Abstract
The efficiency of first-generation adenoviral vectors as gene delivery tools is often limited by the short duration of transgene expression, which can be related to immune responses and to toxic effects of viral proteins. In addition, readministration is usually ineffective unless the animals are immunocompromised or a different adenovirus serotype is used. Recently, adenoviral vectors devoid of all viral coding sequences (helper-dependent or gutless vectors) have been developed to avoid expression of viral proteins. In mice, liver-directed gene transfer with AdSTK109, a helper-dependent adenoviral (Ad) vector containing the human α1-antitrypsin (hAAT) gene, resulted in sustained expression for longer than 10 months with negligible toxicity to the liver. In the present report, we have examined the duration of expression of AdSTK109 in the liver of baboons and compared it to first-generation vectors expressing hAAT. Transgene expression was limited to approximately 3–5 months with the first-generation vectors. In contrast, administration of AdSTK109 resulted in transgene expression for longer than a year in two of three baboons. We have also investigated the feasibility of circumventing the humoral response to the virus by sequential administration of vectors of different serotypes. We found that the ineffectiveness of readministration due to the humoral response to an Ad5 first-generation vector was overcome by use of an Ad2-based vector expressing hAAT. These data suggest that long-term expression of transgenes should be possible by combining the reduced immunogenicity and toxicity of helper-dependent vectors with sequential delivery of vectors of different serotypes.
Numerous studies with adenoviral vectors in a variety of animal models have demonstrated successful gene transfer to many tissues, with high levels of expression of recombinant genes, making these vectors attractive candidates for treating a variety of human diseases (1–5). However, use of first-generation vectors usually results in only transient transgene expression. This is partially due to the development of a cellular immune response triggered by viral proteins expressed from adenovirus genes (1, 4, 6, 7). Other factors that can limit persistence of transgene expression are immune responses to the transgene product (8–10), the dose of virus administered (10, 11), the promoter chosen to drive expression of the recombinant gene (12–14), innate immune mechanisms (15–17), and direct cytotoxicity caused by expression of viral genes (18–20).
One strategy to reduce the immunogenicity of the vector has been to delete all viral coding sequences so that leaky expression of viral proteins is completely eliminated (21–24). Recently, helper-dependent systems have been developed that use a first-generation helper virus to provide the necesary proteins in trans for the packaging of a vector devoid of viral genes (25, 26). One of these helper-dependent vectors has been used in mice to deliver the human α1-antitrypsin gene to the liver, and expression was sustained for longer than 10 months (19). In addition, administration of high doses resulted in negligible toxicity to the liver (18, 27).
Therefore, by using helper-dependent vectors, it may be possible to develop a gene therapy strategy that would require readministration only after long periods of time. Unfortunately, the development of neutralizing antibodies against the adenovirus capsid proteins after the first injection precludes transgene expression with readministration unless the animal is immunocompromised (28–31). One approach to circumvent this problem involves the use of vectors of different serotypes (32, 33).
In the present report, we have examined the duration of expression after intravenous injection aimed at hepatic gene transfer in baboons, using first generation and helper-dependent adenoviral vectors expressing human α1-antitrypsin (hAAT). We have also addressed the feasibility of administering a vector of a different serotype. Our results indicate that alternative delivery of helper-dependent adenoviral vectors from different serotypes is a promising strategy for very long-term gene therapy treatment of human diseases.
Materials and Methods
Vectors.
The construction of adenovirus (Ad) vectors Ad5hAATΔE1 and AdSTK109 has been described (19, 34). Ad5hAATΔE1 is a serotype 5, E1-deleted vector with an expression cassette consisting of the hAAT cDNA under control of the murine phosphoglycerate kinase promoter (34). Ad2hAATΔE1 is a serotype 2 (35), first-generation vector containing an expression cassette identical to that of Ad5hAATΔE1. AdSTK109 is a helper-dependent adenoviral vector containing the complete hAAT gene locus, including the endogenous promoter, all exons and introns, and the natural polyadenylation signal (19). Ad5hAATΔE1 and Ad2hAATΔE1 were produced in 293 cells, and AdSTK109 in 293Cre cells (25, 36). All vector preparations were evaluated by particle count as determined by optical density measurement of DNA. The level of helper contamination in AdSTK109 preparations was determined by plaque assay and found to be <0.1%. The presence of replication-competent adenovirus in AdSTK109 preparations was determined as described (19) and was <1 particle in 2 × 108 particles.
Experimental Animals and Specimen Collection.
