid for partitioning variance among the main effects. We considered variance associated with tree effects to represent spatial variation in larval density attributable to tree quality (Fig. 2, Table 1). Previous sampling and experimental work has shown that budburst phenology is the dominant factor determining the spatial distribution of both species among individual oaks (refs. 28–30; Fig. 6). By combining the estimates of overall variance associated with tree effects (Table 1) with the variance among trees that is explained by budburst phenology (Fig. 6), we calculated how much variance in the census data could be explained by variation among the five trees in their budburst phenology (Table 2). This is one estimate of the power of bottom-up effects.
Table 1.
Variance estimates from analysis of variance of tree effects and year effects from population censuses of O. brumata and T. viridana from Wytham Woods, England
| Sums of squares
|
Variance explained
|
||||||
|---|---|---|---|---|---|---|---|
| Total | Tree | Year | Error | Tree, % | Year, % | Error, % | |
| O. brumata | 121.80 | 27.50 | 83.30 | 11.00 | 22.6 | 68.4 | 9.0 |
| T. viridana | 41.51 | 5.10 | 22.65 | 13.76 | 12.3 | 54.6 | 33.1 |
Data were collected from five Q. robur trees from 1951 to 1966 (80 observations per insect species). The time–series for T. viridana was detrended before analysis (see text for details).
Figure 6.
The relationship between the budburst date of individual Q. robur trees and the densities of (A) O. brumata and (B) T. viridana larvae in Wytham Woods, Oxfordshire. Data are reanalyzed from Hunter (29).
Table 2.
Estimates of the relative roles of top-down and bottom-up forces in determining variation in the populations of O. brumata and T. viridana on five Q. robur trees from 1951 to 1966 in Wytham Woods, England
| Top-down (predation), % | Bottom-up (plant quality), % | Bottom-up (plant quantity), % | Total explained, % | Total unexplained, % | |
|---|---|---|---|---|---|
| O. brumata | 34.2 | 17.2 | Negligible | 51.4 | 48.6 |
| T. viridana | Negligible | 5.7 | 29.4 | 35.1 | 64.9 |
Estimates were derived from partitioning total population variance between tree and year effects, then assessing the relative roles of competition, host-plant phenology, and natural enemies in generating those tree and year effects (see text for details).
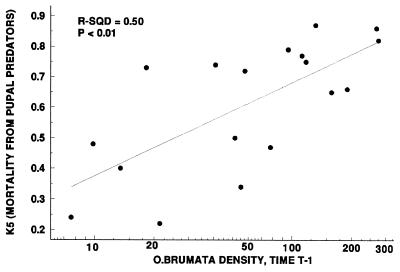
From the time–series analyses, we considered density dependence acting on a time lag of t − 2 or greater as an indication of a delayed density-dependent response typical of the action of a natural enemy (top-down effect) in the system (25). Only O. brumata exhibited such a delayed density-dependent response (Figs. 4A and 5A), and we used the k-factor data for natural enemies in Varley et al. (21) to investigate this further with correlation analyses. Pupal predation (k5) was the only mortality factor that was related to O. brumata density (Fig. 7). The pupal predators responsible for most pupal mortality are Feronia madida (Coleoptera: Carabidae), Abax parallelopipidus (Coleoptera: Carabidae), and Philonthus decorus (Coleoptera: Staphylinidae). Note that a regression between O. brumata density at t − 1 with k5 is equivalent to a t − 2 delayed response because the effects of pupal predation on larval density are not exhibited until the following year. We concluded that the major effect of pupal predation on O. brumata occurs on a time lag and was the likely cause of the apparent cycle in density over time (Fig. 2). We therefore combined the estimates of overall variance associated with “year” effects from analysis of variance (Table 1) with the estimates of variance associated with pupal predation (Fig. 7) to estimate the variance in the O. brumata census data that can be explained by predation (Table 2). This is our estimate of the power of top-down effects on O. brumata.
Figure 7.
The relationship between the density of O. brumata larvae at time t − 1 and pupal predation at time t. Data for pupal predation, described as k5 from k-factor analysis, are from Varley et al. (21).
Finally, we considered density dependence acting on a time-lag of t − 1 as an indication of rapid feedback, consistent with intraspecific competition (a bottom-up effect based on resource quantity). Only T. viridana exhibited such a response (Figs. 4B and 5B). Previous experimental work has shown that T. viridana is particularly susceptible to intraspecific competition because even minor levels of leaf damage interfere with the integrity of T. viridana leafrolls that are essential for osmoregulation (22). We combined the overall estimates of variance in the T. viridana time–series associated with “year” (Table 1) with the variance in the per capita rate of increase of T. viridana that can be explained by a t − 1 density effect (Fig. 5B) to estimate the variance in the T. viridana time–series associated with intraspecific competition, a second bottom-up effect (Table 2).
