Abstract
Background
The function of voltage-gated calcium (Cav) channels greatly depends on coupling to cytoplasmic accessory β subunits, which not only promote surface expression, but also modulate gating and kinetic properties of the α1 subunit. Schistosomes, parasitic platyhelminths that cause schistosomiasis, express two β subunit subtypes: a structurally conventional β subunit and a variant β subunit with unusual functional properties. We have previously characterized the functional properties of the variant Cavβ subunit. Here, we focus on the modulatory phenotype of the conventional Cavβ subunit (SmCavβ) using the human Cav2.3 channel as the substrate for SmCavβ and the whole-cell patch-clamp technique.
Results
The conventional Schistosoma mansoni Cavβ subunit markedly increases Cav2.3 currents, slows macroscopic inactivation and shifts steady state inactivation in the hyperpolarizing direction. However, currents produced by Cav2.3 in the presence of SmCavβ run-down to approximately 75% of their initial amplitudes within two minutes of establishing the whole-cell configuration. This suppressive effect was independent of Ca2+, but dependent on intracellular Mg2+-ATP. Additional experiments revealed that SmCavβ lends the Cav2.3/SmCavβ complex sensitivity to Na+ ions. A mutant version of the Cavβ subunit lacking the first forty-six amino acids, including a string of twenty-two acidic residues, no longer conferred sensitivity to intracellular Mg2+-ATP and Na+ ions, while continuing to show wild type modulation of current amplitude and inactivation of Cav2.3.
Conclusion
The data presented in this article provide insights into novel mechanisms employed by platyhelminth Cavβ subunits to modulate voltage-gated Ca2+ currents that indicate interactions between the Ca2+ channel complex and chelated forms of ATP as well as Na+ ions. These results have potentially important implications for understanding previously unknown mechanisms by which platyhelminths and perhaps other organisms modulate Ca2+ currents in excitable cells.
Background
Voltage-gated calcium (Cav) channels couple membrane depolarisation to the entry of Ca2+ that, in turn, is fundamental in a variety of cellular events such as contraction [1,2], changes in gene expression [3] and neurotransmitter release [4,5]. Cav channels belong to the super-family of voltage-gated ion channels that also include sodium channels and potassium channels [6], and can be broadly classified into high-voltage activated (HVA) and low-voltage activated (LVA) classes. HVA Cav channels are heteromeric protein complexes composed of a pore-forming α1 subunit and auxiliary β and α2δ subunits [7]. In addition to promoting surface expression of the Cavα1 subunit, Cavβ subunits modulate the kinetics of activation and inactivation, gating [8-10] and the rate of recovery from inactivation [11,12].
Schistosomes are parasitic trematode flatworms that cause schistosomiasis, a tropical disease affecting approximately 200 million people worldwide. With the ultimate goal of understanding the molecular basis for neuromuscular transmission in these parasitic flatworms, we have previously cloned three transcripts from Schistosoma mansoni that code for one L-type-like and two non L-type high voltage-activated Cav channel α1 subunits [13]. Heterologous expression of these α1 subunits in Xenopus oocytes and mammalian cell lines has proved problematic, perhaps because of the relatively high A-T content of these coding regions, or the lack of a specific chaperone in these systems. Additionally, we have identified two Cav channel β subunits from schistosomes and other platyhelminths: a conventional β subunit (SmCavβ), and a variant β subunit (SmCavβvar), which appears to be unique to platyhelminths and has unusual structural and functional features [14,15].
When heterologously expressed in Xenopus oocytes, the conventional schistosome Cavβ subunit significantly increases Cav2.3 current amplitude, and shifts the steady state inactivation curve to more hyperpolarized potentials [16] (in these experiments, we use the robustly expressing human Cav2.3 α1 subunit as a "reporter" to assess β subunit function). The actions of this β subunit are consistent with those of mammalian Cavβ subunits [10]. Here, we have reproduced and extended our previous data on modulation of Cav2.3 currents by the schistosome SmCavβ subunit (SmCavβ) in a mammalian cell system, which may better approximate the cellular milieu in which these channels are found in situ. Xenopus oocytes are widely used in expression of ion channels and other proteins, in part because they are primed for high levels of protein translation. However, most other cells are not that strongly geared towards this role. Oocytes are much larger than adult, differentiated cells and contain high amounts of yolk granules. In addition, the mammalian cell line HEK does not express the endogenous Cavβ subunit that complicates analysis of heterologously expressed Cav channels in Xenopus oocytes [17]. Finally, adult S. mansoni live in a mammalian host environment.
Interestingly, during these studies, we observed a rapid run-down of the currents produced by Cav2.3 channels co-expressed with this schistosome Cavβ subunit. Decrease of Ca2+ channel activity under whole-cell patch-clamp, a configuration of the patch-clamp technique that disrupts the contact between membrane and cytoplasm, is a well-known phenomenon [18-21]. However, very few studies have dealt with the structural and biochemical causes for run-down, and those studies focus primarily on L-type Ca2+ channels. Notably, Kameyama and collaborators were able to relate run-down of L-type Ca2+ channels to the Ca2+-binding protein calmodulin [22]. Here we investigate the mechanism of this β subunit-dependent rundown, examining the role of several forms of ATP and cations in run-down of the Cav2.3/SmCavβ complex. Additionally, we use a truncated β subunit protein to provide clues regarding the molecular substrate within the SmCavβ subunit that mediates run-down of Cav2.3/SmCavβ currents.