The baboons used in this study were Papio sp., males, 4 months to 2 years old at the time of the first virus administration. A total of nine animals was used in the study, each with a unique recognition number (Table 1). Before vector administration, the animals were sedated with 10 mg/kg ketamine. A 22 gauge catheter was placed in the right saphenous vein, and the appropriate amount of vector (doses specified in Table 1) was infused, followed by flushing with 5 ml of sterile saline. Five milliliters of blood was obtained from the cephalic vein on days 0, 3, 10, and then weekly after vector administration, to perform blood cell counts and blood chemistries (glucose, blood urea nitrogen, creatinine, total protein, albumin, globulin, A/G ratio, cholesterol, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, Na+, K+, Cl−, CO2, anion gap, total bilirubin), to measure hAAT levels, and to test serum for the presence of neutralizing antibodies to the virus capsid. Baboons receiving AdSTK109 were bled only once a month from the third month after vector administration. The animals were monitored for general health, including appetite, stool consistency, tachycardia, loss of weight, presence of fever, conjunctivitis, or any abnormal sign that could be related to administration of the vectors. The animals were individually caged in animal biosafety level 2 (ABSL2) containment until no vector was recovered from feces for 4 consecutive weeks (approximately 2 months after administration), and were then transferred to an ABSL1 containment.
Table 1.
Experimental design
| Subject | Ad5hAATΔE1
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First injection
|
Second injection
|
Ad2hAATΔE1
|
AdSTK109
|
|||||||||
| Dose, particles per kg | Age, months | Weight, kg | Dose, particles per kg | Age, months | Weight, kg | Dose, particles per kg | Age, months | Weight, kg | Dose, particles per kg | Age, months | Weight, kg | |
| 12402 | 6.2 × 1011 | 6 | 2.06 | 2.8 × 1011 | 12 | 4.13 | 6.2 × 1011 | 24 | 6.10 | — | — | — |
| 12486 | 6.2 × 1011 | 4 | 1.81 | 2.8 × 1011 | 10 | 3.78 | 7.2 × 1011 | 22 | 6.00 | — | — | — |
| 12490 | 1.4 × 1012 | 8 | 2.76 | — | — | — | 6 × 1011 | 27.5 | 6.53 | — | — | — |
| 12497 | 1.4 × 1012 | 8 | 2.65 | — | — | — | 6 × 1011 | 27.5 | 6.80 | — | — | — |
| 13110 | — | — | — | — | — | — | 6.1 × 1011 | 20.5 | 5.77 | — | — | — |
| 13121 | — | — | — | — | — | — | 6.1 × 1011 | 20.5 | 5.65 | — | — | — |
| 13250 | — | — | — | — | — | — | — | — | — | 3.3 × 1011 | 10 | 3.87 |
| 13277 | — | — | — | — | — | — | — | — | — | 3.9 × 1011 | 9 | 3.30 |
| 13729 | — | — | — | — | — | — | — | — | — | 3.6 × 1011 | 7.5 | 2.84 |
For each injection, the dose in particles per kg is given followed by the age in months and the weight of the animal in kg.
Feces were collected at the times of blood withdrawal to be analyzed for the presence of Ad5hAATΔE1 and Ad2hAATΔE1 vectors as described (37).
ELISA for hAAT.
An ELISA was used to measure the levels of hAAT in serum as described for mouse studies (10, 38). Because the antibody against hAAT used was found to cross-react weakly with baboon AAT, a modification was introduced to the assay. The peroxidase-conjugated antibody was treated with naive baboon serum to eliminate the fraction of antibody that cross-reacts with baboon AAT. A 1:300 dilution of naive baboon serum was used for most assays; the dilution was changed to 1:2,400 when a different batch of anti-hAAT antibody was purchased. Naive serum and the secondary antibody were mixed and left at room temperature for 1.5 hours before use.
Anti-hAAT Antibody Detection.
An ELISA assay was used to detect antibodies to hAAT in serum as described (10).
Neutralizing Antibody Titers.
Dilutions of serum samples were tested for the presence of neutralizing antibodies that prevented transduction of wild-type Ad5 and Ad2 on HEp2 cells (37). Serial 1:2 dilutions (starting from a 1:8 dilution) were mixed with wild-type adenovirus and incubated at 37°C for 1 hour. HEp2 cells (2–4 × 104) were then added to the wells and incubated at 37°C. The titer was determined as the highest dilution at which ≥90% reduction in cytopathic effect occurred compared with a negative control (no serum added). Serum samples with undetectable neutralizing antibodies at the 1:8 dilution were assigned a titer of 1:4.
Cytotoxic T Lymphocyte Assay.
Spleen cells were stimulated with 5 × 107 units of Ad2hAATΔE1 for 7 days to promote antigen-specific expansion of cytotoxic T lymphocyte (CTLs). Recombinant human IL-2 was added at 20 units/ml on day 4 of culture. Target fibroblasts derived from skin biopsies of baboons 12490 and 12497 were infected at a multiplicity of infection of 100 for 48 hours and were treated with 500 units/ml recombinant human IFN-γ for the last 24 hours to enhance MHC class I expression and antigen presentation to effector CTLs. Fibroblasts were labeled with 51Cr by overnight incubation at 37°C. Splenocytes and target cells were incubated at ratios of 80:1, 40:1, 20:1, and 10:1 for 5 hours. Infected allogeneic fibroblasts were also used as targets to confirm MHC class I-restricted cytolytic activity. The amount of 51Cr spontaneously released was obtained by incubating target fibroblasts in medium alone; the total amount of 51Cr incorporated was determined by adding 1% Triton X-100. The percentage of lysis was calculated as follows: lysis % = [(sample cpm) − (spontaneous cpm)]/[(total cpm) − (spontaneous cpm)] × 100.
