Abstract
The relative deficiency of T helper type 1 (Th1) and cytotoxic T lymphocyte (CTL) responses in early life is associated with an increased susceptibility to infections by intracellular microorganisms. This is likely to reflect a preferential polarization of immature CD4 T cells toward a Th2 rather than a Th1 pattern upon immunization with conventional vaccines. In this report, it is shown that a single immunization within the first week of life with DNA plasmids encoding viral (measles virus hemagglutinin, Sendai virus nucleoprotein) or bacterial (C fragment of tetanus toxin) vaccine antigens can induce adult-like Th1 or mixed Th1/Th2 responses indicated by production of IgG2a vaccine-specific antibodies and preferential secretion of interferon-γ (IFN-γ) compared with interleukin (IL)-5 by antigen-specific T cells, as well as significant CTL responses. However, in spite of this potent Th1-driving capacity, subsequent DNA immunization was not capable of reverting the Th2-biased responses induced after early priming with a recombinant measles canarypox vector. Thus, DNA vaccination represents a novel strategy capable of inducing Th1 or mixed Th1/Th2 and CTL responses in neonates and early life, providing it is performed prior to exposure to Th2-driving conventional vaccine antigens.
Newborns and young infants are at enhanced risk of severe infection by intracellular microorganisms such as viruses or certain bacteria for which clearance requires the induction of strong cellular immune responses. The immaturity of CD8 cytotoxic T cells, natural killer (NK) cells, and macrophages in early life has long been recognized. However, it was only recently observed that this impairment of cellular responses could derive from a preferential polarization of immature CD4 T cells toward a T helper type 2 (Th2) rather than a Th1 pattern upon early exposure to antigen (1–5). Thus, in contrast to a number of vaccines that successfully induce Th2 responses both in adults and young mice, neonatal Th1 responses are not easily elicited with conventional vaccines capable of eliciting Th1 responses in adults. This immunological bias of early-life responses leads to the generation of antibodies of different isotypes (low IgG2a in presence of significant IgG1 antibodies) and to a low secretion of interferon-γ (IFN-γ) by antigen-specific CD4 T cells, and is associated with an impaired induction of cytotoxic CD8 T cells. As a consequence, mechanisms responsible for the destruction of infected cells and for the intracellular clearance of microorganisms are significantly impaired. Importantly, this Th2 bias may persist later in life (1) in spite of the progressive maturation of the immune system. Persistence of hepatitis B or cytomegalovirus infections after neonatal exposure is a clinical condition that may reflect this phenomenon.
It was recently shown that immunization with plasmid DNA expression vectors can induce strong Th1 vaccine responses in adult animals (reviewed in ref. 6). Would this approach result in the induction of Th1-like responses in newborn or young mice in situations when conventional vaccines failed to do so? This question was addressed using several models of early-life immunization. We investigated the immunogenicity in early life of DNA plasmids encoding two model vaccine antigens, the measles virus hemagglutinin (MV-HA) and the fragment C of tetanus toxin (TetC), which can induce antigen-specific Th1-like or mixed Th1/Th2 responses in adult BALB/c mice (7, 8). We extended our observations to a plasmid encoding another paramyxoviral antigen, the Sendai virus nucleoprotein (SV-NP), injected in the C57BL/6 mouse strain known to differ in its indigenous polarization of immune responses. Finally, we addressed the question of whether DNA vaccines can be used to induce Th1 responses in spite of early-life-triggered Th2 responses.
MATERIALS AND METHODS
Mice.
Specific pathogen-free adult BALB/c and C57BL/6 inbred mice were purchased from Iffa Credo and kept under specific pathogen-free conditions. Breeding cages were checked daily for new births, and the day of birth was recorded as the day the litter was found. Pups were kept with mothers until weaning at the age of 4 weeks.
Vaccines.
The DNA-HA vaccine contains the membrane-bound MV-HA (9) encoding cDNA subcloned into the pV1J (10) plasmid (7). The TetC plasmid contains the synthetic gene encoding fragment C of tetanus toxin (TT) subcloned into the pcDNA3 (Invitrogen, R & D Systems) plasmid (8). The cloned SV-NP gene (11) was inserted into the pSC9 expression vector and shown to express the SV-NP protein by transient transfection assays into LLC-MK2 cells (ref. 12; L. Roux, unpublished data). Endotoxin-free DNA was produced in Escherichia coli and purified by Qiagen DNA purification columns and EndoFree extraction Kit (Qiagen). Purified DNA was stored at −20°C and used at a concentration of 1 mg/ml in sterile isotonic saline. Live attenuated measles virus (Schwarz strain, MV-Schwarz, 5 × 105 CCID50 per dose given with alum), live recombinant canarypox expressing MV-HA [vCp85, ALVAC-HA, 5 × 107 pfu per dose (13)] and TT [3 μg (2 Lf) adsorbed to alum (1)] were generously supplied by Pasteur Mérieux Sérums et Vaccins, Lyon, France.
Immunization Procedures.