Results
SmCavβ modulates activation and inactivation of Cav2.3 in a conventional manner
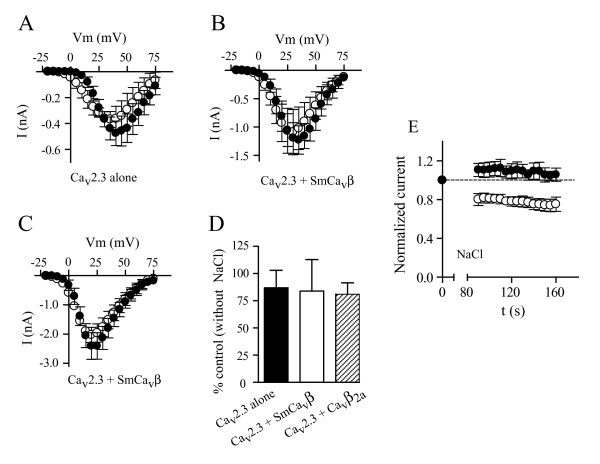
To assess the modulatory phenotype of Cavβ subunits, a HEK-293 cell line stably expressing the human Cav2.3d subunit (GB Acc. # L27745) was used. Using Ca2+ as the charge carrier, currents produced by Cav2.3 alone peaked at +30 mV with an average amplitude of -261 ± 20 pA (n = 15). Co-expression of SmCavβ increased peak amplitudes to -1640 ± 276 pA (n = 5) and shifted the I–V peak leftwards, to +20 mV (Figure 1A,B).
Figure 1.
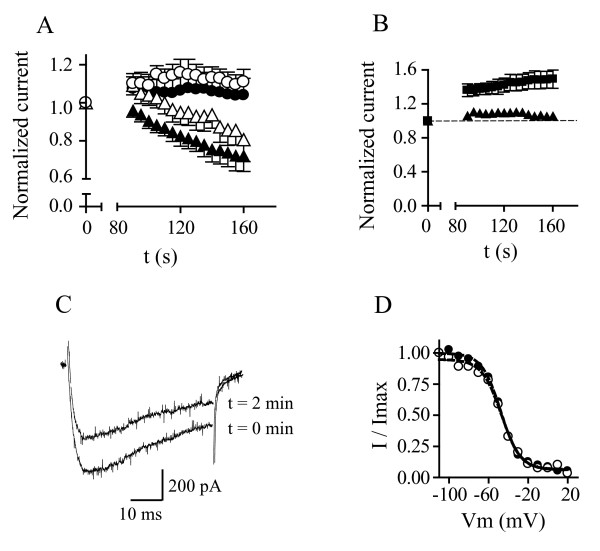
SmCavβ modulates Cav2.3 currents in HEK cells. A. Representative Ca2+ currents generated by voltage steps to -5, +15, +35 and +55 mV from a holding potential of -80 mV in HEK cells expressing Cav2.3 alone and in combination with SmCavβ or Cavβ2a. B. Current-voltage relationships from HEK cells expressing Cav2.3 alone (filled circles) and in combination with SmCavβ (open circles) or Cavβ2a (filled triangles). Peak currents generated by voltage steps from -20 mV to +75 mV in 5 mV steps from a holding potential of -80 mV were measured. Data points represent mean ± s.e.m. N = 5–15. C. Two-variable plot showing the relationships between inactivation time constants and percentages of contribution to total inactivation for Cav2.3 alone (filled circles) and in combination with SmCavβ (open circles). Data points represent mean ± s.e.m. N = 7–9. Slow time constants for the kinetic of recovery are grouped (dotted lines). Fast time constants of recovery from inactivation kinetics are also grouped (dotted lines). D. Voltage-dependence of inactivation (steady-state inactivation) for Cav2.3 alone (filled circles) and in combination with SmCavβ (open circles) or Cavβ2a (filled triangles). Lines represent fits to the Boltzmann function (see Materials and Methods); V50 and slope values are shown in Table 1. Data points represent mean ± s.e.m. N = 3–6. E. Recovery from inactivation from -100 mV for Cav2.3 channels alone (filled circles) or in the presence of SmCavβ (open circles), using Ba2+ as the charge carrier. Solid curves represent single exponential fits to the data. Values are means ± s.e.m. N = 3.
Using Ca2+ as the charge carrier, the decaying phase of Cav2.3 currents produced by Cav2.3 channels alone or with SmCavβ subunits was well fitted by a double exponential function with fast (τfast) and slow (τslow) time constants of inactivation (Table 1). The double exponential fit to the decay of currents produced by Cav2.3 in response to a pulse to +30 mV had a fast time constant of 16 ms that contributed 66% to total current decay, and a slow time constant of 69 ms that represented 34% of total current decay. The double exponential fit to the decay of currents produced by Cav2.3 co-expressed with SmCavβ in response to a depolarizing pulse to +20 mV had a fast time constant of 31 ms, which contributed 55% to total current decay, and a slow time constant of 116 ms that contributed 45% to total inactivation. Fundamentally, SmCavβ slowed macroscopic inactivation of Cav2.3 currents by increasing the time constants of both the fast and slow components while simultaneously decreasing the contribution of the fast component and increasing the contribution of the slow component to total inactivation (Figure 1C and Table 1). Co-expression of SmCavβ markedly shifted the midpoint of steady-state inactivation in the hyperpolarizing direction (Figure 1D). Midpoints of single order Boltzmann fits to steady-state inactivation curves of Cav2.3 and Cav2.3 + SmCavβ currents were, respectively, -8 mV and -49 mV (P < 0.05, Student t-test). The slope factors of these Boltzmann fits to steady-state inactivation were not significantly different: 12 mV/e-fold for Cav2.3 and 10 mV/e-fold for Cav2.3 + SmCavβ (Table 1).
Table 1.
SmCavβ modulates inactivation of Cav2.3 currents
| Cav2.3 alone | Cav2.3 + SmCavβ | |
| Steady state inactivation | ||
| V50 (mV) | -8 ± 4 (3) | -49 ± 3 (3)* |
| Slope (mV/e-fold) | 12 ± 2 (3) | 10 ± 1 (3) |
| Macroscopic inactivation | ||
| τfast (ms) | 16 ± 3 (9) | 31 ± 5 (7)* |
| % Contribution | 66 ± 2% | 55 ± 5%* |
| τslow (ms) | 69 ± 13 (9) | 116 ± 20 (7) |
| % Contribution | 34 ± 2% | 45 ± 5%* |
| Recovery from inactivation | ||
| τrec-80 mV (ms) | 178 ± 28 (3) | 252 ± 17 (4) |
| τrec-100 mV (ms) | 71 ± 7 (3) | 168 ± 16 (3)* |
| τrec-120 mV (ms) | 38 ± 1 (3) | 109 ± 10 (3)* |
Data represent mean ± s.e.m. Number of repeats is indicated in parentheses. Asterisks indicate statistically significant difference (Student t test, P < 0.05).