Proliferation Assay.
Spleen cells from each animal were plated in triplicate in a 96-well plate (5 × 105 cells per well). The cells were incubated with medium alone (background) or stimulated with 5 μg/ml heat-inactivated Ad2 vector in a total volume of 200 μl. Cultures were incubated for 5 days at 37°C in 5% CO2 and pulsed with 1 μCi per well (1 Ci = 37 GBq) [3H]thymidine for the last 18 hours of incubation. Cells were harvested onto glass fiber filters with a 96-well plate cell harvester, and cell-associated radioactivity was measured by liquid scintillation counting.
Results
Short-Term Expression After Administration of a First-Generation Vector.
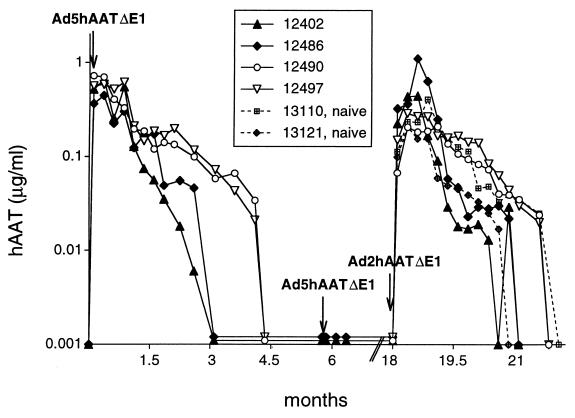
Preliminary data in baboons indicated that intravenous administration of a first-generation vector expressing Escherichia coli β-galactosidase results in transduction primarily in the liver (data not shown). To determine persistence of transgene expression, two baboons (12402 and 12486) were injected intravenously with a dose of 6.2 × 1011 particles per kg of body weight (≈2 × 1010 plaque-forming units/kg) of Ad5hAATΔE1, a first-generation vector expressing hAAT (34) (Table 1). Expression was highest at 3 days and declined slowly for a period of 3 months (Fig. 1). In a second set of experiments, two baboons (12490 and 12497) were injected with 2-fold higher amounts of Ad5hAATΔE1 (Table 1). Expression of hAAT was higher and lasted longer than in the baboons receiving the lower dose (Fig. 1). The differences in the level and persistence of expression between the two groups are unlikely to be significant, because only two animals per group were treated.
Figure 1.
Expression of hAAT in baboons after administration of Ad5hAATΔE1 and Ad2hAATΔE1. Baboons 12402, 12486, 12490, and 12497 received Ad5hAATΔE1 followed by Ad2hAATΔE1 at the doses indicated in Table 1. Two naive animals of similar age and weight (13110 and 13121) were used as controls for Ad2hAATΔE1 administration. Arrows indicate the time of vector injection.
The four animals were monitored at all times for their general health and for the presence of any abnormal behavior that could be related to the administration of the virus. No abnormal symptoms were observed in any of the four animals; all thrived and gained weight normally. Blood chemistries were analyzed at the same time points at which hAAT was measured. No consistent or significant changes in any of the parameters analyzed were observed in any of the four animals, and in no case were they substantially different from normal ranges. Platelets decreased in the four animals 3 days after vector administration [values for preinjection → 3 days (× 103 per μl): 436 → 294, 563 → 280, 569 → 152, 293 → 189] but stayed within normal range (130–400 × 103 per μl). A transient drop in platelet counts has also been reported in mice at doses equal to or higher than 3 × 1012 particles per kg Ad5hAATΔE1 (39). The decrease in platelet counts was believed to be a consequence of endothelial cell damage and correlated with viral dose (39).
Feces were collected for 45 days to determine the period of time of vector shedding. The Ad5hAATΔE1 vector was recovered from the feces of two of the baboons (12402 and 12490) at 3, 10, and 17 days, but after this period virus was not recovered at any time point (data not shown).
Development of Neutralizing Antibodies Prevents a Second Administration.
Neutralizing antibodies against the vector developed in the four animals (Table 2). The titers were between 1:32 and 1:128 (background level of the assay was 1:4). To determine if in vitro neutralization titers correlated with in vivo protection to subsequent infections, we administered a second dose of Ad5hAATΔE1 vector (2.8 × 1011 particles per kg) to baboons 12402 and 12486. No increase in hAAT levels was observed in the two animals (Fig. 1), indicating that the antibodies induced by the first injection precluded expression from a second administration.
Table 2.
