Abstract
G proteins play a major role in signal transduction upon platelet activation. We have previously reported a patient with impaired agonist-induced aggregation, secretion, arachidonate release, and Ca2+ mobilization. Present studies demonstrated that platelet phospholipase A2 (cytosolic and membrane) activity in the patient was normal. Receptor-mediated activation of glycoprotein (GP) IIb-IIIa complex measured by flow cytometry using antibody PAC-1 was diminished despite normal amounts of GPIIb-IIIa on platelets. Ca2+ release induced by guanosine 5′-[γ-thio]triphosphate (GTP[γS]) was diminished in the patient’s platelets, suggesting a defect distal to agonist receptors. GTPase activity (a function of α-subunit) in platelet membranes was normal in resting state but was diminished compared with normal subjects on stimulation with thrombin, platelet-activating factor, or the thromboxane A2 analog U46619. Binding of 35S-labeled GTP[γS] to platelet membranes was decreased under both basal and thrombin-stimulated states. Iloprost (a stable prostaglandin I2 analog) -induced rise in cAMP (mediated by Gαs) and its inhibition (mediated by Gαi) by thrombin in the patient’s platelet membranes were normal. Immunoblot analysis of Gα subunits in the patient’s platelet membranes showed a decrease in Gαq (<50%) but not Gαi, Gαz, Gα12, and Gα13. These studies provide evidence for a hitherto undescribed defect in human platelet G-protein α-subunit function leading to impaired platelet responses, and they provide further evidence for a major role of Gαq in thrombin-induced responses.
G proteins play a major role in signal transduction from the surface heptahelical receptors to effector systems upon platelet activation, and they regulate downstream responses such as aggregation and secretion (1–4). G proteins are a family of heterotrimeric proteins (made up of α, β, and γ subunits) which mediate the interactions between agonist receptors and intracellular enzymes, such as adenylyl cyclase, phospholipase C (PLC), and phospholipase A2 (PLA2). Signaling mechanisms involve a cycle in which αβγ–GDP complex dissociates after replacement of GDP by GTP to produce α–GTP, which then activates the effector molecule. Because of its intrinsic GTPase activity, the α subunit hydrolyzes GTP and reassociates with βγ subunit with termination of the activation process. Multiple forms of Gα have been identified in platelets (1) and are grouped in families; these include Gs, Gi (Gαi1, Gαi2, Gαi3, Gz), Gq (Gαq, Gα11, Gα14, Gα15, Gα16), and Gα12 families (Gα12, Gα13). Gs and Gi mediate the interaction with adenylyl cyclase. There is evidence for both remarkable specificity and potential redundancy in G proteins that mediate interaction between receptors and effectors (1–4). Thromboxane A2-induced activation of PLC-β in platelets is mediated by pertussis toxin-insensitive Gαq (5, 6). Thrombin activates PLC by both pertussis toxin-sensitive (possibly Gαi2) (7, 8) and -insensitive mechanisms (Gαq) (9, 10). Recently, G12 and G13 (members of the G12 family) have been shown to play a role during platelet activation with thrombin and thromboxane A2 (11). Moreover, there is evidence that PLC-β isozymes can be activated by βγ subunits independent of action of αq subunit (4, 12, 13). Abnormalities in G-protein-coupled signal transduction pathways have been described in several human disease states (14–16), and in dog platelets with impaired responses to thromboxane A2 (17).
Congenital abnormalities in platelet aggregation and secretion in response to activation with surface-receptor-mediated agonists may arise by diverse mechanisms, including abnormalities in the surface receptors, membrane glycoproteins, and deficiency of dense and α granule contents (18–22). However, these well recognized abnormalities are observed in a small proportion of patients with abnormal platelet dysfunction; in the majority of such patients, the underlying mechanisms leading to the dysfunction are unknown (18). Evidence is becoming available that at least in some of these patients the primary abnormalities may lie in the signaling mechanisms that follow receptor activation and precede the ultimate responses of aggregation or secretion (18, 19).
We have previously reported (23) a patient with diminished platelet aggregation and secretion in response to multiple agonists despite presence of normal dense granule stores. Further studies showed that receptor-mediated release of arachidonic acid from phospholipids (23) and calcium mobilization (24) were impaired upon platelet activation. We postulated that these abnormal responses may arise due to a defect in signal transduction mechanisms. To delineate the platelet defect in this patient, we investigated receptor-stimulated G-protein function and report an abnormality in Gα subunit function associated with a decrease in immunoreactive Gαq in platelets.¶ To our knowledge a human platelet G-protein defect has hitherto not been described.
MATERIALS AND METHODS
Patient Information and Previous Studies.
The patient is a 46-year-old white female with mild life-long mucocutaneous bleeding diathesis associated with prolonged bleeding times and normal platelet counts (23). The patient’s daughter and father may also have a history of easy bruising. Previous studies in the patient showed the following: (i) platelet aggregation and secretion ([14C]serotonin) in platelet-rich plasma (PRP) were consistently abnormal in response to ADP, epinephrine, collagen, the thromboxane A2 analog U46619, and platelet-activating factor (PAF); (ii) ATP and ADP contents of platelet dense granules were normal; (iii) thromboxane A2 production (measured by thromboxane B2 radioimmunoassay) in response to ADP and thrombin was diminished, but it was normal on exposure to arachidonic acid; (iv) in platelets labeled with [3H]arachidonic acid, the release of free arachidonic acid from phospholipids was impaired upon stimulation with thrombin (23); and (v) calcium mobilization (both internal release and influx of external calcium) was abnormal in response to thrombin and ADP (24). However, calcium release induced by exogenous inositol 1,4,5-trisphosphate (IP3) in saponin-permeabilized platelets was normal (24).
Reagents.
