Abstract
The sunflower alliance of families comprises nearly 10% of all flowering plant species and includes the largest of all plant families, the sunflower family Asteraceae, which has 23,000 species, and the bellflower family Campanulaceae. Both are worldwide in distribution, but the majority of their species occur in the northern hemisphere. Recently it has been shown that a number of small, woody families from the Australian–Southwest Pacific area also belong in this relationship. Here we add yet another such family and present phylogenetic, biogeographic, and chronological analyses elucidating the origin of this large group of plants. We show that the ancestral lineages are confined to Malesia, Australia, New Guinea, and New Zealand and that the sunflower and bellflower families represent phylogenetically derived lineages within a larger group with a Cretaceous and southern-hemisphere, presumably East Gondwana, ancestry. Their highly derived position in the flowering plant phylogeny makes this significant for understanding the evolution of flowering plants in general.
The origin and evolution of flowering plants is a major botanical issue. The time of origin was probably before the Cretaceous and the early diversification during the Cretaceous (1), whereas many specialized families evolved during the Tertiary period. Another aspect of the evolution of flowering plants is their geographic origin (2), a problem that may be too elusive to solve. It should, however, be possible to trace the geographic origins of major groups of flowering plants by analyzing phylogenetic information, fossils, and known distributions. The reconstruction of flowering plant phylogeny is now becoming increasingly feasible, thanks to the cladistic analysis of a rapidly accumulating body of molecular data (3–5). With corroborated phylogenies, it becomes possible to address questions such as when and where major groups of flowering plants evolved.
The sunflower family Asteraceae (Compositae), the bellflower family Campanulaceae, and a number of smaller families together form a monophyletic group known as the order Asterales (or Campanulales; refs. 6–8). Judging by the sunflowers and bellflowers, one could get the impression that this alliance of families was herbaceous and northern hemisphere in origin. We suggest, however, that this is by no means the case.
Many of the families of the sunflower alliance are from the southern hemisphere. This picture has recently been complemented by the addition of three small families confined to Australia, New Guinea, New Zealand, and New Caledonia: Argophyllaceae, Alseuosmiaceae, and Phellinaceae (6, 9). These families of shrubs and trees were formerly associated with or even included in other families from different parts of the system. We also add Carpodetaceae to this alliance, another family of trees and shrubs from Australia, New Zealand, and New Guinea (also in the Solomon Islands). The position of these several families within the sunflower alliance is supported both by rbcL sequences and certain morphological features (refs. 6 and 9; a detailed discussion of the molecular and morphological evidence for the circumscription and systematic position of Carpodetaceae will be published elsewhere; ref. 10).
Macrofossils of the sunflower alliance are unknown or very uncertain, and most of the families have no fossil record. Fossils clearly identifiable as members of the alliance are restricted to pollen of the Asteraceae and Goodeniaceae from Oligocene and later and seeds of the Menyanthaceae and Campanulaceae from Oligocene and Miocene, respectively (11). There are also records of Eocene pollen of the Asteraceae from South America, but they need confirmation (12). Because Oligocene pollen of the Asteraceae is of a comparatively specialized type and is found on several continents (12), it is reasonable to assume that the family dates back at least to the Oligocene-Eocene boundary 38 million years (Myr) B.P. On the basis of the appearance of fossil pollen of the Asteraceae and Goodeniaceae and also of general trends in morphology and pollination syndromes of fossil flowers, DeVore and Stuessy (13) argue that the split between the Australian Goodeniaceae and the originally South American Calyceraceae and Asteraceae occurred in the Eocene and was connected to the early Eocene (43–53 Myr B.P.) isolation of South America from Antarctica.
The geographic origin of any group may be hypothesized from the distributions of its subgroups in relation to the phylogeny (14, 15). Perhaps the most familiar example is that of human geographic origin, inferred from the phylogeny of mitochondrial DNA sequences (16). Areas represented both on phylogenetically basal branches (Africa in the case of human mtDNA) and on several branches of the phylogenetic tree are more likely part of the ancestral area of the group than other regions (14). For the sunflower alliance, our analyses point to an origin in the Australasian region.
Dating the origin of groups, at least approximately, may be done by counting the accumulation of nucleotide substitutions (17, 18). Although rbcL sequence data are not perfectly clock-like (5, 19), there is a roughly linear relationship between time and the amount of nucleotide substitutions in the rbcL gene (20). The substitution rate may be estimated by using fossil data. We have assessed an approximate substitution rate for the rbcL gene in the sunflower alliance using the fossil datings mentioned above and estimate the origin of the alliance to the Cretaceous period.