Neutralizing antibody titer to Ad2 and Ad5
| Subject | Ad5hAATΔE1 administration
|
Ad2hAATΔE1 administration
|
||||
|---|---|---|---|---|---|---|
| day 0 | day 24 | day 87 | day 0 | day 17 | day 52 | |
| Titer to wild-type Ad5 | ||||||
| 12402 | ND | 1:90 | 1:90 | 1:90 | 1:362 | 1:362 |
| 12486 | 1:4 | 1:128 | 1:32 | 1:64 | 1:362 | 1:181 |
| 12490 | 1:4 | ND | 1:64 | 1:64 | 1:256 | 1:90 |
| 12497 | 1:4 | ND | 1:90 | 1:128 | 1:512 | 1:181 |
| Titer to wild-type Ad2 | ||||||
| 12402 | 1:4 | 1:16 | 1:23 | |||
| 12486 | 1:4 | 1:90 | 1:90 | |||
| 12490 | 1:4 | 1:64 | 1:32 | |||
| 12497 | 1:4 | 1:64 | 1:90 | |||
ND, not determined.
A Serotype 2 Vector Circumvents Neutralizing Antibodies to Serotype 5.
To test whether the neutralizing antibodies developed against Ad5hAATΔE1 could be circumvented by using a vector of a different serotype, the four baboons were injected with Ad2hAATΔE1, a serotype 2 vector expressing hAAT. All four previously treated baboons (i.e., 12402, 12486, 12490, and 12497) received a dose of Ad2hAATΔE1 between 6 × 1011 and 7.2 × 1011 particles per kg (Table 1). As controls, two naive baboons received a dose of 6.1 × 1011 particles per kg Ad2hAATΔE1 (Table 1). All four animals previously treated with Ad5hAATΔE1 demonstrated substantial expression of hAAT in response to the Ad2 vector (Fig. 1). Levels of expression declined at approximately the same rate observed after administration of Ad5hAATΔE1 and in a similar way to the naive animals (Fig. 1). All of the animals developed neutralizing antibodies to serotype 2 (Table 2), and interestingly, the titer to serotype 5 also increased after administration of Ad2hAATΔE1, even though no neutralizing antibodies to Ad2 were detected before Ad2 administration (Table 2). Heterotypic antibody responses have been observed in human adults for adenoviruses within the same subgroup (40). Because Ad2 and Ad5 belong to subgroup C, it is very likely that the observed increase in the titer to Ad5 is the result of antigenic relationships between the two serotypes.
One of the naive baboons injected with Ad2hAATΔE1 (number 13121) had high levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ glutamyl transpeptidase, and cholesterol (preinjection and abnormal values on week 6 after injection were 52 → 415 units/liter, 31 → 129 units/liter, 1,632 → 5,070 units/liter, 69 → 779 units/liter, and 96 → 203 mg/dl, respectively) for a period of 60 days, starting 4 weeks after vector administration. However, the animal did not show external signs of disease or discomfort, had normal appetite, and gained weight as expected. The fact that the second baboon injected with the same dose and batch of vector (baboon 13110) did not develop any abnormal symptoms suggests that the altered values were most probably not related to Ad2hAATΔE1 administration.
Cellular Immune Responses to Adenoviral Vectors in Baboons.
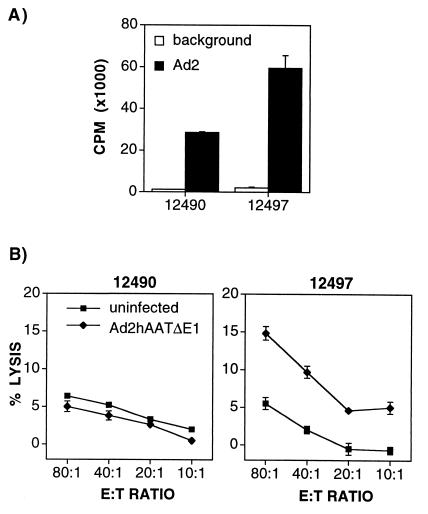
To determine whether a cellular immune response to adenovirus antigens had developed after administration of first-generation vectors Ad5hAATΔE1 and Ad2hAATΔE1, animals 12490 and 12497 were sacrificed 4.5 months after injection of Ad2hAATΔE1 to perform adenovirus-specific proliferation assays and CTL assays. Spleen cells from both baboons responded significantly to Ad2 antigens, as determined in the proliferation assay (Fig. 2A), which mainly indicates the presence of Ad2-specific CD4+ T cells. However, CTLs against cells transduced with vector were only detected in baboon 12497, and the percentage of lysis was relatively low (15% lysis compared with 5.3% lysis in background wells at an E/T ratio of 80:1; Fig. 2B). The inability to detect CTLs in baboon 12490 and the low response in baboon 12497 could be due to the fact that the animals were sacrificed long after the administration of Ad2hAATΔE1 (≈4.5 months), when expression was no longer detectable and CTL stimulation, therefore, was likely to be minimal. Another explanation could be the fact that a relatively low dose of vector was injected in the animals.
Figure 2.