Mice were immunized by groups of six to eight in the neonatal period (<24 h), as young mice (1–2 weeks), or as adults (controls). DNA vaccines were administered i.m. in each quadricep (adults, 50 μl) or into each limb (1 week, 25 μl). Immunization at < 24 h of life was performed by two quadricipital injections (25 μl) of a concentrate (2-fold) DNA solution. For early-life immunization, 29-gauge insulin needles were used, and control adult mice were immunized in parallel. TT, MV-Schwartz, and ALVAC-HA were given i.p. unless otherwise indicated.
Quantification of Vaccine-Specific Antibodies.
Mice were bled at regular intervals for the determination of vaccine-specific serum antibodies. Serum MV-HA and TT antibodies were measured by ELISA as described (1) using either antigen-coated plates or on Ltk-HA transfected cells. Total Sendai-specific antibodies were measured with heat-inactivated, purified Sendai virus-coated plates. Incubation was performed with serial serum dilution starting at 1/100. After washing, the relevant isotype-specific peroxidase-conjugated goat or rabbit anti-mouse antibody (Zymed) was added for 2 h at 37°C prior to washing, incubation with substrate, and reading. Results of MV-HA and TT antibodies were expressed by reference to serial dilution of a titrated serum pool from immunized adult mice. For determination of Sendai-specific antibodies, antibody titers were calculated as the reciprocal of the last serum dilution that gave an OD405 above that of the mean +5 SD of the preimmune sera. Antibody titers below the cutoff of the assay were being given an arbitrary titer of 1/2 the cutoff to allow calculation of geometric mean antibody titers.
Interleukin (IL)-5 and IFN-γ Determination in Supernatant of in Vitro Restimulated T Cells.
Splenocytes (107) were harvested 4 weeks after primary immunization. They were incubated at 37°C with vaccine antigen (TT, 10 μg/ml; MV-Schwarz strain, 2.5 × 105 CCID50/ml) in DMEM/10% FCS or medium alone (control wells), as described (1). Cell supernatants were collected after 48 and 72 h, and IL-5 and IFN-γ content was measured by capture ELISA as described (1, 14). Values for IL-5 and IFN-γ were expressed by reference to a standard curve constructed by assaying serial dilution of the respective mouse cytokines. Values below the cutoff of the assay were being given an arbitrary titer of 1/2 the cutoff. Antigen-specific cytokine secretion was obtained by subtracting the cytokine content of the supernatant from splenocytes incubated with DMEM alone.
Generation of CTLs and Cytotoxicity Assay.
Splenocytes were harvested 4 weeks after immunization. Identical number of splenocytes from DNA-HA-immune mice were pooled and cultured as bulk or under limiting dilution conditions as described (1). For this assay, varying numbers of responding cells were dispensed into 96 round-bottomed microwells together with 5 × 105 irradiated syngeneic stimulator spleen cells, MVHA544–552 CTL peptide (20 μg/ml) (15), DMEM/10% FCS, and EL-4 supernatant as a source of IL-2 (30 units/ml). Fresh medium containing IL-2 was added on day 7. Control bulk cultures were tested on day 7, by adding varying number of effector cells to 51Cr-labeled, HA-transfected or control P815 target cells (5 × 103) in DMEM/10% FCS. After 5 h of incubation at 37°C, cell supernatants were harvested for determination of 51Cr in a γ counter. The percentage of specific lysis was calculated as [(experimental c.p.m. − spontaneous c.p.m.)/(total c.p.m. − spontaneous c.p.m.)] × 100. Spontaneous release and total release were determined from target cells incubated with medium alone or after the addition of 100 μl of 1 M HCl, respectively. Individual wells of limiting dilution analyses were tested at day 10 for CTL activity. 51Cr-labeled P815-HA (H2d) target cells (5 × 103) were added to each well for a 5-h incubation at 37°C. Spontaneous release and total release were determined from target cells incubated with medium alone or after the addition of 100 μl of 1 M HCl, respectively. Cell supernatants were harvested for determination of 51Cr in a γ counter. Wells with a 51Cr-release content superior to the mean value +5 SD of the radioactivity measured in the supernatant of target cells alone were counted as positive wells. CTL precursor (CTLp) frequencies, which represent the mean frequency for the immune mice whose cells were initially pooled, were determined by the intersection of the regression line of the frequency of negative wells with the cutoff frequency of 37%.
Splenocytes from SV-NP-immune mice were cocultured for 5 days with an equivalent number of irradiated (2,500 rad) syngeneic spleen cells infected with the TR-5 Sendai virus strain (16) at a multiplicity of infection of 2/1. The cytotoxic activity of responding cells was determined using 51Cr-labeled, Sendai virus-infected, or control EL-4 (H2b) cells. Sendai-infected target cells were produced by adding TR-5 Sendai virus at a multiplicity of infection of 10/1, and the entire cell suspension was incubated in the presence of chymotrypsin for a further 12–16 h before use. Uninfected cells were given the same treatment, with mock allantoic fluid replacing the Sendai virus-infected allantoic fluid. Cocultures were incubated at various effector–target cell ratios for 4–6 h at 37°C, and specific cytotoxicity was calculated relative to the level of 51Cr release in medium alone.