Iloprost (ZK 36,374), a stable prostacyclin analog, was provided by Berlex Laboratories (Cedar Knolls, NJ). Thrombin receptor agonist peptide (TRAP; SFLLRN) was purchased from Bachem Bioscience (King of Prussia, PA). Bovine thrombin was obtained from Armour Pharmaceutical (Kankakee, IL). PAF was from Avanti Polar Lipids (Alabaster, AL). The 1,2-dioctanoylglycerol was purchased from Biomol Research Laboratories (Plymouth Meeting, PA). Fura 2 pentaacetoxymethyl ester was obtained from Calbiochem (San Diego, CA). U46619, a stable thromboxane A2 analog, was obtained from Cayman Chemical (Ann Arbor, MI). Irish Cream was obtained from T. J. Carolar & Son, Clonmel, Ireland. Radioactive reagents, GTP[γ-32P], 35S-labeled guanosine 5′-[γ-thio]triphosphate (GTPγS], 125I-cAMP radioimmunoassay kit, and 125I-protein A were purchased from DuPont/NEN (Boston, MA).
Antibodies and Disintegrins.
Monoclonal antibody (mAb) PAC1 binds only to the activated form of the GPIIb-IIIa complex (26) and was provided by Sanford Shattil (University of Pennsylvania, Philadelphia). Antibodies 10E5 (27) and A2A9 (28) bind to both resting and activated GPIIb-IIIa and were provided by Barry Coller (Mount Sinai School of Medicine, New York) and Joel Bennett (University of Pennsylvania, Philadelphia), respectively. Monoclonal antibody to ligand-induced binding site (LIBS) Ab62 (29) was donated by Mark Ginsberg (Scripps Research Institute, La Jolla, CA). Albolabrin, a snake venom (Trimeresurus albolabris) peptide containing RGD sequence (disintegrin) (30), was provided by Stefan Niewiarowski (Temple University, Philadelphia). Specific antisera against different Gα subunits, anti-Gαq antibody (no. 941), anti-Gαi (no. 1521, specificity Gαi2 > Gαz; and no. 8729, specificity Gαi1 ≈ Gαi2 > Gαi3), anti-Gαz (no. 2921), anti-Gα12 (no. 121), and anti-Gα13 (no. 120) (31, 32) were generously provided by David Manning (University of Pennsylvania, Philadelphia).
Measurement of PLA2 Activity.
Blood collection and PRP preparation were done as described (33) elsewhere except that 2% EDTA was used as anticoagulant. A platelet pellet was obtained by centrifuging the PRP at 1,000 × g for 15 min at room temperature and was washed with Tyrode’s buffer (pH 7.4) containing 10 mM Hepes, 145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM glucose, and 1 mM EDTA, and centrifuged at 1,000 × g for 15 min. The resulting platelet pellet was resuspended in buffer consisting of 30 mM Tris⋅HCl (pH 7.4), 5 mM EDTA, 5 mM EGTA, 120 mM NaCl, 50 μM leupeptin, 100 μM phenylmethylsulfonyl fluoride (PMSF), and 10 mM benzamidine, and the platelet count was adjusted to 2 × 109 platelets per ml. The platelet suspension was sonicated with five 15-sec bursts on ice and centrifuged at 100,000 × g for 60 min at 4°C in a Beckman ultracentrifuge (model L5–50E). The supernatant containing soluble enzyme was dialyzed overnight against a buffer containing 30 mM Tris⋅HCl and 2 mM EDTA, pH 7.2; this constituted the cytosolic fraction. The pellet containing membrane-bound PLA2 was resuspended in a buffer containing 30 mM Tris⋅HCl (pH 7.2), 2 mM EDTA, 1 mM KCl, and 2 mM benzamidine, to a volume equal to that of supernatant, and sonicated as described above. The membrane suspension was centrifuged at 100,000 × g for 60 min at 4°C, and the supernatant was dialyzed overnight against a buffer consisting of 30 mM Tris⋅HCl and 2 mM EDTA, pH 7.2; this constituted the membrane fraction.
PLA2 activity was determined by measuring the amount of radiolabeled arachidonate hydrolyzed from the 2 position of the substrate 1-stearoyl-2-arachidonoyl phosphatidylcholine as described previously (34). The enzyme activity was calculated as pmol of substrate hydrolyzed per 108 platelets.
GTP[γS]-Induced Ca2+ Release.
These studies were performed as described previously (35). Platelets were resuspended at 5 × 108 platelets per ml in buffer (pH 7.4) consisting of 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 3.3 mM NaH2PO4, 20 mM Hepes, 5 mM glucose, 5 mM creatine phosphate, and 5 units/ml creatine kinase. Just prior to the experiment, 1-ml aliquots of platelets were diluted (1:1) in buffer containing 160 mM KCl, 5.3 mM MgCl2, and 13.3 mM Hepes (pH 7.1) and incubated in the spectrofluorimeter (Perkin–Elmer LS-5) cuvette for 3 min at 37°C with stirring (900 rotations per min) in the presence of 1 mM ATP, 1 μM CaCl2, and 6 μM Quin2 acid. Platelets were permeabilized by using saponin (10–15 μg/ml); 3 min after addition of the saponin, GTP[γS] was added. For the measurement of Ca2+ concentrations, the fluorescence was recorded (excitation wavelength 339 nm; emission 492 nm). At the end of the observation period, 10 μl of 1 M CaCl2 was added to the cuvette (2-ml suspension) to record the maximal fluorescence (Fmax) followed by 10 μl of 1 M MnCl2 to record autofluorescence. Ca2+ concentrations were calculated as described earlier (35).
Platelet Membrane Preparation.