Cellular immune responses to adenoviral antigens in baboons 12490 and 12497. Baboons were treated as indicated in Fig. 1 and Table 1. Both animals were sacrificed 4.5 months after Ad2hAATΔE1 infusion. Splenocytes were restimulated in vitro as described in Materials and Methods section to perform proliferation (A) and CTL (B) assays. Target cells were fibroblasts from skin biopsies of each animal infected with Ad2hAATΔE1 or uninfected. E/T ratio, effector:target ratio.
Long-Term Expression with a Helper-Dependent Adenoviral Vector.
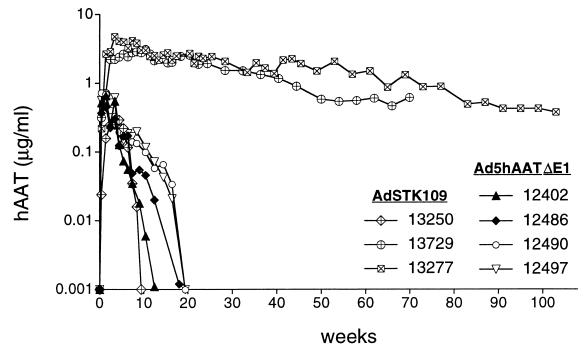
To evaluate whether the helper-dependent vector AdSTK109 would persist in a large animal model, we administered ≈4 × 1011 particles per kg of AdSTK109 to three baboons. In two of the animals (13277 and 13729), maximum levels of hAAT were observed after 3–4 weeks (≈3–4 μg/ml), and these levels decreased very slowly over time (Fig. 3). Both animals expressed hAAT for a period longer than a year and will continue to be studied until expression drops to baseline levels. The concentration of hAAT in these baboons declined approximately to 8% (baboon 13277) and 19% (baboon 13729) of highest levels after 24 and 16 months, respectively.
Figure 3.
Expression of hAAT in baboons after administration of Ad5hAATΔE1 or AdSTK109. Treatment of the four animals that received Ad5hAATΔE1 (12402, 12486, 12490, and 12497) is described in Fig. 1. Baboons 13250, 13277, and 13729 received 3.3 × 1011, 3.9 × 1011, and 3.6 × 1011 particles per kg, respectively, of AdSTK109 vector.
The third baboon injected with AdSTK109 (baboon 13250) had much lower levels of hAAT in the serum, and expression in this animal dropped to undetectable levels after ≈2 months. We hypothesized that the rapid decline in transgene expression in this animal could have been due to the development of antibodies to hAAT, because we previously observed this phenomenon in mice (10). The serum of the three baboons that received AdSTK109 and the serum of baboons that received Ad5hAATΔE1 were analyzed for the presence of antibodies to hAAT. Only samples from baboon 13250 had detectable antibodies, and the titer increased from 1:50 at week 8 to 1:5,000 at week 12, suggesting that the antibodies to hAAT were indeed the likely cause of the decrease in hAAT in this baboon.
Blood cell counts and blood chemistries were within normal values at all times in the three animals. No alterations in platelet counts was observed 3 days after vector administration, as had been observed in animals that were injected with Ad5hAATΔE1.
Discussion
For correction of genetic disorders and many other applications, optimal gene-therapy vectors should be capable of efficiently transducing cells and persisting in the target tissue for a long time. Although first-generation adenoviral vectors have proven to be particularly efficient for targeting liver cells after intravenous infusion (41–45), their usefulness has been limited by the elimination of transduced cells. Deletion of all viral genes from the vector backbone has been a strategy to avoid the production of viral proteins, and therefore, to prolong expression.
Initial in vivo studies with a vector that lacked E1, E2, E3, and late gene expression suggested that the DNA was not stable in the vector-transduced cells, leading to shorter transgene persistence than with first-generation vectors (46). Even though the reasons for the instability of such a construct have not been clearly determined, other studies have demonstrated long-term transgene expression from helper-dependent adenoviral vectors in the liver and in the muscle of immunocompetent hosts (19, 27, 47–49). We show here that helper-dependent vectors result in long-term expression after gene transfer to the liver of a primate model. Two of three animals (13277 and 13729) expressed hAAT for longer than a year. It is relevant that one of them, baboon 13277, expressed hAAT for more than 2 years, although at the present time, the level is <10% of initial values. The decrease in hAAT levels in these two baboons can be partially explained by the fact that the animals were young at the time of injection and growing, and therefore, a decrease in the concentration of hAAT is correlative to the growth of the animals. Another possibility is that cells transduced by the vector are eliminated by immune-related factors. A recent report has shown that psoralen-treated UV-crosslinked first-generation vectors can stimulate the production of CTLs, suggesting that cells infected with inactive vector can present antigens for recognition by MHC class I molecules in the absence of viral replication or de novo protein synthesis (50). Although unlikely, we cannot discount the possibility that the slow decline in transgene expression in baboons 13277 and 13729 might have been due to CTL responses against vector-transduced cells triggered by the proteins present in the capsid of the virus.