Statistical Analysis.
Significance analysis between results obtained from various groups of mice was performed by using the Mann–Whitney U test. Probability values >0.05 were considered insignificant.
RESULTS
Induction of Adult-Like CD4 Th1 or Mixed Th1/Th2 Vaccine Responses by a Single Dose of DNA Vaccine in Early Life.
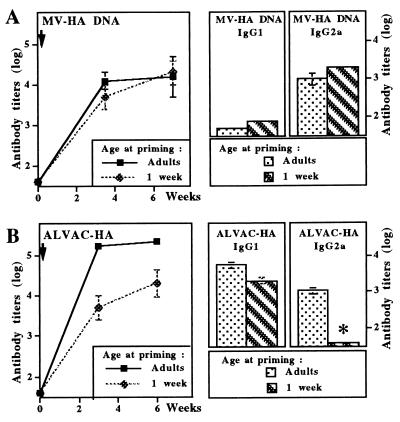
To evaluate capacity of vaccines to induce Th1 responses in early life, a single dose (100 μg) of a DNA plasmid encoding MV-HA was first given i.m. to groups of either adult or 1-week-old BALB/c mice. Similar levels of MV-HA-specific antibodies were reached in either age groups (Fig. 1A). These antibodies were enhanced 10-fold by booster DNA immunization performed 6 weeks later (data not shown). Comparative analysis of the IgG1 and IgG2a subclass distribution of vaccine antibodies, selected as indirect markers of Th1/Th2-type responses, demonstrated that a similar profile of preferential IgG2a compared with IgG1 HA-specific antibodies was induced by DNA immunization in both juvenile and adult mice. In contrast, as previously observed (1), immunization with measles virus induced similar IgG1 (5.1 log10) but significantly lower IgG2a vaccine antibody titers after immunization at 1 week of age (2.3 log10) than in adults (4.0 log10). Both total IgG and, more specifically, IgG2a vaccine antibodies induced by a single immunization of control juvenile BALB/c mice with a live recombinant canarypox vaccine expressing MV-HA (ALVAC-HA) were also significantly reduced compared with adult primed mice (Fig. 1B).
Figure 1.
Adult-like pattern of antibody responses to measles virus hemagglutinin are induced in early life by DNA but not by live recombinant canarypox vaccines. BALB/c mice were immunized at 1 week of age or as adults with the measles virus hemagglutinin encoded either by a DNA plasmid (MV-HA DNA, A) or a live recombinant canarypox vector (ALVAC-HA, B). Vaccine-specific serum IgG antibodies were measured by ELISA at various time intervals after immunization. Serum IgG1 and IgG2a vaccine-specific antibodies were measured by ELISA >6 weeks after priming. Results are expressed as mean vaccine-specific antibody titers (in log10) obtained in groups of 6–10 immunized mice. ∗, P < 0.05.
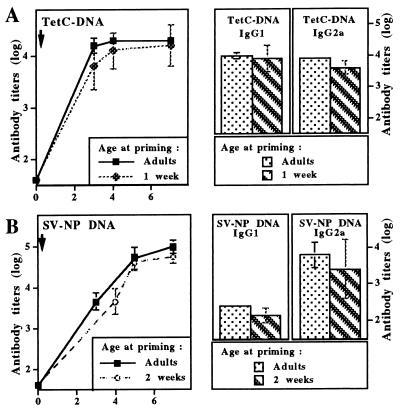
Adult and young BALB/c mice were then immunized with another plasmid (TetC DNA). An antibody profile equally distributed between IgG1 and IgG2a antibodies was induced by this DNA construct regardless of age at immunization (Fig. 2A). This again was in contrast to the lack of detection of IgG2a vaccine antibody titers (<2 log10) observed 2 weeks after TT immunization of 1-week-old vs. adult mice (3.4 log10).
Figure 2.
DNA vaccines induce similar patterns of antibody responses in adults and in early life. Young and adult BALB/c (A) and C57BL/6 (B) mice were immunized i.m. with DNA plasmids encoding either the C fragment of tetanus toxin (TetC-DNA, A), or Sendai virus nucleoprotein (SV-NP DNA, B). Vaccine-specific serum IgG antibodies were measured by ELISA at various time intervals after immunization. Serum vaccine-specific IgG1 and IgG2a antibodies were measured by ELISA >6 weeks after priming. Results are expressed as mean vaccine-specific antibody titers (in log10) obtained in groups of 6–10 immunized mice.
To define whether induction of adult-like antibody responses could be induced in early life in another mouse strain, adult and young C57BL/6 mice were immunized with a different DNA vector encoding SV-NP. A similar vaccine isotype antibody pattern including preferential IgG2a vaccine antibodies compared with IgG1 antibodies was induced in either age group (Fig. 2B). Thus, the vaccine antibody isotype distribution of mice primed in early life was similar in all evaluated cases to responses induced in adult animals with the corresponding DNA vaccine and contrasted to the weaker induction of IgG2a vaccine antibodies following the use of conventional vaccines in early life.