Results and Discussion
A combination of long-term sampling data (20, 21) and experimental studies (22, 28, 29) has allowed us to estimate the relative contributions of top-down and bottom-up forces for the spatial and temporal dynamics of two oak tree insects (Table 2). To our knowledge, these are the first such estimates for a terrestrial insect–plant system. The temporal dynamics of O. brumata appear dominated by a t − 2 delayed density-dependent factor (Figs. 4 A and 5A), consistent with the action of natural enemies such as pupal predators (Fig. 7). Local spatial variation in O. brumata density appears linked to host plant quality, specifically budburst phenology (Figs. 2A and 6A). Partitioning the total spatial and temporal variance in O. brumata density over the 16 years of data, we estimate that top-down forces (predation) account for 34.2% of the variation, bottom-up forces (plant quality) account for 17.2% of the variation, and 48.6% of the total variance in the sample remains unexplained.
The temporal dynamics of T. viridana are more complex. There is clear evidence of a linear decline in density over the 16-year study period (Fig. 2B), and we have no way of assessing whether this represents part of a longer population cycle or a response to a changing environment. This unexplained population trend probably explains Varley and Gradwell’s decision not to publish the T. viridana data along with the O. brumata data in the 1960s. Nonetheless, removing the trend from the data (ref. 25; Fig. 3) has permitted us to examine temporal fluctuations in T. viridana density around the declining mean. The temporal dynamics of T. viridana are dominated by a t − 1 delayed density-dependent factor (Figs. 4B and 5B). Such rapid feedback is usually considered to be consistent with intraspecific competition (25) and previous experimental work has demonstrated the potential of competition to influence T. viridana populations (22). We therefore characterize the variance explained by the t − 1 density-dependent factor as a bottom-up (limitation of resource quantity) effect.
T. viridana larvae exhibit much less spatial variation in density among individual trees than do O. brumata larvae (Fig. 2). Experimental work has demonstrated that neonate T. viridana can withstand starvation for extended periods, and on average survive three times longer than starved neonate O. brumata (28). We suggest that this makes T. viridana larvae less susceptible to variation among individual trees in budburst date: larvae that hatch early are more likely to survive until buds open. This probably explains the weaker relationship between tree budburst and larval density for T. viridana than for O. brumata (r − sqd = 0.46 and 0.76, respectively, Fig. 6). Partitioning the total spatial and temporal variance in T. viridana density over the 16 years of data, we estimate that bottom-up forces (plant quality and quantity combined) account for 35.1% of the total variance in the sample, with effects of resource limitation (plant quantity) by far the more important. We find no convincing evidence of a strong top-down effect in the T. viridana time–series, and 64.9% of the variance remains unexplained.
What factors are likely to contribute to the unexplained variance in the T. viridana and O. brumata samples? For T. viridana, much of the unexplained variance is associated with the general decline in density over the study period (Fig. 2B). A much longer time–series would be required to generate hypotheses to explain this decline (24) and this demonstrates the importance of long-term data sets for understanding population dynamics. One further factor that may contribute to the unexplained variance in T. viridana density is interspecific interactions with O. brumata. Laboratory and field studies have demonstrated occasional effects of interspecific competition between these species (22, 28) with T. viridana suffering more from the interaction than O. brumata. The effect is only strong, however, about 1 year in 10 (22). For O. brumata, some of the unexplained variance is probably what Varley and Gradwell (20) described as “winter disappearance”: in addition to variation in budburst among individual trees, there is weather-related stochastic variation in budburst date among years that will influence colonization levels and larval densities, on trees. In other words, density-independent variation in resource quality among years (a bottom-up effect) may account for some of the unexplained variation in O. brumata density over time. Without data on variation in oak tree quality among years, it is impossible to quantify this effect but, given the susceptibilty of O. brumata to variation in budburst phenology (Fig. 6), it is likely to be substantial. T. viridana is less affected by yearly variation in budburst date (28).
We stress that time–series analysis alone does not provide adequate information to estimate the relative roles of top-down and bottom-up forces on insect populations (24, 31). For example, we cannot rule out the possibility that the t − 1 delayed density-dependent factor that influenced T. viridana populations (Figs. 4B and 5B) reflected a rapid response in space by some natural enemy (32). It is only in combination with experimental studies that have demonstrated the importance of competition that we can be reasonably sure that resource limitation was responsible for the patterns that emerged. Nonetheless, we believe that time–series analysis of data collected at the appropriate spatial scale (i.e., the level of the plant) is a valuable tool, in combination with experiments, for estimating the relative importance of top-down and bottom-up forces for herbivore populations.
Acknowledgments
We would like to thank Gary Polis, Peter Price, and Eugene Odum for helpful comments on an earlier draft of this paper, and Lionel Cole for access to the original raw data.