MATERIALS AND METHODS
We inferred the phylogeny of the sunflower alliance by parsimony analysis (21) of 35 rbcL sequences from all the families. The parsimony analysis comprised a heuristic search with 100 random-addition sequences and tree-bisection-reconnection branch swapping (21). The circumscription of the alliance was corroborated by a prior jackknife analysis (22) of a data matrix comprising 75 sequences from taxa throughout the eudicotyledons (10).
The data matrix corresponded to that of the first analysis of Gustafsson et al. (6), with the addition of Carpodetus serratus J. R. Forst. & G. Forst. and of Chamaedaphne calyculata (L.) Moench and Dracophyllum longifolium (J. R. Forst. & G. Forst.) R. Br. The last two taxa were added to investigate the possibility of a relationship between Carpodetus and the order Ericales (23). European Molecular Biology Laboratory/National Center for Biotechnology Information accession numbers for all sequences are listed by Gustafsson et al. (6), except for Carpodetus serratus [Y08461 (new)], Chamaedaphne calyculata (L12606), Dracophyllum longifolium (L12614), Cuttsia viburnea F. Muell. [Y08462 (new)], and Phelline comosa Labill. (X69748).
The geographic origin of the sunflower alliance was assessed by ancestral area analysis (14) and reversible parsimony analysis (15) of the areas treated as characters to be optimized on the cladogram obtained from the parsimony analysis. In an alternative approach, component analysis (24) of the cladogram was used to estimate the geographic origin by removing possibly dispersed taxa with distributions in conflict with known area interrelationships.
The substitution rate of the rbcL gene was obtained by dividing the mean branch length from the ancestral node of the Asteraceae to its terminals in the cladogram with the minimum age of the family, as given by the fossils. The age of the sunflower alliance was subsequently estimated by dividing the mean branch length from the ancestral node of the whole alliance to its terminals in the cladogram with the substitution rate.
RESULTS
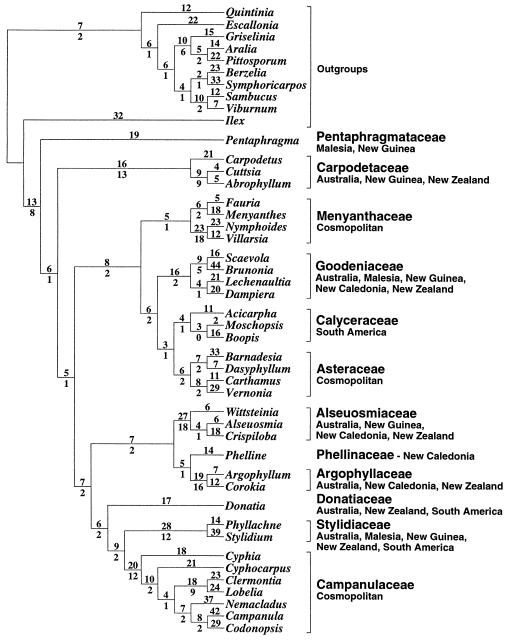
The parsimony analysis resulted in three maximally parsimonious trees with a length of 1,050 steps and a consistency index of 0.41. The alternative trees differ only with respect to the interrelationships within the Calyceraceae. One tree is shown in Fig. 1. Further details of this analysis as well as from the initial jackknife analysis have been reported elsewhere (10). Some of the family groupings, in particular the one comprising the Menyanthaceae, Goodeniaceae, Calyceraceae, and Asteraceae, are also strongly supported by other data sets from analyses of both morphological data (8) and chloroplast DNA ndhF sequences (26). The basal branchings within the alliance are not well supported, but alternative arrangements will not change the overall conclusion drawn from the biogeographic analyses below, because the majority of the branches lead to Australasian groups and because groups outside Australasia are not among the basal branches of the tree.
Figure 1.
Phylogeny of the sunflower alliance of families reconstructed by parsimony analysis (21) of chloroplast DNA rbcL sequences. Numbers above the branches are branch lengths, and those below are measures of branch support, namely the number of extra steps necessary to lose a branch (25). The cosmopolitan Asteraceae are considered to be of South American or South American–Pacific origin (11, 14). Geographic origin of the Campanulaceae is unknown, but phylogenetically basal branches involve Africa, South America, and North America. The widespread Menyanthaceae comprise two groups with different distributions: one found mainly in Australia and the other primarily represented in the northern hemisphere. The Goodeniaceae also comprise a few species on islands of the Pacific, on tropical seashores, and in South America (presumably secondarily dispersed). Biogeographic analysis (14, 15, 24) of the family distributions indicates that the alliance originated in an area related to the Australasian region of today. Fossil evidence (11, 12) and the number of accumulated nucleotide substitutions in the rbcL gene (20) together place the time of origin in the Cretaceous, when Australasia was part of East Gondwana.