SmCavβ significantly slowed the rate of recovery from inactivation. The fractional recoveries of current as a function of time at -80, -100 and -120 mV were well fitted by a single exponential function with recovery time constants from inactivation (τrec). For example, τrec for Cav2.3 and Cav2.3 + SmCavβ from -100 mV were, respectively, 71 and 168 ms (p < 0.05, Student t-test) (Figure 1E; see Table 1 for recovery time constants for the three potentials).
The mammalian (rat) β2a subunit (GB Acc. # M80545) increased current amplitude to a similar extent as the schistosome Cavβ subunit, shifted the steady-state inactivation curve in the hyperpolarizing direction, although to a lesser degree than the schistosome Cavβ subunit, slowed macroscopic inactivation, and shifted the I–V peak in the hyperpolarizing direction (Figure 1).
Cav2.3 currents run-down in the presence of SmCavβ in a manner independent of Ca2+ but dependent on chelated forms of ATP and free Na+
Currents produced by Cav2.3 progressively decrease in amplitude to approximately 75% of their initial values within ~2.5 minutes of establishing the whole-cell configuration, but only when SmCavβ is co-expressed (Figure 2A). Over the same time frame, no run-down was observed for currents produced by Cav2.3 expressed alone, nor in the presence of the mammalian Cavβ2a subunit nor with the structurally atypical schistosome SmCavβvar subunit (Figure 2B). Substitution of CaCl2 by an equimolar concentration of BaCl2 did not prevent run-down, indicating that this phenomenon is Ca2+ independent (Figure 2A).
Figure 2.
SmCavβ-dependent run-down of Cav2.3 currents. A. Relative peak amplitude of currents produced by Cav2.3 channels alone (circles) and in combination with SmCavβ (triangles), plotted as a function of time. Currents were normalized with respect to those measured immediately after rupturing the patch membrane (t = 0). Ca2+ currents (filled symbols) and Ba2+ currents (open symbols) are shown. Data points represent mean ± s.e.m. B. Relative amplitude of currents produced by Cav2.3 in combination with Cavβ2a (squares) or SmCavβvar (triangles), plotted as a function of time. As in A, currents were normalized with respect of those at time 0, measured immediately after break-in. Data points represent mean ± s.e.m, N = 3 – 5. C. Ca2+ currents generated by Cav2.3 in the presence of SmCavβ at t = 0 min and at t = 2 min. Note that the kinetics of macroscopic inactivation remains unchanged. D. Voltage-dependence of inactivation (steady-state inactivation) at t = 0 min (filled circles) and at t = 2 min (open circles). Solid lines represent fits to the Boltzmann function.
We hypothesized that this rapid run-down could be caused by physical dissociation between SmCavβ and Cav2.3. However, as the kinetics of inactivation and the steady state inactivation properties were identical before and after run-down (Figure 2C,D), it seems likely that the association between Cav2.3 and SmCavβ remained intact in these conditions.
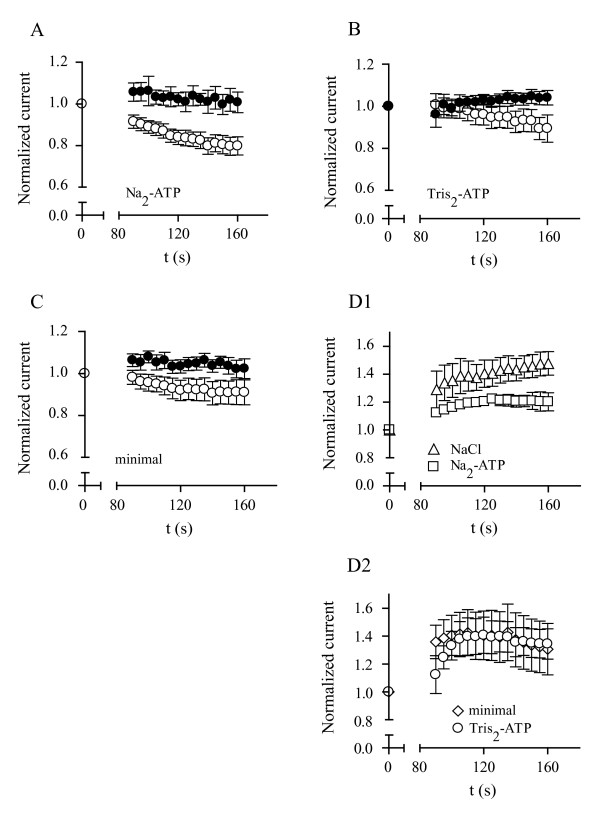
Since Mg2+ is known to block Ca2+ channels from the intracellular side, we replaced Mg2+-ATP with an equimolar concentration (5 mM) of Na+2-ATP, yet under these conditions we still observed significant run-down that, again, was dependent upon co-expression of the schistosome Cavβ subunit (Figure 3A). No run-down was observed in whole-cell patch-clamp experiments using a "minimal" internal solution without ATP (Figure 3C). Further experiments indicate that non-chelated forms of ATP (Tris2-ATP) do not mediate SmCavβ-dependent Cav2.3 run-down (Figure 3B). Currents produced by Cav2.3/Cavβ2a did not run-down in the presence of 5 mM intracellular NaCl, Na2-ATP, Tris2·ATP or in minimal intracellular solution (Figures 3D1 and 3D2).
Figure 3.
Run-down of Cav2.3/SmCavβ currents in the presence of intracellular forms of ATP. Relative current amplitude of currents produced by Cav2.3 (filled circles) channels alone and in combination with SmCavβ (open circles) plotted as a function of time in the presence of 5 mM Na2+-ATP in the patch-pipette solution (A), 5 mM Tris2-ATP (B), and using a "minimal" pipette solution, with no ATP (C). Currents were normalized with respect to those measured immediately after rupturing the patch membrane (t = 0). Panels D1 and D2 show current amplitude produced by Cav2.3/SmCavβ2a channels as a function of time for all conditions explained above. Symbols are as shown on the figure. Values are means ± s.e.m, N = 3 – 8.