References
- 1.Hairston N G, Smith F E, Slobodkin L B. Am Nat. 1960;44:421–425. [Google Scholar]
- 2.Ehrlich P R, Raven P H. Evolution. 1965;18:586–608. [Google Scholar]
- 3.Schindler D W. Limnol Oceanogr. 1978;23:478–486. [Google Scholar]
- 4.Schindler D W, Fee E J, Ruszczynski T. J Fisheries Res Board Can. 1978;35:190–196. [Google Scholar]
- 5.Oksanen L, Fretwell S D, Arruda J, Niemela P. Am Nat. 1981;118:240–261. [Google Scholar]
- 6.Carpenter S R, Kitchell J F. Am Nat. 1987;129:417–433. [Google Scholar]
- 7.Carpenter S R, Kitchell J F. Bioscience. 1988;38:764–769. [Google Scholar]
- 8.Mittelbach G, Osenberg C, Leibold M. In: Size-Structured Populations: Ecology and Evolution. Ebenman B, Persson L, editors. Berlin: Springer; 1988. pp. 219–235. [Google Scholar]
- 9.McQueen D J, Johannes M R S, Post J R, Stewart T J, Lean D R S. Ecol Monogr. 1989;59:289–309. [Google Scholar]
- 10.Leibold M A. Am Nat. 1989;134:922–949. [Google Scholar]
- 11.Power M E. Ecology. 1992;73:733–746. [Google Scholar]
- 12.Menge B A. Ecology. 1992;73:755–765. [Google Scholar]
- 13.Strong D R. Ecology. 1992;73:747–754. [Google Scholar]
- 14.Hunter M D, Price P W. Ecology. 1992;73:724–732. [Google Scholar]
- 15.Menge B A, Daley B, Wheeler P A. In: Food Webs: Integration of Patterns and Dynamics. Polis G A, Winemiller K O, editors. New York: Chapman & Hall; 1995. pp. 258–274. [Google Scholar]
- 16.Harrison S, Cappuccino N. In: Population Dynamics: New Approaches and Synthesis. Cappuccino N, Price P W, editors. San Diego: Academic; 1995. pp. 131–147. [Google Scholar]
- 17.Belovsky G E, Joern A. In: Population Dynamics: New Approaches and Synthesis. Cappuccino N, Price P W, editors. San Diego: Academic; 1995. pp. 359–386. [Google Scholar]
- 18.Carpenter S R, Kitchell J F, Hodgson J R. Bioscience. 1985;35:634–639. [Google Scholar]
- 19.Price P W. In: Population Dynamics of Forest Insects. Watt A D, Leather S R, Hunter M D, Kidd N A C, editors. Andover, U.K.: Intercept; 1990. pp. 221–232. [Google Scholar]
- 20.Varley G C, Gradwell G R. In: Insect Abundance. Southwood T R E, editor. Vol. 4. London: Royal Entomol. Soc. London; 1968. pp. 132–142. [Google Scholar]
- 21.Varley G C, Gradwell G R, Hassell M P. Insect Population Ecology. An Analytical Approach. Oxford: Blackwells; 1973. [Google Scholar]
- 22.Hunter M D, Willmer P G. Ecol Entomol. 1989;14:267–277. [Google Scholar]
- 23.Box G E P, Jenkins G M. Time Series Analysis: Forecasting and Control. San Francisco: Holen–Day; 1976. [Google Scholar]
- 24.Royama T. Analytical Population Dynamics. New York: Chapman & Hall; 1992. [Google Scholar]
- 25.Berryman A A. In: Individuals, Populations, and Patterns in Ecology. Leather S R, Wat A D, Mills N J, Walters K F A, editors. Andover, U.K.: Intercept; 1994. pp. 119–128. [Google Scholar]
- 26.Royama T. Ecol Monogr. 1981;51:473–493. [Google Scholar]
- 27.Turchin P. Nature (London) 1990;344:660–663. [Google Scholar]
- 28.Hunter M D. Ecol Entomol. 1990;15:401–408. [Google Scholar]
- 29.Hunter M D. Ecol Entomol. 1992;17:91–95. [Google Scholar]
- 30.Hunter M D. In: Effects of Resource Distribution on Animal–Plant Interactions. Hunter M D, Ohgushi T, Proce P W, editors. San Diego: Academic; 1992. pp. 287–325. [Google Scholar]
- 31.Hunter M D. In: Individuals, Populations, and Patterns in Ecology. Leather S R, Wat A D, Mills N J, Walters K F A, editors. Andover, U.K.: Intercept; 1994. pp. 443–448. [Google Scholar]
- 32.Gould J R, Elkinton J S, Wallner W E. J Anim Ecol. 1990;59:213–233. [Google Scholar]