Blood was collected in 1/10 vol of 3.8% sodium citrate from the patient and normal donors who had taken no medication for at least 10 days. PRP was prepared by centrifugation at 180 × g for 15 min at room temperature. The platelet pellet obtained by centrifugation of the PRP (20,000 rpm, 30 min, 4°C) in an ultracentrifuge (Beckman, model L3–50) was suspended in 2 ml of TEA buffer, pH 7.4, containing protease inhibitors [10 mM triethanolamine⋅HCl, 5 mM EDTA, 1.8 mM EGTA (pH 6.8), 0.5 mM phenylmethylsulfonyl fluoride, 5 mM benzamidine, 100 ng/ml leupeptin, 10 mM dithiothreitol] and lysed with a sonicator (Heat Systems/Ultrasonics, model W-225R) at 70 W on ice (three 10-sec bursts). The membrane suspension was washed by adding excess TEA buffer and centrifuged at 25,000 rpm for 30 min at 4°C. The membrane pellet was resuspended in minimum volume (1–2 ml) of TEA buffer by sonication and stored at −80°C as 100-μl aliquots. Platelet membrane protein concentrations were determined using Coomassie Plus protein assay reagent (Pierce).
GTPase Assay.
GTPase activity in platelet membranes was measured according to Cassel and Selinger (36) as modified by Johnson et al. (17). In brief, the total reaction mixture (100 μl) contained 0.56 μM [γ-32P]GTP (≈4 μCi; 1 μCi = 37 kBq), 5 mM MgCl2, 100 mM NaCl, 1 mM dithiothreitol, 0.5 mM EGTA, 1 mM ATP, 12.5 mM creatine phosphate, 5 units of creatine kinase, 50 mM Tris⋅HCl (pH 7.4), and agonist. The reaction was initiated by the addition of platelet membrane (5–20 μg) and incubated at 37°C for 7 min. The reaction was terminated with 0.5 ml of 5% (wt/vol) Norit-A charcoal in 20 mM sodium phosphate buffer, pH 7.4. The tubes were vortexed and centrifuged at 8,000 × g for 3 min, and a 200-μl aliquot of supernatant was removed for radioactivity counting. The basal activity was measured in the same way but in the absence of an agonist. The low-affinity or nonspecific GTPase activity was measured in the presence of 100 μM unlabeled GTP. High-affinity or specific GTPase activity was calculated by subtracting the low-affinity (nonspecific activity) activity from total activity. GTPase activity was expressed as pmol of 32P liberated per min per mg of protein.
GTP[γ35S] Binding Assay.
Binding of GTP[γ35S], a nonhydrolyzable analog of GTP, to platelet membranes was measured by the method of Wieland and Jacobs (37). The reaction mixture (100 μl), containing 1 nM GTP[γ35S] (≈50,000 cpm), 50 mM Tris⋅HCl (pH 7.4), 5 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 1 μM GDP, and agonist, was incubated with 5 μg of platelet membrane at 30°C for 30 min. After incubation the samples were filtered rapidly through Whatman GF/C filters in a Millipore vacuum filtration unit. The filters were washed extensively with buffer, pH 7.5, containing 50 mM Tris⋅HCl and 5 mM MgCl2. The radioactivity bound to the filters was measured by using a Beckman scintillation counter (model LS 8000). The basal binding was measured in the absence of agonist. Nonspecific binding was measured in the presence of 100 μM unlabeled GTP[γS]. The membrane bound GTP[γ35S] was calculated as the percent of total radioactivity added.
Immunoblot Analysis.
Platelet membranes prepared as described above were solubilized in Laemmli sample buffer (SDS reducing buffer), resolved on SDS/10% PAGE, and transferred on to Immobilon-P transfer membranes (Millipore) (38). In some studies lysates of whole platelet suspensions in TEA buffer and the cytosolic fraction obtained by centrifugation (40,000 rpm, 30 min, 4°C) were solubilized and subjected to immunoblotting. The blots were incubated with 7.5% Irish Cream in Tris-buffered saline (TBS, pH 7.4) for 2 hr at 4°C to block the nonspecific binding sites, and washed once with 0.1% Tween-20 in TBS (pH 7.4) and twice with TBS (pH 7.4). The blots were incubated overnight at 4°C with specific antisera to Gα subunit(s) in the presence of 1.5% Irish Cream in TBS (pH 7.4). After incubation the blots were washed as described above and probed with 125I-protein A by incubating at room temperature for 1 hr. The blots were washed, dried, and exposed to autoradiography film (Action Scientific, Forest Hill, MD) to visualize Gα subunits. The autoradiograms were scanned using an UXE ScanMaker (Microtek International, Hsinchu, Taiwan) and were subjected to digital image analysis (Kodak Digital Science, Image Analysis Package, Rochester, NY).
Adenylyl Cyclase Assay and cAMP Measurement.
Adenylyl cyclase activity was assayed as described by Simonds et al. (39) with modifications. The reaction mixture (100 μl) contained 0.1 mM ATP, 1 mM dithiothreitol, creatine kinase (0.2 mg/ml), creatine phosphate (1.8 mg/ml), 2 mM MgCl2, 50 mM NaCl, 10 μM GTP, 1 mM isobutylmethylxanthine, BSA (0.25 mg/ml), and 50 mM Tris⋅HCl (pH 7.5), and was incubated with 30 μg of platelet membrane in the presence and absence of agonist for 20 min at 30°C. Iloprost (10 nM) was used as agonist for activation of adenylyl cyclase. To study inhibition of adenylyl cyclase, cAMP levels were determined in the presence of thrombin (1 unit/ml). The reaction was terminated by adding equal volume of 10% cold trichloroacetic acid, and the samples were centrifuged at 12,000 × g for 15 min at 4°C. The supernatants were extracted with 5 vol of water-saturated ether and lyophilized. cAMP levels were measured by using a 125I-cAMP radioimmunoassay kit (DuPont/NEN). The results were expressed as fold increase over basal levels or as percent inhibition, taking iloprost-induced cAMP levels as 100%.
Flow Cytometry Analysis of Activation of GPIIb-IIIa.