Next we explored the possibility that this particular form of run-down was caused by the cations associated with the ATP molecule (Mg2+ and Na+). Free Mg2+ reduced currents produced by Cav2.3 alone by about 75%, and by about 90% when SmCavβ was co-expressed (Figure 4); therefore, since it would be very challenging to distinguish Ca2+ current decrease due to run-down from Ca2+ current decrease due to blockade by internal Mg2+, we did not pursue run-down studies in the presence of free Mg2+. However, in contrast with Mg2+, free Na+ did not suppress the activity of Cav2.3 channels expressed alone or co-expressed with the schistosome SmCavβ or with the mammalian Cavβ2a subunit (Figure 5A–D). However, substituting Mg2+-ATP with NaCl resulted in significant run-down (Figure 5E), indicating Na+-dependent modulation of channels containing SmCavβ.
Figure 4.
Mg2+ block of Cav2.3 channels expressed alone and co-expressed with SmCavβ or Cavβ2a. Current-voltage relationships from HEK cells expressing Cav2.3 subunits alone (A), co-expressing Cav2.3 and SmCavβ subunits (B) or co-expressing Cav2.3 and Cavβ2a subunits (C) in minimal intracellular solution (filled circles) and in intracellular solution containing 5 mM MgCl2 (open circles). Peak currents generated by voltage steps from -20 mV to +75 mV in 5 mV steps from a holding potential of -80 mV are plotted. Data points represent mean ± s.e.m. D. Peak current amplitude in the presence of 5 mM internal MgCl2 relative to peak current amplitude in the absence of MgCl2 for Cav2.3 subunits alone, co-expressed with SmCavβ subunits or co-expressed with Cavβ2a subunits. Asterisks denote statistically significant difference with respect to Mg2+ block of Cav2.3 channels alone (p < 0.05), N = 5 – 7.
Figure 5.
Intracellular free Na+ does not block Cav2.3 channels expressed alone or co-expressed with SmCavβ or Cavβ2a but induces SmCavβ-dependent run-down of Cav2.3 currents. Current-voltage relationships from HEK cells expressing Cav2.3 subunits alone (A), co-expressing Cav2.3 and SmCavβ subunits (B) or co-expressing Cav2.3 and Cavβ2a subunits (C) in minimal intracellular solution (filled circles) and in intracellular solution containing 5 mM NaCl (open circles). Peak currents generated by voltage steps from -20 mV to +75 mV in 5 mV steps from a holding potential of -80 mV are plotted. Data points represent mean ± s.e.m. (D) Peak current amplitude in the presence of 5 mM internal NaCl relative to peak current amplitude in the absence of NaCl for Cav2.3 subunits alone, co-expressed with SmCavβ subunits or co-expressed with Cavβ2a subunits. (E) Relative current amplitude of currents produced by Cav2.3 (filled circles) channels alone and in combination with SmCavβ (open circles) plotted as a function of time in the presence of 5 mM NaCl in the patch-pipette solution. Values are means ± s.e.m, N = 3 – 9.
The structural basis for run-down mediated by SmCavβ resides in its acidic N-terminal domain
The N-terminus of the SmCavβ subunit contains an atypical domain that is rich in glutamic acid and aspartic acid residues (Figure 6A). Since BLAST screens for similar domains in other proteins did not yield any hits, we set to investigate whether this N-terminal domain represents the structural base for the atypical modulation of the SmCavβ subunit. Furthermore, there is precedence for important modulatory effects on Cav channels localizing to the N-terminal regions of β subunits [23-26]. To this end, we created a truncated version of SmCavβ that lacks the first forty-six amino acids containing this acid-rich domain (Figure 6A), and tested whether it still caused Cav2.3 currents to run-down. The N-terminally truncated SmCavβ subunit enhanced Cav2.3 currents, slowed their macroscopic inactivation and shifted their steady-state inactivation to the same extent as the wild type SmCavβ subunit (Figure 6B,C). However, unlike the wild-type version of SmCavβ, the N-terminally truncated SmCavβ subunit did not induce run-down of Ca2+ currents within 2–3 minutes of establishing the whole-cell configuration with an internal solution containing either 5 mM Mg2+-ATP (Figure 6D,F), or 5 mM NaCl (Figure 6E,F).
Figure 6.
The N-terminal domain of SmCavβ is the molecular substrate for Na+ and Mg2+-ATP mediated block of Cav2.3 currents. A. Diagram showing the portion of the N-terminal domain, including a domain rich in aspartic acid and glutamic acid (DE-rich domain), that is removed by the truncation. Acidic residues are in red. B. Current-voltage relationships from HEK cells expressing Cav2.3 alone (filled circles) and in combination with SmCavβ (open circles) or N-terminally truncated SmCavβ (filled triangles). Peak currents generated by voltage steps from -20 mV to +75 mV in 5 mV steps from a holding potential of -80 mV were measured. Data points represent mean ± s.e.m. N = 6–7. C. Voltage-dependence of inactivation (steady-state inactivation) for Cav2.3 alone co-expressed with SmCavβ (filled circles) or with its N-terminally truncated version (open circles). Lines represent fits to the Boltzmann function. D. Relative peak current amplitude of currents produced by Cav2.3 channels in the presence of internal 5 mM Mg2+-ATP co-expressed with the wild type SmCavβ subunit (filled circles) or with the N-terminally truncated SmCavβ subunit (open circles) plotted as a function of time. Currents were normalized with respect to those measured immediately after rupturing the patch membrane (t = 0). Data points represent mean ± s.e.m. N = 5. E. Relative current amplitude of currents produced by Cav2.3 channels in the presence of internal 5 mM NaCl co-expressed with the wild type SmCavβ subunit (filled circles) or with the N-terminally truncated SmCavβ subunit (open circles) plotted as a function of time. Currents were normalized with respect to those measured immediately after rupturing the patch membrane (t = 0). Data points represent mean ± s.e.m. N = 10–13. F. Comparison between the relative current amplitudes produced by Cav2.3 channels co-expressed with SmCavβ (in black) or with its N-terminally truncated version (in grey), in the presence of intracellular 5 mM Mg2+-ATP (left), or in the presence of 5 mM NaCl (right), after 2.5 minutes of establishing the whole-cell configuration. Asterisks denote statistical difference with respect to current amplitude for Cav2.3 co-expressed with wild type SmCavβ (Student t test, p < 0.05).
