Abstract
Magnesium inadequacy affects more than half of the U.S. population and is associated with increased risk for many age-related diseases, yet the underlying mechanisms are unknown. Altered cellular physiology has been demonstrated after acute exposure to severe magnesium deficiency, but few reports have addressed the consequences of long-term exposure to moderate magnesium deficiency in human cells. Therefore, IMR-90 human fibroblasts were continuously cultured in magnesium-deficient conditions to determine the long-term effects on the cells. These fibroblasts did not demonstrate differences in cellular viability or plating efficiency but did exhibit a decreased replicative lifespan in populations cultured in magnesium-deficient compared with standard media conditions, both at ambient (20% O2) and physiological (5% O2) oxygen tension. The growth rates for immortalized IMR-90 fibroblasts were not affected under the same conditions. IMR-90 fibroblast populations cultured in magnesium-deficient conditions had increased senescence-associated β-galactosidase activity and increased p16INK4a and p21WAF1 protein expression compared with cultures from standard media conditions. Telomere attrition was also accelerated in cell populations from magnesium-deficient cultures. Thus, the long-term consequence of inadequate magnesium availability in human fibroblast cultures was accelerated cellular senescence, which may be a mechanism through which chronic magnesium inadequacy could promote or exacerbate age-related disease.
Keywords: oxygen tension, telomeres, tumor suppressor
Magnesium is an essential micronutrient required for an extensive range of metabolic, regulatory, and structural activities. It is predominantly obtained from diet by eating green leafy vegetables and unprocessed grains (1). The typical U.S. diet has drifted away from these food sources in favor of more refined and often nutrient-poor food options, thus magnesium inadequacy has become quite common (2). Intake estimates generated by national surveys including the National Health and Nutrition Examination Survey (NHANES) have predominantly indicated that more than half of the U.S. population has magnesium intakes below the Estimated Average Requirement (EAR), a measure of population adequacy that is 2 SD below the Recommended Daily Allowance (3). Moreover, the incidence and severity of magnesium inadequacy is even greater in at-risk groups, including children, the poor, and the elderly (4, 5).
Despite growing appreciation of the prevalence of magnesium inadequacy, essentially no immediate clinical symptoms are known, due in part to the lack of robust biomarkers of magnesium status in vivo. However, there is a sizeable literature on the functional consequences linked with long-term magnesium inadequacy. Epidemiological data have associated increased risk of several aging-related diseases with chronic magnesium inadequacy, including cardiovascular disease, hypertension, diabetes, osteoporosis, and some cancers (1, 6–8). Numerous animal studies on magnesium inadequacy have supported these findings, along with additional morbidities including increased oxidative stress levels, altered calcium homeostasis, aberrant inflammatory response, diminished glucose sensitivity, seizures, and tetany (9–18).
Given the numerous functions of magnesium, a prolonged inadequacy would likely impair many metabolic pathways leading to decrements in cellular processes. Several studies on the effects of acute and severe magnesium deficiency on cells in culture have demonstrated reduced oxidative stress defense, cell cycle progression, culture growth, and cellular viability (13, 19–24, 60), whereas the expression of protooncogenes (e.g., c-fos, c-jun) and activation of transcription factors (e.g., NF-κB) were increased (25). Few studies have investigated the cellular consequences of long-term and moderate magnesium deficiency in normal human cells. Therefore, we tested human fibroblasts continuously cultured in magnesium-deficient conditions to determine the effects on cellular physiology. Both primary and immortalized cell types grew in magnesium-deficient conditions and demonstrated no loss of viability. However, primary human fibroblast populations appeared to undergo accelerated senescence as a function of available extracellular magnesium. This may be an important finding because several studies now indicate that cellular senescence in vivo can lead to tissue remodeling, resulting in tissue damage and a prooncogenic environment (26).