This analysis was performed using PRP as described by us previously (33). Platelets (50 μl, 1 × 108 per ml) were incubated with fluorescein isothiocyanate (FITC)-conjugated mAbs PACl (50 μg/ml), 10E5 (50 μg/ml), A2A9 (50 μg/ml), or Ab62 (anti-LIBS; 50 μg/ml) in the presence of a specific agonist at room temperature for 15 min without stirring. Samples were then diluted to 0.5 ml with Tyrode’s buffer and analyzed on an Epics Elite flow cytometer (Coulter). Platelets (10,000 in each sample) were analyzed with a 100-mW laser beam (coherent Innover 300) for FITC fluorescence to quantitate the amount of platelet-bound antibody. Results were expressed as histograms of platelet fluorescence intensity in arbitrary units on a logarithmic scale on the abscissa and platelet number on the ordinate.
RESULTS
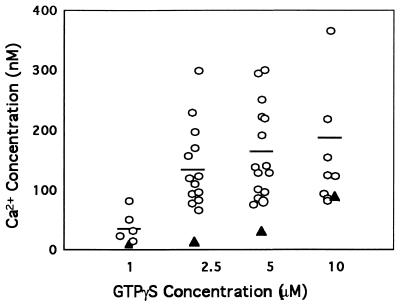
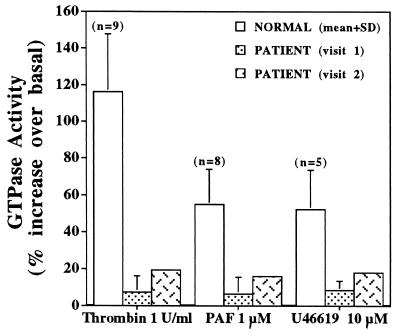
To delineate the mechanisms leading to the impaired platelet responses to receptor activation, we assessed G-protein function upon platelet activation. In initial studies, GTP[γS]-induced calcium release was measured in patient and normal platelets permeabilized with saponin. The rise in intracellular Ca2+ in patient platelets on exposure to increasing GTP[γS] concentrations (from 1 to 10 μM) was markedly decreased (Fig. 1), with the exception of the highest concentration tested. To assess G-protein α-subunit function, GTPase activity was measured in platelet membranes in the basal state and after receptor activation. The basal GTPase activity in the patient’s platelet membranes was 29.5 and 26.8 pmol/min per mg of protein (average of four and two assays, respectively, using platelet membranes from two separate visits), which is similar to that observed in nine normal subjects (25.2 ± 3.1 pmol/min per mg, mean ± SD). Upon stimulation with thrombin (1 unit/ml), PAF (1 μM), or U46619 (10 μM), the increase over basal in GTPase activity in the patient’s platelet membranes was markedly decreased on both occasions (Fig. 2). Even at a higher thrombin concentration (20 units/ml) there was no further increase in GTPase activity in patient platelets. Studies on GTP[γ35S] binding to platelet membranes revealed the basal binding in patient platelet membranes to be 6.3% (mean of three separate experiments), which was lower than in normal subjects, 13.5% ± 1.9% (mean ± SD, n = 4). With thrombin stimulation, the GTP[γS] binding in the patient was 7% (mean of three experiments), and this was lower than that observed in normal subjects (23% ± 3%, n = 4). These findings suggested that the patient’s platelets had an abnormality in G-protein α-subunit function.
Figure 1.
GTP[γS]-induced Ca2+ release in saponin-permeabilized platelets from normal subjects (○) and the patient (▴). Platelet suspensions were permeabilized with saponin (10–15 μg/ml) in the presence of 1 mM ATP, 1 μM CaCl2, and 6 μM Quin2 acid. For measurement of Ca2+ concentrations, fluorescence was recorded (excitation, 339 nm; emission, 492 nm) using a Perkin–Elmer spectrofluorimeter and calculated as described in the text.
Figure 2.
GTPase activity in platelet membranes from the patient and normal subjects. GTPase activity was measured in platelet membrane suspensions (5–20 μg) from normal subjects (mean ± SD) and the patient (mean ± SD of four and two experiments using membranes from two separate visits) in response to thrombin, PAF, or U46619. The basal activity was measured in the absence of an agonist. Shown is the increase in GTPase activity over basal levels upon activation.
Platelet membranes from the patient and normal subjects were analyzed by immunoblotting using specific antibodies against different Gα subunits. A marked decrease in immunoreactive Gαq subunit was observed in patient platelet membranes assessed in four experiments using membranes from two separate visits (Fig. 3A); all other Gα subunits studied, including Gαi2 (Fig. 3B), Gα12, Gα13, and Gαz (not shown) were comparable to those in normal subjects. By digital image analysis of the autoradiograms the intensity of the Gαq band for the patient showed a mean value of 45% compared with the values in nine normal subjects studied concurrently. Studies using whole platelet lysates also revealed a decrease in immunoreactive Gαq in the patient compared with normal subjects, and no Gαq was detected in the cytosolic fraction from either the patient or the normal subjects (data not shown).
Figure 3.
(A) Immunoblot (autoradiogram) analysis of Gαq subunit in platelet membranes from patient (P) and two normal subjects (N1 and N2). Platelet membrane proteins (22.5 and 45 μg) resolved by SDS/10% PAGE were transferred to Immobilon-P transfer membrane, and the blot was incubated with Gαq antisera. The blot was probed with 125I-protein A to visualize the Gαq protein band on autoradiography. (B) Immunoblot (autoradiogram) analysis of Gαi2 subunit in platelet membranes from patient (P) and five normal subjects (N1–N5). Platelet membrane proteins (22.5 μg) resolved on SDS/10% PAGE were transferred to Immobilon-P transfer membrane, and the blot was incubated with Gαi2 antiserum (no. 1521). The blot was washed and probed with 125I-protein A to visualize the Gαi2 protein band on autoradiography.