Discussion
In this study we set out to characterize the modulatory phenotype of a Cavβ subunit from the parasitic trematode S. mansoni. This β subunit is phylogenetically close to other Cavβ subunits [14]. The mammalian HEK-293 cell line was used as the expression system and the mammalian Cav2.3 channel splice variant "d" as our "test" α1 subunit. This splice variant is susceptible to modulation by Cavβ subunits [27] and is the longest of all known versions of the Cav2.3 subunit [28]. Our data show that SmCavβ exhibits similar modulatory phenotypes in HEK cells and Xenopus oocytes. Additionally, SmCavβ slows recovery from inactivation of Cav2.3 currents using Ba2+ as the charge carrier, i.e. in the absence of intracellular Ca2+ accumulation. Using strontium as the charge carrier, mammalian and jellyfish Cavβ subunits also slow recovery from inactivation of this same channel in conditions in which Ca2+ does not accumulate in the intracellular compartment [11]. However, it is important to note that recovery from inactivation appears to depend heavily on the identity of the Cavα1 subunit being assayed: all jellyfish and mammalian Cavβ subunits delayed recovery from inactivation of Cav2.3 channels, but accelerated the recovery of the jellyfish L-type Cav channel [11].
SmCavβ-dependent run-down of Cav2.3 currents
While characterizing the modulatory phenotype of SmCavβ, we consistently observed a rapid run-down of Cav2.3 currents only in the presence of SmCavβ. This contrasts with previous studies, in which run-down appears to occur in the α1 subunit, independently of accessory subunits [29-31]. Down-regulation of Ca2+ currents caused by interaction of Cavβ subunits with intracellular proteins is well documented. For example, interaction of Cavβ subunits with small GTPases of the RGK family [32], with large GTPases of the dynamin family [33], or with the nuclear protein HP1 [34] results in down-regulation of Ca2+ currents. Our data represent the first case, to our knowledge, of down-regulation of Ca2+ currents by interactions between Cavβ subunits and chelated forms of ATP or Na+ ions, which are likely relevant to platyhelminth physiology. Unlike previous studies, in which hydrolysable forms of ATP suppress Ca2+ current run-down [35,36], here we show that two physiologically relevant, hydrolysable forms of ATP induce SmCavβ-mediated decrease in Cav2.3 activity. The possibility that run-down was caused by Mg2+ ions that dissociate from the ATP molecule was considered, but the fact that free Mg2+ dramatically suppressed Cav2.3 currents posed a significant challenge to explore this possibility. These results are reminiscent of the effects of Mg2+ ions at a similar concentration on L-type Cav channels, which occurs by their binding to a low-affinity site at the pore of the α 1 subunit [37]. Since ATP associates with Na+ ions under physiological conditions [38], we also measured run-down of Cav2.3/SmCavβ channels in the presence of intracellular Na+2-ATP. Because we detected significant run-down under these conditions, and knowing that the binding constant between Na+ ions and ATP is relatively low, about 13 M-1 (in contrast to 9000 M-1 for Mg2+, [39]), we hypothesized that Na+ ions that had dissociated from ATP were causing this run-down. Experiments using NaCl instead of Na+2-ATP confirmed this hypothesis.
Our study is not the first to show Cavβ-dependent run-down of non-L type Ca2+ current. The mammalian Cavβ subunit appears to enhance run-down of the non-L-type, Cav2.1 α1 subunit [40]. However, it is important to note that in that study, run-down occurred under the two-electrode voltage clamp configuration on Xenopus oocytes, where the connection between cytoplasm and plasma membrane remains largely intact. Therefore the mechanism(s) of run-down employed by the mammalian Cavβ subunit is likely to be distinct from that used by the schistosome Cavβ subunit.
Molecular substrate for SmCavβ-dependent Mg2+-ATP and Na+ sensitivity
Since the conventional SmCavβ subunit contains a string of acidic residues in the N-terminal domain that is not found in its mammalian counterparts, we generated a version of this subunit that lacked this region, to test whether this unique domain is the molecular substrate for this particular form of run-down. Deletion of this acidic, N-terminal fragment did not change the modulatory phenotype of SmCavβ on current amplitude, inactivation kinetics or steady-state inactivation. However, in contrast to our results with wild type SmCavβ, Cav2.3 currents did not run down when co-expressed with this mutated subunit, using Ca2+ as the charge carrier and in the presence of intracellular free Na+ or Mg2+-ATP. It seems likely that sensitivity to intracellular Mg2+-ATP and free Na+ resides in all or part of the string of acidic residues of the SmCavβ subunit.
Physiological relevance
Several reports have shown that the Ca2+ currents of platyhelminth muscle and nerve cells are very labile, running down within minutes or even seconds after establishing the whole-cell patch-clamp configuration. In previous work, the intracellular solution used to record voltage-gated Ca2+ currents from muscle cells of S. mansoni was titrated with NaOH to bring the pH to a physiological value [41]. We have empirically calculated that this action would bring the concentration of Na+ to approximately 19 mM, which is sufficient to reduce Ca2+ currents modulated by SmCavβ significantly, according to our data. Similarly, Ca2+ currents recorded from isolated muscle cells of the free-living flatworm Bdelloura candida run-down within 20 seconds of establishing the whole-cell configuration [42]: It is tempting to speculate that this run-down was caused by the relatively high concentration of NaCl (30 mM) added to the intracellular solution in these experiments.