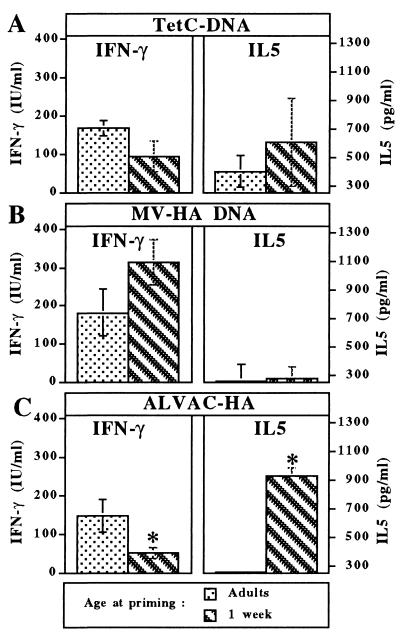
The vaccine antigen-driven cytokine secretion pattern of in vitro restimulated T cells was analyzed next, selecting IFN-γ and IL-5 as markers for the Th1 and Th2 pattern of CD4 T cell phenotype, respectively. In accordance with the respective antibody profiles, a pattern of mixed IFN-γ and IL-5 secretion was present in the supernatant of antigen-specific T cells from mice immunized with TetC DNA (Fig. 3A), whereas IFN-γ was preferentially produced by antigen-specific T cells from mice immunized with MV-HA DNA (Fig. 3B). Importantly, cytokine production patterns were similar in mice immunized either as adults or at 1 week of age. In contrast, early-life immunization with TT induced a much higher IL-5 production (data not shown), whereas the use of live recombinant ALVAC-HA induced lower IFN-γ and a much higher IL-5 production by T cells from mice primed at 1 week of age as compared with adults (Fig. 3C).
Figure 3.
Similar cytokine pattern production by antigen-specific T cells from mice primed in early life or as adults. Splenocytes were harvested 4 weeks after priming at various ages with either TetC-DNA (A), MV-HA DNA (B), or ALVAC-HA (C). Cytokine content was measured by capture ELISA after in vitro restimulation with the corresponding vaccine antigen and expressed by reference to standards. ∗, P < 0.05.
Thus, in contrast to conventional vaccines, a single early DNA immunization induced vaccine antibody responses, including significant IgG2a antibodies and IFN-γ production characteristics of Th1 or mixed Th1/Th2 responses, as efficiently in early life as in adult animals.
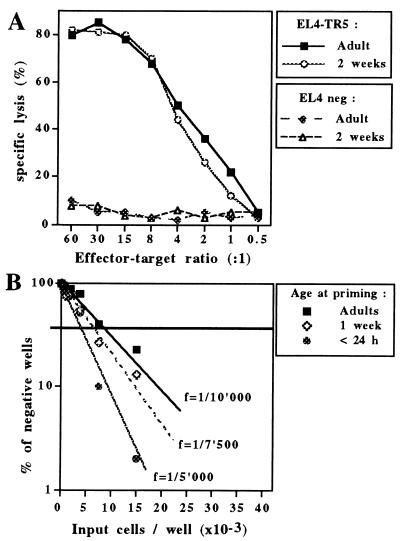
Induction of Vaccine-Specific Cytotoxic Responses by DNA Immunization in Newborns and Early Life.
The capacity of DNA vaccines to induce vaccine-specific CTLs before the age of 3–4 weeks was evaluated next. When C57BL/6 mice were immunized with a single dose of SV-NP DNA (100 μg) either as adults or as young mice, a cytolytic activity similarly resulting in the specific killing of Sendai-infected EL-4 target cells was observed (Fig. 4A). The induction of CTLs by early DNA immunization was confirmed in the MV-HA model by determination of the CTLp frequency through limiting-dilution analyses. In contrast to early life immunization with the live recombinant canarypox vector ALVAC-HA, which induced weak CTL responses in animals <3 weeks of age (ref. 1; data not shown), experiments repeatedly indicated that a single immunization of BALB/c mice by MV-HA DNA resulted in similar CTLp frequencies (varying from 1/3,000 to 1/10,000 in various experiments) whether performed in adult mice or at 1 week of age (Fig. 4B). Control lysis of untransfected P815 cells remained <20% under all conditions tested. This successful induction of CTLs by MV-HA DNA compared with ALVAC-HA was not due to a preferential CTL induction through the i.m. compared with the i.p. immunization route: in adult mice immunized i.m. with ALVAC-HA, CTLp frequency was significantly lower (f = 1/12,500) compared with i.p.-immunized animals (f > 1/3,000).
Figure 4.