Stimulation of platelets with prostacyclin (iloprost) activates adenylyl cyclase and results in a rise in cAMP levels that is mediated by Gαs (1, 2); in the presence of thrombin or epinephrine, adenylyl cyclase is inhibited by a Gαi-mediated mechanism (1, 2). To demonstrate that the functional responses mediated by Gαi and Gαs were intact in the patient’s platelets, we studied the regulation of adenylyl cyclase. In the patient the basal cAMP, the iloprost-stimulated (10 nM) cAMP, and thrombin-induced decrease in cAMP levels were within the range observed in platelet membranes from six normal subjects (Table 1). Because one of the major abnormalities described initially (23) in this patient was an abnormality in thrombin-induced release of free arachidonic acid from phospholipids, we measured PLA2 activity in platelet membrane and cytosolic fractions. Platelet PLA2 activity in both membrane and cytosolic fractions from the patient’s platelets was within the normal range obtained from studies in nine normal subjects (Table 1).
Table 1.
Platelet cAMP and PLA2 levels
| Subject | cAMP, nmol per mg of protein
|
Thrombin-induced cAMP decrease, % | Platelet PLA2, pmol hydrolyzed per 108 platelets
|
||
|---|---|---|---|---|---|
| Basal | Iloprost (10 nM) | Cytosol | Membrane | ||
| Normal | 3.3 ± 1.1 (6) | 28.8 ± 11.6 (6) | 41.2 ± 16.2 (6) | 666 ± 303 (9) | 121 ± 48 (9) |
| Patient | 2.5 ± 1.0 (3) | 22.4 ± 10.9 (3) | 66 (2) | 1,064 | 128 |
Results are shown mean ± SD. Numbers in parenthesis indicate the number of normal subjects studied or the number of studies in the patient.
Platelet activation results in a conformational change in platelet surface GPIIb-IIIa complex and binding of fibrinogen, a prerequisite for platelet aggregation. Because the patient’s platelets showed an abnormality even in primary aggregation in response to multiple agonists, we studied the expression and activation of the GPIIb-IIIa complex on four separate occasions,and the results were similar. The binding of mAbs 10E5 or A2A9 (which bind to both activated and unactivated GPIIb-IIIa complex) to patient platelets was comparable to that in normal subjects (Fig. 4), indicating that the patient’s platelets have normal amounts of GPIIb-IIIa complex on their surface. Surface-receptor-mediated activation of GPIIb-IIIa was measured using mAb PAC1, which binds only to the activated form of GPIIb-IIIa complex (26), and this was diminished in patient platelets in response to ADP, TRAP, or PAF (Fig. 4), indicating that receptor-mediated signal transduction-dependent activation of GPIIb-IIIa was abnormal. The most striking abnormality was in response to PAF (Fig. 4). The expression of LIBS on GPIIb-IIIa complex upon activation of platelets with agonists was monitored using anti-LIBS mAb Ab62 (29). The binding of Ab62 to platelets in response to ADP (Fig. 4) and TRAP (not shown) was normal, but it was decreased with PAF, the agonist which induced least expression of PAC1 binding (Fig. 4). RGD-peptide-containing albolabrin (a snake venom protein) or RGDS peptide (33) can induce Ab62 binding in a signal-transduction independent manner, such as in the presence of prostacyclin (which inhibits signal transduction processes in platelets) (33). The binding of Ab62 induced by albolabrin (5 μg/ml) or RGDS (1 mM) in the presence of iloprost (28 nM) was normal in the patient’s platelets. Taken together, these results suggest that surface-receptor-mediated signal transduction-dependent activation of GPIIb-IIIa was abnormal in the patient’s platelets despite the presence of normal amounts of GPIIb-IIIa complex present on their surface. The normal expression of LIBS suggests that patient platelet GPIIb-IIIa complexes have intact ligand-binding capacity (40). A similar abnormality in activation of GPIIb-IIIa complex but normal ligand-binding capacity has been recently reported (33) by us in another patient with impaired platelet signal transduction mechanisms.
Figure 4.
Flow cytometry analysis of GPIIb-IIIa complex on platelets from normal donors (N) and patient (P) in the resting (R) state and after activation. (Top Left) Binding of FITC-labeled mAb 10E5 (which recognizes both activated and resting forms of GPIIb-IIIa). (Top Right and Middle) Binding of PAC1 (which binds only to activated form of GPIIb-IIIa) after activation with TRAP, ADP, or PAF. (Bottom) Binding of Ab62 (anti-LIBS) after activation with ADP and PAF. Samples were analyzed on an Epics flow cytometer. Antibody binding to platelets was measured as fluorescence intensity, shown on the abscissa, and platelet count is on the ordinate. The data are representative of one to four experiments performed on separate occasions.
DISCUSSION
Our studies provide evidence for a hitherto undescribed defect in G-protein α-subunit function in a patient with impaired platelet aggregation, secretion, release of arachidonic acid, and Ca2+ mobilization, in response to a number of agonists. This is based on the following lines of evidence: (i) GTP[γS]-induced Ca2+ release was diminished (Fig. 1) in the patient’s platelets, suggesting a defect either in the G protein per se or in the involved downstream effector systems; this observation makes it unlikely that the primary abnormality is in the surface agonist receptors. (ii) The basal GTPase activity in the platelet membranes from the patient was comparable to that in normal platelets; however, the increase in GTPase activity upon activation with thrombin, U46619, or PAF was blunted (Fig. 2). (iii) The binding of GTP[γ35S] to patient platelet membranes in response to thrombin stimulation was markedly diminished. These findings suggest an abnormality distal to the surface receptor, specifically in the G-protein α-subunit function in the patient’s platelets. Immunoblotting studies demonstrated that Gαq subunit (Fig. 3A) is decreased in the platelet membranes, whereas the amounts of Gαi2 (Fig. 3B), Gα12, Gα13, and Gαz (not shown) are normal. These findings suggest a reduction in the quantity of Gαq in the membranes or the presence of substantial amounts of an abnormal Gαq that is less immunoreactive to the antisera and is dysfunctional.