Our previous studies have identified a different schistosome Cavβ subunit (SmCavβvar; [14]), which does not exhibit the hallmark action of Cavβ subunits, namely, to increase Ca2+ current density. Together with the data presented here, this raises the question of whether schistosomes employ unique strategies to modulate excitability via atypical modulation of HVA Cav channels, information that could be useful in the design of targeted therapies to treat schistosomiasis.
Conclusion
We have identified novel functions for a schistosome Cavβ subunit, namely to confer Ca2+ currents with sensitivity to intracellular Mg2+-ATP and Na+ ions, which translates into a reduced ability on the part of this Cavβ subunit to increase Ca2+ currents. We conclude that the molecular basis for this atypical sensitivity to both Mg2+-ATP and Na+ ions resides in a domain or domains located within the first forty-six amino acids of SmCavβ, which contains a string of twenty-two aspartic and glutamic acid residues not present in other Cavβ subunits.
Methods
Materials
Tissue culture dishes were purchased from Corning (NY, USA), Dulbecco's modified Eagle's media (DMEM) was purchased from Invitrogen, poly-L-lysine and ATP salts were purchased from Sigma. The transfection reagent, Tfx, was purchased from Promega. Restriction enzymes were from NEB, and oligonucleotide primers were from MWG Biotech.
Preparation of eukaryotic expression plasmids encoding SmCavβ
Using standard methods, we cloned all Cavβ subunits into the pXOOM vector [43], which is optimized for expression of inserts in mammalian cells (under control of a cytomegalovirus promoter), and contains the gene for green fluorescent protein (GFP) as a marker for transfection. For SmCavβ, the insert from the original SmCavβ clone in pCR4-TOPO (Invitrogen) was amplified using Phusion high-fidelity DNA polymerase (NEB). Primers were designed against the beginning and end of the coding regions of the sequence, and included appropriate restriction sites for insertion into pXOOM. The primers were: Forward: 5'-GGAAGCTTATGGCTGGTGATCGAGGATATTCA-3', which includes two G residues and a Hind III site at the 5' end; and Reverse: 5'-GGGCGGCCGCTTAAATCATGATTGAACCTTGACGA-3', which includes two G residues and a Not I site at the 5' end. Following an initial 98° denaturation for 30 seconds and 25 cycles of 98° for 10 s, 68° for 30 s, and 72° for 2 min, the reaction was purified over a QiaQuick spin column (Qiagen), and digested with Hind III and Not I. The digested band was gel-purified using Quantum Prep Freeze-n-Squeeze columns (BioRad), Pellet Paint (Novagen) was added as carrier, and the product was ethanol-precipitated and ligated to pXOOM that had been digested with Hind III and Not I and gel-purified. All constructs were sequenced to verify the absence of PCR errors.
Cell culture and transfection of HEK293-Cav2.3 cells
HEK293 cells stably transfected with Cav2.3d [44] were cultured in DMEM supplemented with L-glutamine, glucose and 10% foetal bovine serum in a humidified atmosphere (95%) at 5% CO2 and 37°C. Cells were used for up to 20 passages and were split every 2–4 days. For electrophysiological recordings, cells were seeded in Petri dishes coated with poly-l-lysine, and transfection of auxiliary β subunits was performed with Tfx on cells at a confluence of 50–60%, using 1 μg of the construct and a DNA: Tfx ratio of 1:2. Cells exhibiting green fluorescence were used for further study.
Construction of a N-terminal deletion mutant of the SmCavβ subunit
To generate a mutant subunit lacking amino acids 2–46 of the N terminus domain, a diluted sample of the SmCavβ clone was used as template for amplification of the truncated insert by PCR. The forward primer (5'-GGGGATCCATGGAAAATGCTCGTCAGGGAACGG-3') was designed to bind to the SmCavβ clone, starting from nucleotide 142. This forward primer contains a start codon, a Kozak sequence and a BamHI restriction site. The reverse primer was the same one used to amplify the full-length sequence. These PCR products were inserted into pXOOM and transformed into E. coli. Clones were sequenced to verify the deletion and to detect possible PCR errors.
Electrophysiology
Whole-cell recordings were obtained at room temperature 24 hours following transfection using an Axopatch 200B (Molecular Devices). Cell capacitance was 12–25 pF. Series resistance was compensated by 70%. Voltage pulses from -20 mV to +70 mV were delivered in 5 mV increments from a holding potential of -80 mV. Data were acquired at sampling intervals of 50 μs and filtered at 5 kHz during acquisition. The pipette solution contained (mM): cesium methane sulfonate (110), HEPES (10), EGTA (9), Mg2+-ATP (5); pH (CsOH) 7.3, with variations, as noted. The bath solution contained (mM): CaCl2 (10), TEA-Cl (160), HEPES (10), EGTA (0.1); pH (TEA-OH) 7.4. Patch pipettes were pulled from borosilicate glass and fire polished before each experiment. To ensure a fast dialysis of the intracellular compartment, only pipettes with resistances between 0.8 and 1.2 MΩ were used. Membrane seals were obtained by applying negative pressure. All experiments were performed at room temperature (22°C). The voltage-dependence of steady state inactivation was determined by measuring the peak current evoked with a depolarising pulse to elicit the maximum current as a function of the voltage of a preceding 1.5 s pre-pulse test (between -110 and +20 mV). Steady-state inactivation curves were fitted by a sigmoid (Boltzmann) distribution of the form:
| F (V) = Imax/{1 + exp [V0.5 - V)/K]} |
Where Imax is the maximal current, V is the pre-pulse voltage, K is the slope factor and V0.5 is the voltage at which inactivation is half-maximal.