Results
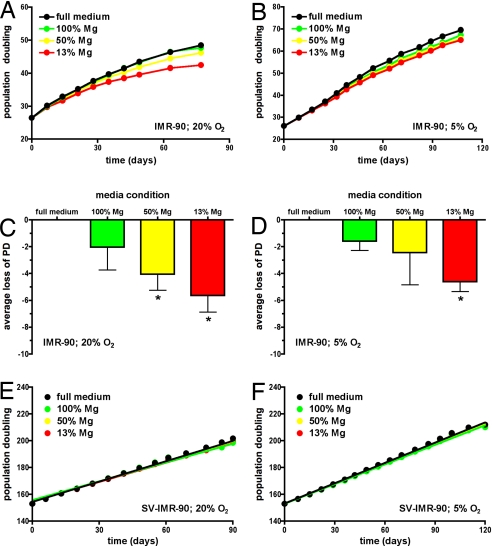
IMR-90 cells were thawed and continuously passaged in full or magnesium-deficient media that was repleted to ≈13% (0.1 mM), 50% (0.4 mM), or 100% (0.8 mM) of the magnesium level found in full culture medium; 0.8 mM is also within the clinical reference range for magnesium in normal human serum. The normal lifespan of IMR-90 populations under standard conditions is ≈3 months, with steady growth between 20 and 40 population doublings (PD), slowing between 40 and 50 PD, and finally, population senescence between 50 and 60 PD (Fig. 1A). Cell populations maintained in magnesium-deficient conditions demonstrated loss of PD compared with cell populations cultured in full medium (Fig. 1 A and C). The results from five independent lifespan studies indicated that populations cultured in 50% of normal Mg levels lost ≈4 PD, whereas populations cultured in 13% of normal Mg levels lost ≈6 PD. Loss of 6 PD is equivalent to a 10% shortening of population lifespan.
Fig. 1.
Long-term exposure to magnesium deficiency causes accelerated cellular senescence in primary but not immortalized IMR-90 cells. Cells were cultured in full medium (black circles) or magnesium-deficient media repleted to 13% (red circles), 50% (yellow circles), or 100% (green circles) of normal magnesium content. (A) Representative lifespan curves show change in PD over time cultured at ambient (20%) oxygen levels with data fit by point-to-point function. (B) Representative lifespan curves show change in PD over time cultured at physiologic (5%) oxygen levels with data fit by point-to-point function. (C) Average loss of PD (mean ± SD) for five independent lifespan curves from cells cultured at ambient (20%) oxygen levels is shown. The 99% confidence intervals were −1.46–5.54 for 100% Mg, 1.53–6.55 for 50% Mg, and 3.13–8.17 for 13% Mg conditions; asterisks indicate loss of PD values outside the 99% confidence interval of full medium condition. (D) Average loss of PD (mean ± SD) for four independent lifespan curves from cells cultured at physiologic (5%) oxygen levels is shown. The 99% confidence intervals were −0.43–3.61 for 100% Mg, −4.55–9.45 for 50% Mg, and 2.44–6.76 for 13% Mg conditions; asterisks indicate loss of PD values outside the 99% confidence interval of full medium condition. (E) Representative (n = 4) growth curves of SV-IMR-90 cells for comparable time of IMR-90 cells show change in PD over time cultured at ambient (20%) oxygen levels. Data for all conditions were fit to a linear regression function that converged with r2 > 0.98 and indicated that slopes for each condition were not statistically different. (F) Representative (n = 2) growth curves of SV-IMR-90 cells for comparable time of IMR-90 cells show change in PD over time cultured at physiologic (5%) oxygen levels. Data for all conditions were fit to a linear regression function that converged with r2 > 0.98 and indicated that slopes for each condition were not statistically different.