The mechanisms whereby hAAT expression can persist for over a year after a single intravenous dose of helper-dependent adenoviral vector are not entirely clear. The most likely possibility is that the vector genome can persist in the nucleus for prolonged intervals, so long as it is not diluted by cell division. Integration into the host genome is unlikely for the majority of cells, given the very stable persistence of expression compared with levels seen shortly after injection. It is possible that chromosomal integration occurs at a low level, and further studies are needed to assess this possibility.
We have previously shown in mice that the level of transgene expression does not linearly correlate with the dose of AdSTK109 (18). We observed a striking threshold effect with peak levels of hAAT of 50 μg/ml at the lowest dose tested (6.3 × 1011 particles per kg) compared with 6,407 μg/ml at the highest dose injected (168 × 1011 particles per kg) (18). This value represents a 128-fold increase in expression in response to a 27-fold increase in dose. Based on these data, the amount of hAAT that would be expected in mice at the dose injected in the baboons (3.75 × 1011 particles per kg) would be ≈10 μg/ml, which is only slightly higher than what we observed in baboons (3–4 μg/ml). Thus, the level of expression in baboons is relatively consistent with what would be expected for a similar dose per kg body weight in mice. Since helper-dependent vectors have been substantially less toxic than first-generation vectors in all instances studied in mice to date (18, 19, 27), studies to evaluate the toxicity and expression with higher doses of helper-dependent vector administered to primates are of great importance.
Expression by first-generation vectors in baboons lasted only 3–5 months. The lack of detection of antibodies against hAAT in these animals suggests that cellular immune responses directed against viral proteins may have been generated, with the potential to subsequently eliminate vector-transduced cells. This idea is supported by the presence of reactive CD4+ T cells after in vitro stimulation with viral antigens of spleen cells from baboons 12490 and 12497, which received Ad5hAATΔE1 followed by Ad2hAATΔE1. It is interesting that we did not observe a faster decrease in hAAT expression after Ad2hAATΔE1 delivery in animals previously challenged with Ad5hAATΔE1. Human CTLs raised against serotype 5 have been found to cross-react with other serotypes in vitro, suggesting that in vivo, the memory cytotoxic response raised against one serotype might not be evaded by switching to a different serotype (51). We would have expected shorter transgene expression if memory T cells activated after delivery of Ad5hAATΔE1 played an important role in eliminating Ad2hAATΔE1-transduced liver cells. However, it is possible that the cytotoxic T cell response depends on the viral dose administered and that at the dose of vector used in our experiments, the response is not robust enough to observe a rapid decline in transgene expression after Ad2hAATΔE1 delivery. It is worth noting that the potential amount of replication-competent adenovirus contamination in the vector preparations is negligible (103 particles) and that in mice it is not sufficient to induce a detectable CTL response (J.K., unpublished results).
If helper-dependent vectors of different serotypes were used for gene transfer, cellular immune responses after multiple administrations would not be anticipated, because these vectors are devoid of viral coding sequences. Recently, we have shown that expression of hAAT is not affected by CTLs generated from three previous administrations of serotype 5-based first generation vectors in mice (W.O., unpublished results). In addition, mice lacking Ig production treated with four consecutive doses of AdSTK109 did not have decreased hAAT expression, indicating that cytotoxic immune responses to helper-dependent vectors are unlikely to be developed (W.O., unpublished results).
The pattern of hAAT expression observed in the animals that received AdSTK109 suggests that readministration of vector will probably be necessary to achieve lifetime expression of a therapeutic gene with helper-dependent adenoviral vectors, although it may be possible to achieve adequate therapy in some cases for 1–2 years after a single injection. Delivery of a serotype 5 followed by administration of a serotype 2 vector has been used for repeated administration to the lung (52). In the present study, we have shown that intravenous readministration of first-generation vectors in baboons is possible using the two most common adenovirus serotypes used in gene therapy studies, serotypes 2 and 5. Recently, an Ad2-based helper-dependent vector has been developed (53). It should be noted that Ad5 and Ad2 helper-dependent vectors can be produced from the same starting vector plasmid by changing only the helper virus. Therefore, multiple serotypes of helper-dependent vectors may provide the opportunity for multiple years of corrective therapy in many gene-therapy settings. This possibility, combined with the previously reported improved safety, makes helper-dependent vectors promising tools for the treatment of diseases that require persistent expression of therapeutic genes.
Acknowledgments
We thank Gustavo A. Poveda for technical assistance and Drs. R. C. Eisensmith and L. Pastore for helpful comments on the manuscript. This work was supported by grants from the National Institutes of Health (P01 HL 51754) and the Medical Research Council of Canada, by research awards from Merck & Co. (S.K. and F.L.G.) and the Parent Project for Muscular Dystrophy Research (S.K.), and fellowships from the Ministerio de Educación y Cultura (Spain) to N.M., the Cystic Fibrosis Foundation to H.Z., the Natural Sciences and Engineering Research Council of Canada, and the Medical Research Council of Canada (R.J.P.). F.L.G. is a Terry Fox Research Scientist of the National Cancer Institute of Canada. A.L.B. was an investigator with the Howard Hughes Medical Institute.