(A) Significant induction of vaccine-specific cytotoxic responses after early immunization. Splenocytes were harvested 4 weeks after immunization with SV-NP. Identical numbers of cells per mice were pooled and restimulated in vitro with irradiated syngeneic spleen cells infected with the TR-5 Sendai virus strain. On day 5, varying numbers of effector cells were added to 51Cr-labeled Sendai virus-infected EL-4 (EL-4-TR5) cells or control, uninfected (EL-4 neg) cells. 51Cr release was measured after 4–6 h of incubation at 37°C. The percentage of specific lysis was calculated as [(experimental c.p.m. − spontaneous c.p.m.)/(total c.p.m. − spontaneous c.p.m.)] × 100. Results are representative of the entire group (pool of splenocytes) and have been reproduced in more than four independent experiments that simultaneously assessed groups of young and adult mice. (B) MV-HA DNA immunization induces CTLp at similar frequencies after priming at various stages of immune maturation. Splenocytes harvested 4 weeks after primary immunization were cultured under limiting dilution conditions. Identical numbers of splenocytes were pooled and various numbers of responding cells were incubated with 5 × 105 irradiated syngeneic stimulator spleen cells and MV HA544–552 CTL peptide (20 μg/ml) in IL-2-supplemented medium. Individual wells were tested at day 10 for CTL activity on P815-HA cells. Wells with a 51Cr-release content superior to the mean + 5 SD of the radioactivity measured in the supernatant of target cells alone were counted as positive wells. CTLp frequencies (f) were determined by the intersection of the regression line of the frequency of negative wells with the cutoff frequency of 37%. Results are representative of more than six independent experiments that simultaneously assessed groups of young and adult mice.
To further determine whether DNA vaccines could induce CTL responses independently of the stage of immune maturation, a single dose of MV-HA DNA (100 μg) was administered to newborn BALB/c mice within their first 24 h of life. When assessed 4 weeks after immunization, the CTLp frequency of mice immunized immediately after birth was similar to the one observed after immunization at 1 week of age or as adults (Fig. 4B). Vaccine antibody profile and IFN-γ production by T cells of newborn primed mice were also similar to those seen in mice immunized later in life (data not shown). Thus, strong Th1 and CTL vaccine responses can be triggered by neonatal DNA immunization before 24 h of life, at a time classically considered as favoring the induction of tolerance rather than immunity.
Importance of Age at Priming for the Pattern of Adult Responses.
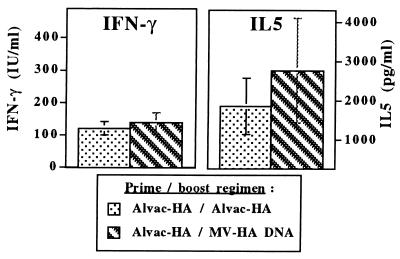
DNA vaccines have been reported as capable to induce Th1 responses in adult mice previously primed with a Th2 polarizing agent (17). We thus wondered whether they could similarly induce Th1 responses in mice exhibiting a Th2-biased vaccine response following early-life immunization with another antigen presentation system. One-week-old BALB/c mice were thus primed with ALVAC-HA and boosted 3 weeks later with either ALVAC-HA or MV-HA DNA. Boosting with MV-HA DNA did not increase MV-HA IgG2a antibodies, which remained at a very low titer of 2.1 log10. Comparison of the cytokine production by antigen-specific T cells of mice from either groups revealed a similar pattern consisting of low levels INF-γ in the presence of very high levels of IL-5 (Fig. 5). This significantly differed from the antibody and cytokine patterns induced by a single MV-HA DNA immunization given at any age (Figs. 1A and 3B). Finally, MV-HA DNA boosting of mice primed with ALVAC-HA at 1 week of age did not increase CTLp frequencies, which remained similar to the low frequencies observed after early-life ALVAC-HA priming and boosting (data not shown).
Figure 5.
Boosting with MV-HA DNA or ALVAC-HA in mice primed at 1 week of age with ALVAC-HA induces similarly high levels of IL-5 and low levels of IFN-γ secretion by vaccine T cells. Splenocytes were harvested 3 weeks after boosting with ALVAC-HA or MV-HA DNA. Cytokine content was measured by capture ELISA after in vitro restimulation with the corresponding vaccine antigen and expressed by reference to standards. Antigen-specific cytokine secretion was obtained by subtracting the cytokine content present in the supernatant of splenocytes incubated with DMEM alone.
Thus, early-life responses of BALB/c mice to vaccines can be polarized toward the Th2 phenotype to such an extent that later exposure to a strong Th1-driving DNA vaccine could not circumvent the influence of neonatal priming.
DISCUSSION
This report provides preclinical evidence of the potential advantages of DNA vaccines for the induction of cellular vaccine responses in early life. It demonstrates that adult-like Th1 and CTL vaccine responses against various viral or bacterial antigens can be raised by DNA immunization at any stage of the immune maturation of young mice, even immediately after birth. However, it also stresses the fact that the Th2 polarization following neonatal exposure to conventional vaccines extends into adult age to such a degree that it cannot be reverted by Th1-driving DNA vaccines.