An abnormality or deficiency in Gαq can provide a cogent explanation for the observed defective responses for the following reasons. Three of the agonists used in our studies, U46619 (5), PAF (41), and thrombin, are recognized to activate platelets by mechanisms mediated by means of Gαq. Earlier studies (7, 8) have suggested that thrombin-induced phosphatidylinositol hydrolysis is mediated by a pertussis toxin-sensitive G protein (presumably Gαi2). Recent studies (9, 10, 42) indicate that thrombin-induced responses are also mediated by Gαq. Our findings provide further corroborative evidence that Gαq, indeed, plays a major role in platelet responses to thrombin. The stimulation of adenylyl cyclase by the prostacyclin analog iloprost (mediated by Gαs) and its inhibition by thrombin (mediated by Gαi) were both normal, indicating an intact function of Gαs and Gαi subunits. Moreover, we have previously described (23) that platelet aggregation and secretion in response to ADP and epinephrine are also abnormal in this patient. The present studies suggest that Gαq plays a role in mediating the responses to these agonists also, either directly or indirectly, possibly via thromboxane A2. Last, on the basis of the observation in Albright hereditary osteodystrophy that a 50% decrement in Gαs leads to the endocrine phenotype of pseudohypoparathyroidism (43), it is likely that the magnitude of decrease noted in Gαq in our patient may be sufficient to induce the functional aberration.
We had originally described (23) this patient in 1984 because the studies showed an impaired thrombin-stimulated release of free arachidonic acid from phospholipids. The major mechanism of arachidonate mobilization following thrombin activation of platelets is mediated by PLA2, a Ca2+-dependent enzyme (44, 45). Activation of PLA2 may occur by several mechanisms, including (i) activation by a rise in cytosolic Ca2+ secondary to PLC activation; (ii) direct activation of PLA2 by a G-protein-mediated mechanism; (iii) activation of PLA2 by phosphorylation involving protein kinase C and mitogen-activated protein (MAP) kinase (46). However, little is known regarding the relative importance of these mechanisms in platelets. In our patient, PLA2 activities in platelet extracts (cytosolic and membrane) were comparable to those in normal subjects. One explanation for the observed defect in the agonist-stimulated arachidonate release is that PLA2 activation is blunted because of impaired Ca2+ mobilization, an abnormality previously documented in this patient (24). Whether a G-protein-mediated direct activation of PLA2 is also abnormal remains unknown. Receptor-mediated activation of PLC and PLA2 can be dissociated (47, 48), and Gαi2 has been implicated in the direct activation of PLA2 by a G-protein-mediated mechanism (49). However, in our patient Gαi2 levels were normal (Fig. 3B).
A number of naturally occurring abnormalities in G-protein-coupled signal transduction mechanisms have been linked to human diseases (14–16). Loss-of-function mutations encompass those that disrupt synthesis or targeting of the components (receptor, G protein, effectors) of the signal transduction pathways as well as those that impair receptor–G-protein coupling, activation of G protein by GTP, or G-protein–effector coupling (14–16). To our knowledge, a naturally occurring human platelet dysfunction associated with a specific G-protein abnormality has hitherto not been described. Johnson et al. (17) have described receptor-linked G-protein dysfunction in dog platelets in relation to impaired responsiveness to thromboxane A2 but not other agonists. In contrast, the observed abnormality in response to several agonists in our patient suggests an aberration in signal transduction pathways common to different agonists. Moreover, in the canine model (17), although a defect in the Gq family was postulated, immunoblotting studies showed normal levels of Gq in the platelet membranes. In our patient the diminished agonist-stimulated GTPase activity is associated with decreased binding of GTP and with decreased amount of immunoreactive Gαq in platelet membranes. Structural studies with G α subunit have defined five distinct noncontiguous GTP-binding regions, G-1, G-2, G-3, G-4, and G-5, which collectively form the GTP-binding pocket (50). Several distinct conserved amino acids in this region have been identified as critical residues involved in GDP/GTP binding and GTPase activity (50). The observed reduced levels of immunoreactive Gαq in our patient’s platelets suggest the possibility of a mutation which is defining the stability of the α subunit. In fact, such a mutation has been described in pseudohypoparathyroidism (PHP-1a) involving αs (51). The αs-A366S mutation of the G-5 region confers temperature sensitivity at 37°C and alters the GTP-binding characteristics of αs (51); at 37°C the mutant αs is rapidly degraded. In the patient, a similar mutation in the αq G-5 region can explain the reduced levels of αq as well as the decreased GTP binding. Site-directed mutations in G-5 regions of other GTPases also alter GDP-binding affinity (52). It is worth noting that although platelets have different G proteins as well as low molecular weight GTPases, the basal GTP binding in the patient was significantly reduced. The mechanism for this is presently unclear.
Activation-induced conformational change in the platelet GPIIb-IIIa complex and resulting fibrinogen binding are essential for platelet aggregation. Our studies indicate that although GPIIb-IIIa complexes are present in normal amounts on the platelets, their activation is impaired, as evidenced by diminished PAC-1 binding (Fig. 4) following exposure to ADP, PAF, or TRAP. The binding of antibody Ab62 was normal following exposure to ADP and TRAP, indicating that expression of LIBSs and the ability of the GPIIb-IIIa complexes to bind the fibrinogen are preserved. We conclude from these studies that the signal transduction abnormality leads not only to diminished secretion but also to impaired expression of fibrinogen-binding sites on activation, with the impairment being more severe with PAF than ADP (Fig. 4). These findings provide further evidence that G-protein-mediated mechanisms modulate expression of GPIIb-IIIa-binding sites on platelets. The present findings are similar to the abnormality in activation of GPIIb-IIIa reported by us in a patient with abnormal pleckstrin phosphorylation (33).