Ambient cell culture conditions expose cells to ≈20% O2, but this is hyperoxic compared with physiological oxygen levels that are thought to range between 1% and 6% O2 in vivo (27). Because it was possible that magnesium-deficient conditions sensitized cells to this preexisting stress, IMR-90 cells were cultured as described above, except at a more physiological oxygen tension of 5% O2. Reduced oxygen tension alone caused an extension in lifespan for IMR-90 populations by ≈1 additional month with senescence occurring between 70 and 80 PD (Fig. 1B), similar to published data from our laboratory (28). Yet continuous exposure to magnesium-deficient conditions still resulted in a loss of PD compared with cells cultured in full medium (Fig. 1 B and D). The results of four independent lifespan studies indicated that populations cultured in 50% of normal Mg levels lost ≈2.5 PD, whereas populations cultured in 13% of normal Mg levels lost ≈4.5 PD.
To determine whether the shortened population lifespan was due to cell loss, cells cultured in full and magnesium-deficient media were assessed for cellular viability and plating efficiency. Cell samples taken at points along the population lifespan did not demonstrate a significant difference in MTT reduction between cultures maintained in full or magnesium-deficient media, regardless of whether they were maintained in 20% or 5% O2 [supporting information (SI) Table S1]. Results from similar experiments measuring vital dye exclusion supported these findings (data not shown). Additionally, cell samples did not demonstrate significant differences in plated cell numbers between cultures maintained in full or magnesium-deficient media, regardless of whether they were maintained in 20% or 5% O2 (Table S2).
If the changes in lifespan induced by magnesium-deficient conditions depended on cellular aging pathways, then inactivation of these pathways should abrogate the effects of magnesium deficiency. All current evidence indicates that p53 and the retinoblastoma protein (pRb) tumor-suppressor pathways integrate and control cellular senescence (29, 30). Therefore, the effect of magnesium deficiency was measured in an immortalized line created from SV40-transformed IMR-90 cells (SV-IMR-90), which have inactivated p53 and pRb proteins (29, 31). SV-IMR-90 cells were continuously cultured in media with varying magnesium levels and oxygen tension for the same amount of time as the lifespan of primary IMR-90 populations. The results of four (at 20% O2) and two (at 5% O2) independent cell populations indicated that these immortalized cells demonstrated no significant reduction in cellular growth rates, even in the most magnesium-deficient conditions (Fig. 1 E and F).
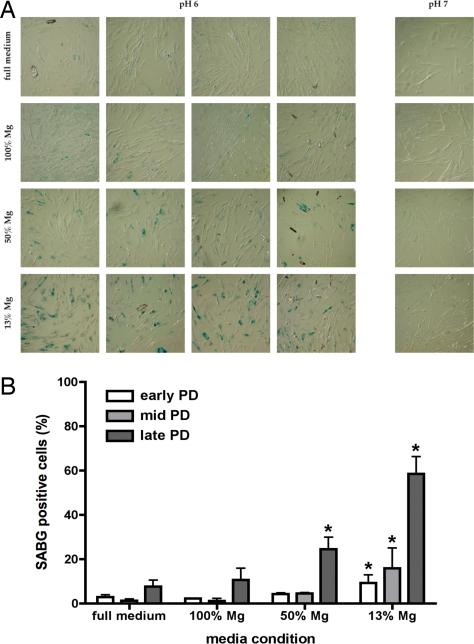
If the changes in lifespan induced by magnesium-deficient conditions were due to activation of cellular aging pathways, then expression of biomarkers of cellular senescence should also be accelerated. Therefore, senescence-associated β-galactosidase activity was measured in IMR-90 cells continuously cultured in full and magnesium-deficient media. In three independent cell populations, exposure to magnesium-deficient conditions resulted in an increased percentage of cells expressing senescence-associated β-galactosidase activity compared with cells cultured in full medium (Fig. 2 A and B). SV-IMR-90 cells were not tested because they are known not to have senescence-associated β-galactosidase activity (32).
Fig. 2.
Long-term exposure to magnesium deficiency causes greater expression of senescence-associated β-galactosidase activity in IMR-90 cells. Cells were cultured in full medium or magnesium-deficient media repleted to 13% (13% Mg), 50% (50% Mg), or 100% (100% Mg) of normal magnesium content at ambient oxygen levels. (A) Representative micrographs show senescence-associated β-galactosidase activity as a function of media condition in late PD IMR-90 cells; four replicates at pH 6 (senescence-associated β-galactosidase activity) and one control at pH 7 (negative control) are shown for each media condition. (B) Percentage of cells that are positive senescence-associated β-galactosidase activity in three independent IMR-90 populations from early, middle, and late PD are shown. Asterisks indicate significant elevation over full medium control (P < 0.05, one-way ANOVA, Dunnett's multiple comparison post hoc test).