Abbreviations
- hAAT
human α1-antitrypsin
- Ad
adenovirus
- CTL
cytotoxic T lymphocyte
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Stratford-Perricaudet L D, Levrero M, Chase J F, Perricaudet M, Briand P. Hum Gene Ther. 1990;1:241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld M A, Siegfried W, Yoshimura K, Yoneyama K, Fukayama M, Stier L E, Pääkkö P K, Gilardi P, Stratford-Perricaudet L D, Perricaudet M, et al. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe H, Danel C, Longenecker G, Metzger M, Setoguchi Y, Rosenfeld M A, Gant T W, Thorgeirsson S S, Stratford-Perricaudet L D, Perricaudet M, et al. Nat Genet. 1992;1:372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- 4.Quantin B, Perricaudet L D, Tajbakhsh S, Mandel J L. Proc Natl Acad Sci USA. 1992;89:2581–2584. doi: 10.1073/pnas.89.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hitt M, Addison C, Graham F L. In: Advances in Pharmacology-Gene Therapy. August J T, editor. Vol. 40. San Diego: Academic; 1997. pp. 137–206. [DOI] [PubMed] [Google Scholar]
- 6.Engelhardt J F, Simon R H, Yang Y, Zepeda M, Weber-Pendleton S, Doranz B, Grossman M, Wilson J M. Hum Gene Ther. 1993;4:759–769. doi: 10.1089/hum.1993.4.6-759. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Li Q, Ertl H C, Wilson J M. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozarsky K F, Jooss K, Donahee M, Strauss J F, III, Wilson J M. Nat Genet. 1996;13:54–62. doi: 10.1038/ng0596-54. [DOI] [PubMed] [Google Scholar]
- 9.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 10.Morral N, O’Neal W, Zhou H, Langston C, Beaudet A. Hum Gene Ther. 1997;8:1275–1286. doi: 10.1089/hum.1997.8.10-1275. [DOI] [PubMed] [Google Scholar]
- 11.Connelly S, Gardner J M, Lyons R M, McClelland A, Kaleko M. Blood. 1996;87:4671–4677. [PubMed] [Google Scholar]
- 12.Armentano D, Zabner J, Sacks C, Sookdeo C C, Smith M P, St. George J A, Wadsworth S C, Smith A E, Gregory R J. J Virol. 1997;71:2408–2416. doi: 10.1128/jvi.71.3.2408-2416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brough D E, Hsu C, Kulesa V A, Lee G M, Cantolupo L J, Lizonova A, Kovesdi I. J Virol. 1997;71:9206–9213. doi: 10.1128/jvi.71.12.9206-9213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loser P, Jennings G S, Strauss M, Sandig V. J Virol. 1998;72:180–190. doi: 10.1128/jvi.72.1.180-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff G, Worgall S, van Rooijen N, Song W R, Harvey B G, Crystal R G. J Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worgall S, Wolff G, Falk-Pedersen E, Crystal R. Hum Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 17.Kuzmin A I, Finegold M J, Eisensmith R C. Gene Ther. 1997;4:309–316. doi: 10.1038/sj.gt.3300377. [DOI] [PubMed] [Google Scholar]
- 18.Morral N, Parks R, Zhou H, Langston C, Schiedner G, Quinones J, Graham F L, Kochanek S, Beaudet A L. Hum Gene Ther. 1998;9:2709–2716. doi: 10.1089/hum.1998.9.18-2709. [DOI] [PubMed] [Google Scholar]
- 19.Schiedner G, Morral N, Parks R, Wu Y, Koopmans S C, Langston C, Graham F L, Beaudet A L, Kochanek S. Nat Genet. 1998;18:180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- 20.Muruve D A, Barnes M J, Stillman I E, Libermann T A. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 21.Fisher K J, Choi H, Burda J, Chen S-J, Wilson J M. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- 22.Haecker S E, Stedman H H, Balice-Gordon R J, Smith D B J, Greelish J P, Mitchell M A, Wells A, Sweeney H L, Wilson J M. Hum Gene Ther. 1996;7:1907–1914. doi: 10.1089/hum.1996.7.15-1907. [DOI] [PubMed] [Google Scholar]
- 23.Kochanek S, Clemens P R, Mitani K, Chen H-H, Chan S, Caskey C T. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar-Singh R, Chamberlain J S. Hum Mol Genet. 1996;5:913–921. doi: 10.1093/hmg/5.7.913. [DOI] [PubMed] [Google Scholar]