To study inactivation as a function of time, the decaying phases of the inward currents evoked by a test pulse to +20 or +30 mV were fitted to a double exponential equation of the form: I (t) = I0 + I1 exp (-t/τ1) + I2 exp (-t/τ2), where τ1 and τ2 represent the fast and the slow time constants of inactivation, respectively, I1 and I2 represent the relative contribution of each component to inactivation and I0 is the offset. To assess the rate of recovery from inactivation from the closed state, a two-pulse protocol was used. Two test pulses to +20 or +30 mV were separated by a recovery step to -80, -100 or -120 mV for varying amounts of time (from 10 ms to 1 second). The length and voltage of the first test pulse was adjusted accordingly for each particular channel combination. For example, currents produced by Cav2.3 channels alone were maximal at approximately +30 mV and inactivated completely within 100 ms, whereas currents produced by Cav2.3/SmCavβ were maximal at approximately +20 mV and required several seconds to inactivate fully. In the latter case, recovery from inactivation was measured using Ba2+ instead of Ca2+ as the charge carrier (Ba2+ currents inactivate faster than Ca2+ currents produced by Cav2.3 [45]), thereby decreasing the need to use an excessively long depolarising first pulse protocol, which could compromise cell viability. The currents evoked by the second pulse of this double-pulse protocol were normalized to the currents produced by the first pulse and plotted against the duration of the inter-pulse interval.
Statistical analyses
Statistical comparisons were carried out using the Student t-test. Data are presented as means ± s.e.m. Number of repeats is indicated in parentheses.
Authors' contributions
VSR carried out the molecular and electrophysiological studies, made substantial contributions to conception and experimental design, and drafted the manuscript. TS provided the HEK cell line stably transfected with Cav2.3 and made a significant contribution to the interpretation of the data. RMG carried out molecular work and contributed to experimental design as well as to the writing of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We are grateful to Dr. Edward Perez-Reyes (Department of Pharmacology, University of Virginia, Charlottesville, Virginia 22904) for the donation of the mammalian Cavβ2a subunit.
This work was supported by NIH grant #s R01 AI-40522 and R01 AI-73660 to RMG and by NIH-NCRR grant # P41 RR001395 to the Biocurrents Research Center (BRC) at MBL.
Contributor Information
Vicenta Salvador-Recatalà, Email: vsalvador@mbl.edu.
Toni Schneider, Email: toni.schneider@uni-koeln.de.
Robert M Greenberg, Email: rgree@vet.upenn.edu.
References
- Fabiato A, Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol. 1979;41:473–484. doi: 10.1146/annurev.ph.41.030179.002353. [DOI] [PubMed] [Google Scholar]
- Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci. 2007;104:109–115. doi: 10.1254/jphs.CP0070027. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, et al. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchitel OD, Protti DA, Sanchez V, Cherksey BD, Sugimori M, Llinas R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci USA. 1992;89:3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Zamponi GW. Presynaptic Ca2+ channels – integration centers for neuronal signaling pathways. Trends Neurosci. 2006;29:617–624. doi: 10.1016/j.tins.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different beta subunits in the membrane expression of the alpha1A and alpha2 calcium channel subunits: studies using a depolarization-sensitive alpha1A antibody. Eur J Neurosci. 1997;9:749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Beta subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. doi: 10.1023/B:JOBB.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- Jeziorski MC, Greenberg RM, Anderson PA. Calcium channel beta subunits differentially modulate recovery of the channel from inactivation. FEBS Lett. 2000;483:125–130. doi: 10.1016/S0014-5793(00)02098-6. [DOI] [PubMed] [Google Scholar]
- Takahashi SX, Mittman S, Colecraft HM. Distinctive modulatory effects of five human auxiliary beta2 subunit splice variants on L-type calcium channel gating. Biophys J. 2003;84:3007–3021. doi: 10.1016/S0006-3495(03)70027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AB, Lea J, Roberts-Misterly JM, Anderson PA, Greenberg RM. Structure of three high voltage-activated calcium channel alpha1 subunits from Schistosoma mansoni. Parasitology. 2001;123:489–497. doi: 10.1017/S0031182001008691. [DOI] [PubMed] [Google Scholar]
- Kohn AB, Anderson PA, Roberts-Misterly JM, Greenberg RM. Schistosome calcium channel beta subunits. Unusual modulatory effects and potential role in the action of the antischistosomal drug praziquantel. J Biol Chem. 2001;276:36873–36876. doi: 10.1074/jbc.C100273200. [DOI] [PubMed] [Google Scholar]
- Jeziorski MC, Greenberg RM. Voltage-gated calcium channel subunits from platyhelminths: potential role in praziquantel action. Int J Parasitol. 2006;36:625–632. doi: 10.1016/j.ijpara.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AB, Roberts-Misterly JM, Anderson PA, Greenberg RM. Creation by mutagenesis of a mammalian Ca2+ channel beta subunit that confers praziquantel sensitivity to a mammalian Ca2+ channel. Int J Parasitol. 2003;33:1303–1308. doi: 10.1016/S0020-7519(03)00209-1. [DOI] [PubMed] [Google Scholar]
- Tareilus E, Roux M, Qin N, Qin N, Olcese R, Zhou J, Stefani E, Birnbaumer L. A Xenopus oocyte beta subunit: evidence for a role in the assembly/expression of voltage-gated calcium channels that is separate from its role as a regulatory subunit. Proc Natl Acad Sci USA. 1997;94:1703–1708. doi: 10.1073/pnas.94.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalie A, Ochi R, Pelzer D, Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflugers Arch. 1983;398:284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG. Intracellular perfusion of nerve cells and its effects on membrane currents. Physiol Rev. 1984;64:435–454. doi: 10.1152/physrev.1984.64.2.435. [DOI] [PubMed] [Google Scholar]
- McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Xu JJ, Hao LY, Kameyama A, Kameyama M. Calmodulin reverses rundown of L-type Ca2+ channels in guinea pig ventricular myocytes. Am J Physiol Cell Physiol. 2004;287:C1717–24. doi: 10.1152/ajpcell.00105.2004. [DOI] [PubMed] [Google Scholar]
- Olcese R, Qin N, Schneider T, Neely A, Wei X, Stefani E, Birnbaumer L. The amino terminus of a calcium channel β subunit sets rates of channel inactivation independently of the subunit's effect on activation. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- Takahashi SX, Mittman S, Colecraft HM. Distinctive modulatory effects of five human auxiliary β2 subunit splice variants on L-type calcium channel gating. Biophys J. 84:3007–3021. doi: 10.1016/S0006-3495(03)70027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry JB, Kobrinsky E, Abernethy DR, Soldatov NM. New short splice variants of the human cardiac Cavβ2 subunit: redefining the major functional motifs implemented in modulation of the Cav1.2 channel. J Biol Chem. 2004;279:46367–46372. doi: 10.1074/jbc.M409523200. [DOI] [PubMed] [Google Scholar]
- Vendel AC, Terry MD, Striegel AR, Iverson NM, Leuranguer V, Rithner CD, Lyons BA, Pickard GE, Tobet SA, Horne WA. Alternative splicing of the voltage-gated Ca2+ channel β4 subunit creates a uniquely folded N-terminal protein binding domain with cell-specific expression in the cerebellar cortex. J Neurosci. 2006;26:2635–2644. doi: 10.1523/JNEUROSCI.0067-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LP, Wei SK, Yue DT. Mechanism of auxiliary subunit modulation of neuronal alpha1E calcium channels. J Gen Physiol. 1998;112:125–143. doi: 10.1085/jgp.112.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereverzev A, Leroy J, Krieger A, Malécot CO, Hescheler J, Pfitzer G, Klöckner U, Schneider T. Alternate splicing in the cytosolic II–III loop and the carboxy terminus of human E-type voltage-gated Ca2+ channels: electrophysiological characterization of isoforms. Mol Cell Neurosci. 2002;21:352–365. doi: 10.1006/mcne.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hao LY, Kameyama A, Kuroki S, Nishimura S, Kameyama M. Run-down of L-type Ca2+ channels occurs on the alpha 1 subunit. Biochem Biophys Res Commun. 1998;247:844–850. doi: 10.1006/bbrc.1998.8886. [DOI] [PubMed] [Google Scholar]
- Zhen XG, Xie C, Yamada Y, Zhang Y, Doyle C, Yang J. A single amino acid mutation attenuates rundown of voltage-gated calcium channels. FEBS Lett. 2006;580:5733–5738. doi: 10.1016/j.febslet.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepplinger KJ, Förstner G, Kahr H, Leitner K, Pammer P, Groschner K, Soldatov NM, Romanin C. Molecular determinant for run-down of L-type Ca2+ channels localized in the carboxyl terminus of the 1C subunit. J Physiol. 2000;529:119–130. doi: 10.1111/j.1469-7793.2000.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin P, Nagashima K, Gonoi T, Shibasaki T, Takahashi K, Kashima Y, Ozaki N, Geering K, Iwanaga T, Seino S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gutierrez G, Miranda-Laferte E, Neely A, Hidalgo P. The Src homology 3 domain of the beta-subunit of voltage-gated calcium channels promotes endocytosis via dynamin interaction. J Biol Chem. 2007;282:2156–2162. doi: 10.1074/jbc.M609071200. [DOI] [PubMed] [Google Scholar]
- Hibino H, Pironkova R, Onwumere O, Rousset M, Charnet P, Hudspeth AJ, Lesage F. Direct interaction with a nuclear protein and regulation of gene silencing by a variant of the Ca2+-channel beta 4 subunit. Proc Natl Acad Sci USA. 2003;100:307–312. doi: 10.1073/pnas.0136791100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa K, Kameyama A, Yasui K, Li JM, Kameyama M. ATP regulates cardiac Ca2+ channel activity via a mechanism independent of protein phosphorylation. Pflugers Arch. 1997;433:557–562. doi: 10.1007/s004240050314. [DOI] [PubMed] [Google Scholar]
- Elhamdani A, Bossu JL, Feltz A. ADP exerts a protective effect against rundown of the Ca2+ current in bovine chromaffin cells. Pflugers Arch. 1995;430:401–409. doi: 10.1007/BF00373916. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Hess P. Block of the L-type Ca2+ channel pore by external and internal Mg2+ in rat phaeochromocytoma cells. J Physiol. 1993;466:683–706. [PMC free article] [PubMed] [Google Scholar]
- Melchior NC. Sodium and potassium complexes of adenosinetriphosphate: equilibrium studies. J Biol Chem. 1954;208:615–627. [PubMed] [Google Scholar]
- Wilson JE, Chin A. Chelation of divalent cations by ATP, studied by titration calorimetry. Anal Biochem. 1991;193:16–19. doi: 10.1016/0003-2697(91)90036-S. [DOI] [PubMed] [Google Scholar]
- De Waard M, Campbell KP. Subunit regulation of the neuronal alpha 1A Ca2+ channel expressed in Xenopus oocytes. J Physiol. 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett P, Day TA. Functional voltage-gated Ca2+ channels in muscle fibers of the platyhelminth Dugesia tigrina. Comp Biochem Physiol A Mol Integr Physiol. 2003;134:593–605. doi: 10.1016/S1095-6433(02)00350-1. [DOI] [PubMed] [Google Scholar]
- Blair KL, Anderson PAV. Physiological and pharmacological properties of muscle cells isolated from the flatworm Bdelloura candida (Tricladia) Parasitology. 1994;109:325–335. [Google Scholar]
- Jespersen T, Grunnet M, Angelo K, Klaerke DA, Olesen SP. Dual-function vector for protein expression in both mammalian cells and Xenopus laevis oocytes. BioTechniques. 2002;32:536–8. doi: 10.2144/02323st05. 540. [DOI] [PubMed] [Google Scholar]
- Mehrke G, Pereverzev A, Grabsch H, Hescheler J, Schneider T. Receptor-mediated modulation of recombinant neuronal class E calcium channels. FEBS Lett. 1997;408:261–270. doi: 10.1016/S0014-5793(97)00437-7. [DOI] [PubMed] [Google Scholar]
- Leroy J, Pereverzev A, Vajna R, Qin N, Pfitzer G, Hescheler J, Malécot CO, Schneider T, Klöckner U. Ca2+-sensitive regulation of E-type Ca2+ channel activity depends on an arginine-rich region in the cytosolic II–III loop. Eur J Neurosci. 2003;18:841–844. doi: 10.1046/j.1460-9568.2003.02819.x. [DOI] [PubMed] [Google Scholar]