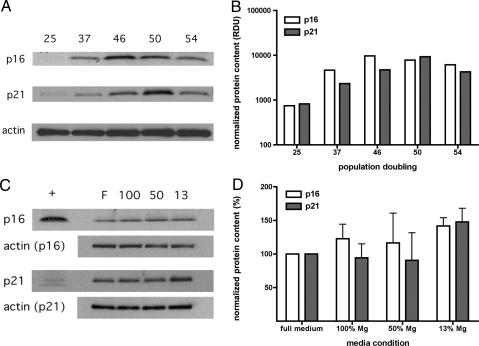
Additionally, activation of cellular aging (p53 and pRb) pathways by magnesium-deficient conditions should also increase the expression of proteins associated with cellular senescence, including p16INK4a and p21WAF1. These proteins are potent inhibitors of cyclin/cyclin-dependent kinase complexes that are known to be expressed in senescing populations of human fibroblasts (33–35). Therefore, expression levels of p16INK4a and p21WAF1 were measured in IMR-90 cells cultured in full and magnesium-deficient conditions in ambient oxygen from the middle of the population lifespan when expression of these proteins begins to become elevated in IMR-90 cells (Fig. 3 A and B). In two independent cell populations, continuous exposure to magnesium-deficient conditions resulted in increased p16INK4a and p21WAF1 levels compared with cells cultured in full medium (Fig. 3 C and D). SV-IMR-90 cells were not tested because the p53 and pRb pathways are inactivated (29, 30).
Fig. 3.
Long-term exposure to magnesium deficiency causes greater expression of senescence-associated protein expression in IMR-90 cells. Cells were cultured in different media conditions at ambient oxygen levels. (A) Autoradiographs show hybridization of p16INK4a, p21WAF1, or actin as a function of PD indicated across the top. (B) Expression levels of p16INK4a and p21WAF1 in relative densitometric units (RDU) were normalized to actin levels as a loading control, illustrating increased expression as a function of PD. (C) Representative autoradiographs show hybridization of p16INK4a, p21WAF1, or actin as a function of media condition in a population set with PD ranging from 40 to 45. Cells were cultured in full medium (F) or magnesium-deficient media repleted to 13% (13), 50% (50), or 100% (100) of normal magnesium content across the top; positive control peptide for antibody indicated by “+.” (D) Relative expression levels of p16INK4a and p21WAF1 expression were normalized to actin levels as a loading control and expressed as a percentage (mean ± SD, n = 2) of youngest PD (25), illustrating increased expression with decreasing magnesium condition.