Each of the three DNA vaccines tested proved capable of inducing adult-like vaccine responses in young mice. Vaccine antibody patterns and cytokine production indicated that immunized mice preferentially raised Th1 responses in response to MV-HA or SV-NP DNA and responded to TetC DNA with a mixed Th1/Th2 response. Importantly, these vaccine responses to DNA immunization, which probably depend on the biochemical nature of the antigen itself, were not affected by age at immunization. This is in striking contrast to the Th2-biased early-life responses already observed with other antigen presentation systems (1). Use in early life of alum-adjuvanted peptide/protein vaccines [tetanus toxoid, whole measles virus, inactivated Sendai virus (data not shown)], of live attenuated measles virus (data not shown), or even of live recombinant canarypox ALVAC-HA vaccines generated limited IgG2a antibody responses and IFN-γ production in contrast to a burst of antigen-driven IL-5 production. These observations occurred independently of the nature of the antigen or of the antigen presentation systems.
Defective induction of CD8 cytotoxic responses (CTLs) had previously been observed in mice immunized up to the age of 3–4 weeks (1). CTLp frequency in 1-week-old mice immunized with live recombinant ALVAC-HA remained below 1/20,000 compared with >1/3,000 in adult primed mice. This CTL deficiency could represent merely a consequence of the defective induction of CD4 Th1 responses. Alternatively, additional deficiencies of the lytic machinery could have been present in early life. Our observation that DNA vaccines that induce strong CD4 Th1 responses also successfully induce vaccine-specific CTLs in early life suggests that the Th2 polarization of early vaccine responses is the main obstacle to the early generation of cytotoxic effector cells. Thus, selective vaccine strategies inducing Th1 and CTL responses in adults could be expected to generate CTLs in early life if strong enough Th1 responses can be induced.
CD4 Th1 and CD8 vaccine responses were thus elicited against various antigens, by several DNA plasmids, at various stages of the early immune maturation of either BALB/c or C57BL/6 mice. These observations are in accordance with preliminary observations describing induction of adult-like antibody patterns (18) or of CTL responses (19, 20) after neonatal immunization with plasmids encoding rabies virus glycoprotein, influenza nucleoprotein, or a murine retroviral antigen.
In contrast, they do not support the hypothesis by Mor et al. (21) based on immunization with a malaria circumsporozoite protein (CSP)-encoding plasmid, that neonatal DNA immunization favors neonatal tolerance induction. An explanation would be that this CSP DNA construct, whose immunogenicity is clearly weaker and different from the native protein, encodes only a few epitopes cross-reacting with self-antigens. An alternative explanation, which we favor, is that its weak immunogenicity might not be sufficient for priming in early life. In either case, our observations demonstrate that the immunogenic potential of DNA vaccines is worth assessing in the many situations in which the induction of early life Th1 and/or CD8 responses would be desirable.
The demonstration that neonatal and early-life Th1 responses can be induced by DNA vaccines provides new insight into the mechanisms possibly at the basis of the Th2 bias of neonatal responses. Steps considered instrumental in the induction of primary Th1/Th2 immune responses and thus potentially involved in the polarization of early immune responses have been identified at the level of (i) antigen-presenting cell (APC) activation and IFN-α/IL-12 production (2, 22, 23), (ii) NK cell activity and IFN-γ secretion (24), and (iii) T cell activation requirements (25). It was also considered possible that the Th2 cytokine milieu prevailing during pregnancy at the materno-fetal interface (26) could extend its influence to a period of time after birth and thus contribute to the shaping of immune responses in newborn and juvenile mice. The generic property of nucleic acids to raise strong Th1 responses in adult animals has been recently linked to the presence in bacterial DNAs of specific, unmethylated CpG motives capable of rapidly enhancing transcription of IL-6, IFN-α, IFN-γ, and IL-12 in antigen-presenting cells (27–29). This APC activation was found to result in NK cell/T cell activation. The demonstration of the capacity of DNA plasmids to induce Th1 neonatal responses indicates that DNA-triggered mechanisms should already function early in life. Thus, the essential basic defect underlying the Th2 polarization of early responses can be found within the initial steps of neonatal APC activation, rather than in the “downstream pathway” of neonatal NK cell/T cell activation. This APC activation defect could involve (i) variation in antigen presentation by immature APC, (ii) impaired activation of IL-12/IFN-α by viral or bacterial agents, or (iii) impaired molecular responses to yet undefined “danger” signals required for full activation of APC (2).