- 25.Parks R J, Chen L, Anton M, Sankar U, Rudnicki M A, Graham F L. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps M L. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morsy M A, Gu M C, Motzel S, Zhao J, Su Q, Allen H, Franlin L, Parks R J, Graham F L, Kochanek S, et al. Proc Natl Acad Sci USA. 1998;95:7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai Y, Schwarz E M, Gu D, Zhang W W, Sarvetnick N, Verma I M. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith T A, White B D, Gardner J M, Kaleko M, McClelland A. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- 30.Yang Y, Greenough K, Wilson J M. Gene Ther. 1996;3:412–420. [PubMed] [Google Scholar]
- 31.Jooss K, Turka L A, Wilson J M. Gene Ther. 1998;5:309–319. doi: 10.1038/sj.gt.3300595. [DOI] [PubMed] [Google Scholar]
- 32.Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pedersen E. Gene Ther. 1996;3:154–162. [PubMed] [Google Scholar]
- 33.Mastrangeli A, Harvey B G, Yao J, Wolff G, Kovesdi I, Crystal R G, Falck-Pedersen E. Hum Gene Ther. 1996;7:79–87. doi: 10.1089/hum.1996.7.1-79. [DOI] [PubMed] [Google Scholar]
- 34.Zhou H, O’Neal W, Morral N, Beaudet A L. J Virol. 1996;70:7030–7038. doi: 10.1128/jvi.70.10.7030-7038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadsworth S C, Zhou H, Smith A E, Kaplan J M. J Virol. 1997;71:5189–5196. doi: 10.1128/jvi.71.7.5189-5196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Anton M, Graham F. Somatic Cell Mol Genet. 1996;22:477–488. doi: 10.1007/BF02369439. [DOI] [PubMed] [Google Scholar]
- 37.Piedra P A, Poveda G A, Ramsey B, McCoy K, Hiatt P W. Pediatrics. 1998;101:1013–1019. doi: 10.1542/peds.101.6.1013. [DOI] [PubMed] [Google Scholar]
- 38.Kay M, Graham F, Leland F, Woo S L. Hepatology. 1995;21:815–819. [PubMed] [Google Scholar]
- 39.O’Neal W K, Zhou H, Morral N, Aguilar-Cordova E, Pestaner J, Langston C, Mull B, Wang Y, Beaudet A L, Lee B. Hum Gene Ther. 1998;9:1587–1598. doi: 10.1089/hum.1998.9.11-1587. [DOI] [PubMed] [Google Scholar]
- 40.Kasel J A, Banks P A, Wigand R, Knight V, Alling D W. Proc Soc Exp Biol Med. 1965;119:1162–1165. doi: 10.3181/00379727-119-30404. [DOI] [PubMed] [Google Scholar]
- 41.Smith T A G, Mehaffey M G, Kayda D B, Saunders J M, Yei S, Trapnell B C, McClelland A, Kaleko M. Nat Genet. 1993;5:397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- 42.Fang B, Eisensmith R C, Li X H C, Finegold M J, Shedlovsky W, Dove W, Woo S L C. Gene Ther. 1994;1:247–254. [PubMed] [Google Scholar]
- 43.Kass-Eisler A, Falk-Pedersen E, Elfenbein D H, Alvira M, Buttrick P M, Leinwand L A. Gene Ther. 1994;1:395–402. [PubMed] [Google Scholar]
- 44.Huard J, Lochmüller H, Acsadi G, Jani A, Massie B, Karpati G. Gene Ther. 1995;2:107–115. [PubMed] [Google Scholar]
- 45.Vrancken Peeters M-J T F D, Lieber A, Perkins J, Kay M A. BioTechniques. 1996;20:278–285. doi: 10.2144/96202rr05. [DOI] [PubMed] [Google Scholar]
- 46.Lieber A, He C, Kirillova I, Kay M A. J Virol. 1996;70:8944–8960. doi: 10.1128/jvi.70.12.8944-8960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H H, Mack L M, Kelly R, Ontell M, Kochanek S, Clemens P R. Proc Natl Acad Sci USA. 1997;94:1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar-Singh R, Farber D B. Hum Mol Genet. 1998;7:1893–1900. doi: 10.1093/hmg/7.12.1893. [DOI] [PubMed] [Google Scholar]
- 49.Burcin M M, Schiedner G, Kochanek S, Tsai S Y, O’Malley B W. Proc Natl Acad Sci USA. 1999;96:355–360. doi: 10.1073/pnas.96.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kafri T, Morgan D, Krahl T, Sarvetnick N, Sherman L, Verma I. Proc Natl Acad Sci USA. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith C A, Woodruff L S, Rooney C, Kitchingman G R. Hum Gene Ther. 1998;9:1419–1427. doi: 10.1089/hum.1998.9.10-1419. [DOI] [PubMed] [Google Scholar]
- 52.Mack C A, Song W R, Carpenter H, Wickham T J, Kovesdi I, Harvey B G, Magovern C J, Isom O W, Rosengart T, Falck-Pedersen E, et al. Hum Gene Ther. 1997;8:99–109. doi: 10.1089/hum.1997.8.1-99. [DOI] [PubMed] [Google Scholar]
- 53.Parks R J, Evelegh C M, Graham F L. Gene Ther. 1999;6:1565–1573. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]