In most patients with congenital abnormalities in platelet aggregation and secretion responses the underlying mechanisms leading to the abnormal end responses remain undefined. The generally considered entities such as thrombasthenia, Bernard Soulier syndrome, and storage pool deficiency occur in only a small fraction of these patients. Evidence is beginning to emerge that some of these patients have defects in early signal transduction events, including at the level of the receptors (20–22, 47), Ca2+ mobilization (24, 53), PLC activation, and pleckstrin phosphorylation (33, 54–57). We now document a hitherto undescribed abnormality in platelet G-protein function. Further studies in such patients should lead to a better definition of the role of specific G proteins, particularly Gq, in mediating responses to the different agonists as well provide insights into the mechanisms leading to the platelet dysfunction in the substantial group of patients that remain poorly characterized at present.
Acknowledgments
We thank Dr. David Manning for generously providing antisera against different Gα subunits, Drs. Joel Bennett, Barry Coller, Sanford Shattil, and Mark Ginsberg for providing mAbs against GPIIb-IIIa and the anti-LIBS antibody, and Ms. JoAnn Hamilton and Ms. Mary Merrick for excellent secretarial assistance. This work was supported by a Grant-in-Aid from the Southeastern Pennsylvania Affiliate of the American Heart Association and by Grant GM 49897 (to N.D.) from the National Institutes of Health. A.K.R. is the recipient of an Academic Award in Vascular Disease (National Heart, Lung, and Blood Institute K077HL02658). X.Y. was supported by Award T32 HL07777 (National Heart, Lung, and Blood Institute). The use of the General Clinical Research Center facilities was supported by Grant RR349 from the National Institutes of Health.
ABBREVIATIONS
- FITC
fluorescein isothiocyanate
- GP
glycoprotein
- GTP[γS]
guanosine 5′-[γ-thio]triphosphate
- LIBS
ligand-induced binding site
- mAb
monoclonal antibody
- PAF
platelet-activating factor
- PLA2
phospholipase A2
- PLC
phospholipase C
- PRP
platelet-rich plasma
- TEA
triethanolamine
- TRAP
thrombin receptor agonist peptide
Footnotes
This work was presented in part at the 69th Scientific Sessions of the American Heart Association in New Orleans, November 1996, and has been published in abstract form (25).
References
- 1.Brass L F, Hoxie J A, Manning D R. Thromb Haemostasis. 1993;70:217–223. [PubMed] [Google Scholar]
- 2.Hepler J R, Gilman A G. Trends Biochem Sci. 1992;17:383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaumer L. Cell. 1992;71:1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- 4.Clapham D E, Neer E J. Nature (London) 1993;365:403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- 5.Shenker A, Goldsmith P, Unson C G, Spiegel A M. J Biol Chem. 1991;266:9309–9313. [PubMed] [Google Scholar]
- 6.Baldassare J J, Tarver A P, Henderson P A, Mackin W M, Sahagan B, Fisher G J. Biochem J. 1993;29l:235–240. doi: 10.1042/bj2910235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brass L F, Manning D R, Williams A, Woolkalis M J, Poncz M. J Biol Chem. 1991;266:958–965. [PubMed] [Google Scholar]
- 8.Brass L F, Laposata M, Banga H S, Rittenhouse S E. J Biol Chem. 1986;261:16838–16847. [PubMed] [Google Scholar]
- 9.Hung D T, Wong Y H, Vu T-K H, Coughlin S R. J Biol Chem. 1992;267:20831–20834. [PubMed] [Google Scholar]
- 10.Benka M L, Lee M, Wang G R, Buckman S, Burlacu A, et al. FEBS Lett. 1995;363:49–52. doi: 10.1016/0014-5793(95)00278-h. [DOI] [PubMed] [Google Scholar]
- 11.Offermanns S, Laugwitz K L, Spicher K, Schultz G. Proc Natl Acad Sci USA. 1994;91:504–508. doi: 10.1073/pnas.91.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S B, Shin S H, Hepler J R, Gilman A G, Rhee S G. J Biol Chem. 1993;268:25952–25957. [PubMed] [Google Scholar]
- 13.Katz A, Wu D, Simon M I. Nature (London) 1992;360:686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- 14.Clapham D E. Cell. 1993;75:1237–1239. doi: 10.1016/0092-8674(93)90609-t. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel A M, Weinstein L S, Shenker A. J Clin Invest. 1993;92:1119–1124. doi: 10.1172/JCI116680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coughlin S R. Curr Opin Cell Biol. 1994;6:191–197. doi: 10.1016/0955-0674(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson G J, Leis L A, Dunlop P C. J Clin Invest. 1993;92:2469–2479. doi: 10.1172/JCI116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao A K. Hematol Oncol Clin N Amer. 1990;4:65–87. [PubMed] [Google Scholar]
- 19.Weiss H J. In: Hemostasis and Thrombosis, Basic Principles and Clinical Practice. Colman R W, Hirsh J, Marder V J, Salzman E W, editors. Philadelphia: Lippincott; 1994. pp. 673–741. [Google Scholar]
- 20.Cattaneo M, Lecchi A, Randi A M, McGregor J L, Mannucci P M. Blood. 1995;80:2787–2796. [PubMed] [Google Scholar]