The role of antigen presentation by neonatal APC in the shaping of neonatal responses can be evaluated in our MV-HA immunization model. In spite of a similar Th1-driving immunogenicity in adult mice, a DNA vaccine and a live viral vaccine (ALVAC-HA) encoding the same MV-HA antigen completely differ in their capacity to induce Th1 and CTL responses in early life. It is therefore likely that two different activation patterns of neonatal/juvenile dendritic cells are triggered by these two antigen-presenting systems. Injections of DNA plasmids and of live recombinant canarypox share common features such as direct introduction and immediate transcription and expression of the encoded antigen within the cell. They differ, however, in the type of antigen exposure they generate: canarypox vectors have been reported as resulting in a strong and transient production of antigen (30), whereas DNA vaccines induce low but prolonged antigen synthesis (31). The pattern of antigen exposure to the immune system, including antigen load and/or persistence, could thus play a crucial role in the shaping of early-life responses. The role of the dose of antigen had been identified in two reports, demonstrating that lowering the dose of antigen [adult male splenic cells (2) or infectious murine leukemia virus (3)] was sufficient to prime neonatal mice for Th1 responses. However, dose reduction failed to induce Th1 responses in all our models of early immunization with “conventional” vaccine antigens (1). We had postulated this discrepancy to reflect differences between the antigenic stimulation following neonatal infection with a replicating pathogenic live retrovirus and immunization with subunits or replication-deficient live viral vaccines. The observations reported here suggest that induction of Th1 responses in early life could require a prolonged exposure to low doses of antigen, as is achieved either by the use of infectious agents with prolonged replication patterns (3) or by DNA vaccines. This could be linked to the recently described requirement for prolonged antigen exposure to maintain expression of the IL-12 receptor β2 chain and thus drive CD4 T cells along the Th1 differentiation pathway (32, 33).
Whether impaired activation of IL-12/IFN-α by viral or bacterial agents also plays a role in the bias of neonatal responses cannot be inferred from these studies and is currently being assessed. However, our observations imply that if specific “danger” activation signals are required for induction of early Th1 responses, they would be similarly induced by live replicating agents and DNA vaccines but not by other Th1-driving vaccines such as replication-deficient live vectors. In this period of early life associated with immune immaturity, the use of DNA vaccines rather than live replicating agents would represent an obvious advantage.
We had previously shown that the influence of early priming with conventional vaccines or even live recombinant vector tends to persist in adult life (1). This occurred even if a vaccine boost was given with a Th1-driving adjuvant (34). In adult mice, Th1-driving DNA vaccines were found capable of circumventing the Th2 response induced by priming with antigen [β-galactosidase (β-gal)] in alum: a change to a mixed Th1/Th2 response was achieved by boosting with a β-gal-encoding plasmid (17). We show here that this is not the general case for Th2-biased responses induced in early life. Neither the low IgG2a responses, the IL-5 burst, nor the weak CTL induction typical of early ALVAC-HA priming were modified by boosting with MV-HA DNA. Thus, memory cells induced by early immunization appear strongly committed toward the Th2 pattern, at least in the more Th2-prone BALB/c strain. Their IL-4/IL-5 cytokine production probably maintains its domination over that of IL-12 and thus limits the polarization of newly induced Th1 cells by either specific adjuvant formulations (34) or DNA vaccines. Whether this is also the case in mice strains with an indigenous bias toward Th1 responses remains to be evaluated. This observation of the prolonged influence of Th2-biased CD4 T cells triggered in early life implies that vaccine strategies capable of inducing early Th1 and CD8 cytotoxic responses might have to include administration of a Th1-driving vaccine prior to exposure to either wild-type agents or Th2-driving conventional vaccine antigens.
In conclusion, the capacity of DNA vaccine to circumvent the tendency to develop preferential Th2 patterns of responses in early life is a promising observation for the development of vaccines against diseases caused in early life by viruses (e.g., cytomegalovirus, respiratory syncytial virus, or herpes simplex virus) or by other intracellular microorganisms such as mycobacteria or leishmania. Appropriate preclinical safety/efficacy assessment should thus be conducted in newborn and young animals at initial stages of vaccine development.
Acknowledgments
We are grateful to L. Roux for his essential contribution to the development of the Sendai virus immunization model and to M. Berney and M. Córdova for excellent technical assistance. We also thank M. Homma for kindly providing the TR-5 Sendai vaccine strain. This work was supported by grants from the Swiss National Science Foundation, from the World Health Organization Global Programme for Vaccines and Immunization, from the Sandoz Foundation, and from Pasteur-Mérieux Serums et Vaccins. This work was also supported by the Deutsche Forschungsgemeinschaft (C.B.), the Roche and the Wolfermann-Nageli Foundations (F.S.), and the Swiss National Science Foundation (C.A.S.).