Ancestral area analysis (14) indicates that Australia, New Guinea, and New Zealand are most likely to be part of the ancestral area, followed by Malesia with a likelihood of 54% of that of the former areas. Area-character optimization (reversible parsimony; ref. 15) distinguishes New Guinea and possibly also Malesia, Australia, and New Zealand as part of the ancestral area. Both methods indicate the other areas as less likely or less parsimoniously to be part of the ancestral area. Tracing possible dispersal from an ancestral area by checking conflicting distributions in component analysis (24) yields similar results. The method is not decisive; even a cursory inspection of Fig. 1 suggests that the origin is related to the Australasian region. We realize that any future addition of hitherto misplaced taxa may affect these results. The number of such taxa that are even remotely possible candidates for a position in the sunflower alliance is small, however, and the few that are known to us (but have yet to be sequenced because of the scarcity of material) are all from the southern hemisphere, and most of them are from the Australasian region. (One such southern hemisphere taxon is Roussea from the Indian Oceanic island of Mauritius; refs. 4 and 27.) The general conclusion, that the alliance is of Australasian origin, is thus unlikely to be changed.
As noted above, the age of the Asteraceae may be assessed to at least 38 Myr. The mean branch length from the ancestral node of this family to its terminals in Fig. 1 is 28 ± 6.5 (standard error) steps or substitutions. The substitution rate may then be estimated at at most (28 ± 6.5)/38 = 0.74 ± 0.17 substitutions per Myr within the whole rbcL gene. Almost exactly the same rate is obtained by calibrating with DeVore and Stuessy’s (13) dating of the split between the Goodeniaceae and the Calyceraceae and Asteraceae to 48 ± 5 Myr B.P. The mean branch length from the ancestral node of the sunflower alliance to the terminals in Fig. 1 is 66 ± 4.4 steps. The age of the alliance thus may be estimated to be at least (66 ± 4.4)/(0.74 ± 0.17) = 96 ± 28 Myr, which is within the Cretaceous period (65–145 Myr B.P.). These calculations are based on patristic distances as summarized branch lengths on cladograms. Using plain sequence dissimilarity results in slightly lower age estimates, but corrections for multiple substitutions (28) increase the estimates. The general conclusion of a Cretaceous origin is likely to be the same.
DISCUSSION
A Cretaceous origin of the sunflower alliance is significant for timing flowering plant evolution in general (1), because the sunflower and bellflower families are among phylogenetically the most specialized of all flowering plants (2, 3). These two families are represented by branches very high up in the flowering plant phylogeny, the basal branchings of which are considered to be from the Cretaceous (1). If phylogenetically specialized groups such as the sunflower alliance date back to the Cretaceous, early angiosperm diversification may have been even older. This conclusion also is supported by other analyses using molecular data (18).
The distribution and phylogenetic interrelationships of extant members indicate that the ancestor of the alliance occurred in an area including what is now Australasia. There is, of course, always the possibility that the small families today restricted to Australasia may have had much wider distributions in the past, the recent members representing relict survivors in an area where such relicts are common. One such example may be provided by the Escalloniaceae, which is outside the sunflower alliance. Extant members of this family (Escallonia and Quintinia in Fig. 1) are restricted to the southern hemisphere, but there is a Cretaceous fossil from Sweden, Silvianthemum (29). Notably, there is no fossil or other evidence from the sunflower alliance itself to support a scenario of relictual occurrence in Australasia, and an Australasian origin is therefore the best-supported hypothesis.
In the Cretaceous, Australasia was connected to eastern Antarctica, the two areas constituting most of East Gondwana (as opposed to West Gondwana, comprising western Antarctica, Africa, and South America; ref. 30). Given the available evidence of distributions, fossils, and sequence data, the sunflower alliance therefore seems to have originated in East Gondwana. If so, the group evidently diversified and expanded to West Gondwana before the final breakup of the supercontinent and the isolation of Australasia and South America from Antarctica. The Australasian–South American connection displayed by the Goodeniaceae and the Calyceraceae and Asteraceae (13), as well as, for example, by the Stylidiaceae (and many other groups of organisms; refs. 13 and 31), is a remnant from the early, but apparently not original, distribution of the alliance in both East and West Gondwana. The current cosmopolitan distributions of the sunflower and bellflower families are the results of more recent events.
Acknowledgments
We thank Birgitta Bremer for access to her laboratory and James S. Farris, Else Marie Friis, Jeff J. Doyle, Martin Naylor, John Peel, Fredrik Ronquist, Christopher Talbot, Anders Backlund, Maria Backlund, Birgitta Bremer, Nadina Laurent, Johannes Lundberg, and Ulf Swenson for advice, suggestions, and support. This research was funded by the Swedish Natural Science Research Council.