- 21.Nurden P, Savi P, Heilmann E, Bihour C, Herbert J M, Maffrand J P, Nurden A. J Clin Invest. 1995;95:1612–1622. doi: 10.1172/JCI117835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirata T, Kakizuka A, Ushikubi F, Fuse I, Okuma M, Narumiya S. J Clin Invest. 1994;94:1662–1667. doi: 10.1172/JCI117510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao A K, Koike K, Willis J, Daniel J L, Beckett C, Hassell B, Day H J, Smith J B, Holmsen H. Blood. 1984;64:914–921. [PubMed] [Google Scholar]
- 24.Rao A K, Disa J, Yang X. J Lab Clin Med. 1993;121:52–63. [PubMed] [Google Scholar]
- 25.Gabbeta J, Yang X, Sun L, Dhanasekaran N, Rao A K. Circulation. 1996;94:I–580. (abstr.). [Google Scholar]
- 26.Shattil S J, Hoxie J A, Cunningham M, Brass L F. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- 27.Coller B S, Peerschke E I, Scudder L E, Sullivan C A. J Clin Invest. 1983;72:325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett J S, Hoxie J A, Leitman S F, Vilaire G, Cines D B. Proc Natl Acad Sci USA. 1983;80:2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Toole T E, Loftus J C, Du X, Glass A, Ruggeri Z M, Shattil S J, Plow E F, Ginsberg M H. Cell Regul. 1990;1:883–893. doi: 10.1091/mbc.1.12.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lasz E C, McLane M A, Trybulec M, Kowalska M A, Khan S, Budzynski A Z, Niewiarowski S. Biochem Biophys Res Commun. 1993;190:118–124. doi: 10.1006/bbrc.1993.1019. [DOI] [PubMed] [Google Scholar]
- 31.Carlson K E, Brass L F, Manning D R. J Biol Chem. 1989;264:13298–13305. [PubMed] [Google Scholar]
- 32.Lounsbury K M, Schlegel B, Poncz M, Brass L F, Manning D R. J Biol Chem. 1993;268:3494–3498. [PubMed] [Google Scholar]
- 33.Gabbeta J, Yang X, Sun L, McLane M A, Niewiarowski S, Rao A K. Blood. 1996;87:1368–1376. [PubMed] [Google Scholar]
- 34.Kramer R M, Checani G C, Deykin A, Pritzker C R, Deykin D. Biochim Biophys Acta. 1986;378:394–403. doi: 10.1016/0005-2760(86)90248-1. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Disa J, Rao A K. Thromb Res. 1992;65:549–558. doi: 10.1016/0049-3848(92)90205-o. [DOI] [PubMed] [Google Scholar]
- 36.Cassel D, Selinger Z. Biochim Biophys Acta. 1976;452:538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- 37.Wieland T, Jakobs K H. Methods Enzymol. 1994;237:3–13. doi: 10.1016/s0076-6879(94)37048-6. [DOI] [PubMed] [Google Scholar]
- 38.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonds W F, Goldsmith P K, Codina J, Unson C G, Spiegel A M. Proc Natl Acad Sci USA. 1989;86:7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frelinger A L, Du X, Plow E F, Ginsberg M H. J Biol Chem. 1991;266:17106–17111. [PubMed] [Google Scholar]
- 41.Honda Z, Takano T, Hirose N, Suzuki T, Muto A, Kume S, Mikoshiba K, Itoh K, Shimizu T. J Biol Chem. 1995;270:4840–4844. doi: 10.1074/jbc.270.9.4840. [DOI] [PubMed] [Google Scholar]
- 42.Baffy G, Yang L, Raj S, Manning D R, Williamson J R. J Biol Chem. 1994;269:8483–8487. [PubMed] [Google Scholar]
- 43.Patten J L, Levine M A. J Clin Endocrinol Metab. 1990;71:1208–1214. doi: 10.1210/jcem-71-5-1208. [DOI] [PubMed] [Google Scholar]
- 44.Rittenhouse S E, Deykin D. In: Platelets in Biology and Pathology-2. Gordon J L, editor. Amsterdam: Elsevier/North Holland; 1982. pp. 349–362. [Google Scholar]
- 45.Purdon D, Smith J B. J Biol Chem. 1985;260:12700–12706. [PubMed] [Google Scholar]
- 46.Dennis E A. J Biol Chem. 1994;269:13057–13061. [PubMed] [Google Scholar]
- 47.Fuse I, Mito M, Hattori A, Higuchi W, Shibata A, Ushikubi F, Okuma M, Yahata K. Blood. 1993;81:994–1000. [PubMed] [Google Scholar]
- 48.Nakashima S, Hattori H, Shirato L, Takenaka A, Nozawa Y. Biochim Biophys Acta. 1987;148:971–978. doi: 10.1016/s0006-291x(87)80227-9. [DOI] [PubMed] [Google Scholar]
- 49.Gupta S K, Diez E, Heasley L E, Osawa S, Johnson G L. Science. 1990;249:662–666. doi: 10.1126/science.2166341. [DOI] [PubMed] [Google Scholar]
- 50.Bourne H R, Sanders D A, McCormick F. Nature (London) 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 51.Iiri T, Herzmark P, Nakamoto J M, Dop C V, Bourne H R. Nature (London) 1994;371:164–168. doi: 10.1038/371164a0. [DOI] [PubMed] [Google Scholar]
- 52.Thomas T C, Schmidt C J, Neer E J. Proc Natl Acad Sci USA. 1993;90:10295–10299. doi: 10.1073/pnas.90.21.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rao A K, Kowalska M A, Disa J. Blood. 1989;74:664–672. [PubMed] [Google Scholar]
- 54.Yang X, Sun L, Ghosh S, Rao A K. Blood. 1996;88:1676–1683. [PubMed] [Google Scholar]
- 55.Lee S B, Rao A K, Lee K H, Yang X, Bae Y S, Rhee S G. Blood. 1996;88:1684–1691. [PubMed] [Google Scholar]
- 56.Cartwright I J, Hampton K K, Macneil S, Colvin B T, Preston F E. Br J Haematol. 1994;88:129–136. doi: 10.1111/j.1365-2141.1994.tb04988.x. [DOI] [PubMed] [Google Scholar]
- 57.Speiser-Ellerton S, Weiss H J. J Lab Clin Med. 1990;115:104–111. [PubMed] [Google Scholar]