ABBREVIATIONS
- Th
T helper
- CTL
cytotoxic T lymphocyte
- IFN-γ
interferon-γ
- IL
interleukin
- NK
natural killer
- MV
measles virus
- HA
hemagglutinin
- TetC
fragment C of tetanus toxin
- SV-NP
Sendai virus nucleoprotein
- ALVAC-HA
measles-HA recombinant canarypox
- TT
tetanus toxoid
- APC
antigen-presenting cells
References
- 1.Barrios C, Brawand P, Berney M, Brandt C, Lambert P H, Siegrist C A. Eur J Immunol. 1996;26:1489–1496. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 2.Ridge J P, Fuchs E J, Matzinger P. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 3.Sarzotti M, Robbins D S, Hoffman P M. Science. 1996;271:1726–1728. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 4.Forsthuber T, Yip H C, Lehman P V. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 5.Singh R R, Hahn B H, Sercarz E E. J Exp Med. 1996;183:1613–1621. doi: 10.1084/jem.183.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulmer J B, Sadoff J C, Liu M A. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso A I, Blixenkrone-Moller M, Fayolle J, Liu M, Buckland R, Wild T F. Virology. 1996;225:293–299. doi: 10.1006/viro.1996.0603. [DOI] [PubMed] [Google Scholar]
- 8.Anderson R, Gao S M, Papakonstantinopoulou A, Roberts M, Dougan G. Infect Immun. 1996;64:3168–3173. doi: 10.1128/iai.64.8.3168-3173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerald C, Buckland R, Barker R, Freeman G, Wild T F. J Gen Vir. 1986;67:2695–2703. doi: 10.1099/0022-1317-67-12-2695. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery D I, Shiver J W, Leander K R, Perry H C, Friedman A, Martinez D, Ulmer J B, Donnelly J J, Liu M A. DNA Cell Biol. 1993;12:777–783. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 11.Kast W M, Roux L, Curran J, Bloom H J J, Voordouw A C, Meloen R H, Kolakofsky D, Melief C J. Proc Natl Acad Sci USA. 1991;88:2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakrabarti S, Brechling K, Moss B. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor J, Pincus S, Tartaglia J, Richardson C, Alkhatib G, Briedis D, Appel M, Norton E, Paoletti E. J Virol. 1991;65:4263–4274. doi: 10.1128/jvi.65.8.4263-4274.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramar G, Schurmans S, Berney M, Izui S, del Giudice G, Lambert P H. J Autoimmunol. 1995;8:177–192. doi: 10.1006/jaut.1995.0014. [DOI] [PubMed] [Google Scholar]
- 15.Galletti R, Beauverger P, Wild T F. Vaccine. 1995;13:197–201. doi: 10.1016/0264-410x(95)93136-w. [DOI] [PubMed] [Google Scholar]
- 16.Maru M, Haraguchi M, Sato K, Hotta H, Homma M. Vet Microbiol. 1992;30:1–12. doi: 10.1016/0378-1135(92)90089-c. [DOI] [PubMed] [Google Scholar]
- 17.Raz E, Tighe H, Sato Y, Corr M, Dudler J A, Roman M, Swain S, Spiegelberg H L, Carson D A. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Xiang X, Pasquini S, Ertl H E. Virology. 1997;228:278–284. doi: 10.1006/viro.1996.8384. [DOI] [PubMed] [Google Scholar]
- 19.Bot A, Bot S, Garcia-Sastre A, Bona C. Viral Immunol. 1996;9:207–210. doi: 10.1089/vim.1996.9.207. [DOI] [PubMed] [Google Scholar]
- 20.Sarzotti, M., Dean, T. A., Remington, M. P., Ly, C. D., Furth, P. A. & Robbins, D. S. (1997) Vaccine, in press. [DOI] [PubMed]
- 21.Mor G, Yamshchikov G, Sedegah M, Takeno M, Wang R, Houghten R A, Hoffman S, Klinman D M. J Clin Invest. 1996;98:2700–2705. doi: 10.1172/JCI119094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duncan D D, Swain S L. Eur J Immunol. 1994;24:2506–2514. doi: 10.1002/eji.1830241037. [DOI] [PubMed] [Google Scholar]
- 23.Macatonia S E, Hosken N A, Litton M, Vieira P, Hsieh C S, Culpepper J A, Wysocka M, Trinchieri G, Murphy K M, O’Garra A. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 24.Lee S M, Suen Y, Chang L, Bruner V, Quian J, Indes J, Knoppel E, van de Ven C, Cairo M S. Blood. 1996;88:945–954. [PubMed] [Google Scholar]
- 25.Adkins B, Ghanei A, Hamilton K. J Immunol. 1994;153:3378–3385. [PubMed] [Google Scholar]
- 26.Lin H, Mosmann T R, Guilbert L, Tuntipopipat S, Wegmann T G. J Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- 27.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. Proc Nat Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M D, Silverman G J, Lotz M, Carson D A, Raz E. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 29.Ballas Z K, Rasmussen W L, Krieg A M. J Immunol. 1996;157:1840–1845. [PubMed] [Google Scholar]
- 30.Taylor J, Meignier B, Tartaglia J, Languet B, VanderHoeven J, Franchini G, Trimarchi C, Paoletti E. Vaccine. 1995;13:539–549. doi: 10.1016/0264-410x(94)00028-l. [DOI] [PubMed] [Google Scholar]
- 31.Wolff J A, Ludtke J J, Acsadi G, Williams P, Jani A. Hum Mol Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 32.Szabo S J, Dighe A S, Gubler U, Murphy K M. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky D H, Gubler U, Sinigaglia F. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrios C, Brandt C, Berney M, Lambert P H, Siegrist C A. Eur J Immunol. 1996;26:2666–2670. doi: 10.1002/eji.1830261118. [DOI] [PubMed] [Google Scholar]