ABBREVIATIONS
- rbcL
ribulose-1,5-biphosphate carboxylase/oxygenase large subunit
- ndhF
NADH dehydrogenase subunit F
Footnotes
References
- 1.Crane P R, Friis E M, Pedersen K R. Nature (London) 1995;374:27–33. [Google Scholar]
- 2.Takhtajan A. Flowering Plants: Origin and Dispersal. Edinburgh: Oliver & Boyd; 1969. [Google Scholar]
- 3.Chase M W, Soltis D E, Olmstead R G, Morgan D, Les D H, et al. Ann Mo Bot Gard. 1993;80:528–580. [Google Scholar]
- 4.Soltis D E, Soltis P S, Nickrent D L, Johnson L A, Hahn W J, Hoot S B, Sweere J A, Kuzoff R K, Kron K A, Chase M W, Swensen S M, Zimmer E A, Chaw S-M, Gillespie L J, Kress W J, Sytsma K J. Ann Mo Bot Gard. 1997;84:1–49. [Google Scholar]
- 5.Clegg M T. Proc Natl Acad Sci USA. 1993;90:363–367. doi: 10.1073/pnas.90.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafsson M H G, Backlund A, Bremer B. Plant Syst Evol. 1996;199:217–242. [Google Scholar]
- 7.Cosner M E, Jansen R K, Lammers T G. Plant Syst Evol. 1994;190:79–95. [Google Scholar]
- 8.Gustafsson M H G, Bremer K. Am J Bot. 1995;82:250–265. [Google Scholar]
- 9.Backlund, A. & Bremer, B. (1997) Plant Syst. Evol., in press.
- 10.Gustafsson, M. H. G. & Bremer, K. (1997) Aust. Syst. Bot., in press.
- 11.Benton M J. The Fossil Record 2. London: Chapman & Hall; 1993. [Google Scholar]
- 12.Graham A. In: Compositae: Systematics. Hind D J N, Beentje H J, editors. Kew, U.K.: Royal Botanic Gardens; 1996. pp. 123–140. [Google Scholar]
- 13.DeVore M L, Stuessy T F. In: Advances in Compositae Systematics. Hind D J N, Jeffrey C, Pope G V, editors. Kew: Royal Botanic Gardens; 1995. pp. 23–40. [Google Scholar]
- 14.Bremer K. Syst Biol. 1992;41:436–445. [Google Scholar]
- 15.Ronquist F. Syst Biol. 1994;43:267–274. [Google Scholar]
- 16.Vigilant L, Stoneking M, Harpending H, Hawkes K, Wilson A C. Science. 1991;253:1503–1507. doi: 10.1126/science.1840702. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe K H, Gouy M, Yang Y-W, Sharp P M, Li W H. Proc Natl Acad Sci USA. 1989;86:6201–6205. doi: 10.1073/pnas.86.16.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin W, Lydiate D, Brinkmann H, Forkmann G, Saedler H, Cerff R. Mol Biol Evol. 1993;10:140–162. doi: 10.1093/oxfordjournals.molbev.a039989. [DOI] [PubMed] [Google Scholar]
- 19.Bousquet J, Strauss S H, Doerksen A H, Price R A. Proc Natl Acad Sci USA. 1992;89:7844–7848. doi: 10.1073/pnas.89.16.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albert V A, Backlund A, Bremer K, Chase M W, Manhart J, Mishler B D, Nixon K C. Ann Mo Bot Gard. 1994;81:534–567. [Google Scholar]
- 21.Swofford D L. paup: Phylogenetic Analysis Using Parsimony. Champaign: Illinois Natural History Survey; 1993. , Version 3.1.1. [Google Scholar]
- 22.Farris J S. jac. Stockholm: Swedish Museum of Natural History; 1996. , Version 4.4. [Google Scholar]
- 23.Praglowski J, Grafström E. Grana. 1985;24:11–21. [Google Scholar]
- 24.Page R D M. component. London: The Natural History Museum; 1993. , Version 2.0. [Google Scholar]
- 25.Bremer K. Cladistics. 1994;10:295–304. [Google Scholar]
- 26.Jansen R K, Kim K J. In: Compositae: Systematics. Hind D J N, Beentje H J, editors. Kew: Royal Botanic Gardens; 1996. pp. 317–339. [Google Scholar]
- 27.Soltis D E, Soltis P S. Am J Bot. 1997;84:504–522. [PubMed] [Google Scholar]
- 28.Zharkikh A. J Mol Evol. 1994;39:315–329. doi: 10.1007/BF00160155. [DOI] [PubMed] [Google Scholar]
- 29.Friis E M. Biol Skr Dan Vid Selsk. 1990;36:1–35. [Google Scholar]
- 30.Archangelsky S. In: Antarctic Paleobiology. Taylor T N, Taylor E L, editors. New York: Springer; 1990. pp. 102–117. [Google Scholar]
- 31.Thorne R F. Notes Royal Bot Gard Edinburgh. 1978;36:297–315. [Google Scholar]