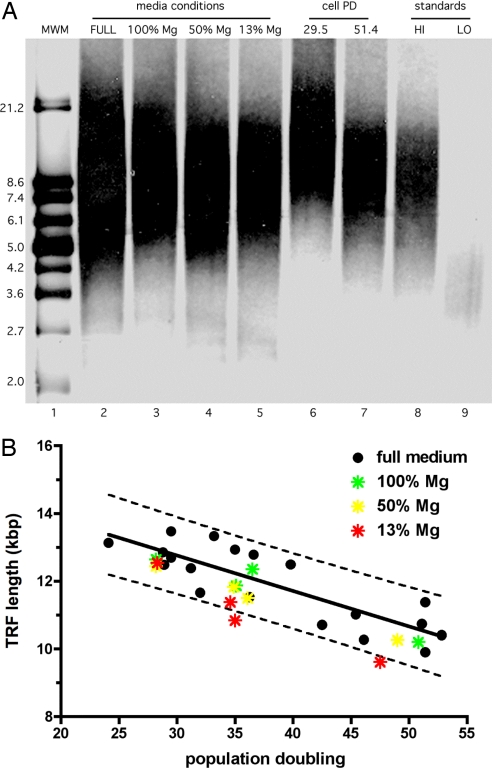
Telomere attrition rates play an important, although complex, role in the regulation of cellular senescence. The rate of telomere attrition in IMR-90 cells cultured in standard conditions was determined by analysis of mean telomere restriction fragment (TRF) of cells from varying PD cultured in full medium (Fig. 4 A and B). Telomere attrition was measured at 105 ± 17 bp lost per PD in ambient oxygen. Then, the average telomere length was measured in IMR-90 cells cultured in full or magnesium-deficient conditions in ambient oxygen from four independent cell populations at early, middle, and late points in the population lifespan. The mean TRF of cells cultured in magnesium-deficient conditions at middle and late points within the population lifespan fell outside the 99% confidence interval (data not shown) and the 90% prediction band (Fig. 4B) for normal telomere lengths in IMR-90 cells cultured in full conditions, suggesting higher levels of telomere attrition of cells in magnesium-deficient conditions. Preliminary data indicated that IMR-90s cultured in physiologic 5% oxygen had a similar telomere attrition rate (91 ± 10 bp/PD) and also demonstrated shorter telomeres in cultures maintained in magnesium-deficient compared with full media (data not shown).
Fig. 4.
Long-term exposure to magnesium deficiency causes accelerated telomere attrition in IMR-90 cells. Cells were cultured in different media conditions at ambient oxygen levels. (A) Representative autoradiograph shows mean TRF as a function of media condition (lanes 2–5) and PD (lanes 6–7) in IMR-90 cells. Cells were cultured in full medium (FULL) or magnesium-deficient media repleted to 13% (13% Mg), 50% (50% Mg), or 100% (100% Mg) of normal magnesium content (lanes 2–5) with PD ranging from 35 to 38. High (10.2 kbp) and low (3.9 kbp) TRF DNA standard was used as positive control for TRF quantitation. (B) The average telomere attrition rate derived from seven independent experiments with a wide range of PD (black circles) is shown; the solid line indicates fit to a linear regression function (r2 = 0.72) and the dotted line corresponds to a 90% prediction band. The attrition rate was determined at 105 ± 17 bp lost per population doubling. Four independent experiments using populations from magnesium-deficient media repleted to 13% (red stars), 50% (yellow stars), or 100% (green stars) at early, middle, and late PD are overlaid. Mean TRF from middle and late PD populations cultured in 10% Mg medium conditions were outside the 90% prediction band (dotted line).
Discussion
Continuous culture of primary fibroblasts in magnesium-deficient media resulted in a loss of replicative capacity with accelerated expression of senescence-associated biomarkers (Figs. 1–3). Several studies have reported alterations in cell physiology during magnesium deficiency, but exposure times ranged from hours to days, resulting in detection of acute responses to (typically severe) reductions in magnesium levels (19–24). Recently, Maier and colleagues have shown the development of senescence features in endothelial cell cultures maintained in low magnesium in just 3–5 days (60). In this study, cells were cultured throughout the complete population lifespan (3–4 months) in conditions where magnesium levels were only moderately reduced, as evidenced by the lack of viability and plating efficiency deficits. Moreover, intracellular magnesium levels were similar in cells from standard and magnesium-deficient conditions; no consistant loss in total magnesium content over time was detected (Fig. S1). This finding is similar to other reports showing that several human cell types maintained a constant intracellular magnesium level even when cultured in very high or very low extracellular magnesium (13, 36). However, it is also possible that some loss of magnesium did occur that was too small to detect, relative to the total magnesium content in the cell. Intracellular magnesium is divided among cytosolic, nuclear, endoplasmic reticulum, and mitochondrial compartments (13). Loss of magnesium from one cellular compartment would be difficult to detect by measuring only whole-cell magnesium content but may significantly affect cellular response. Additionally, even small changes in extracellular magnesium can result in significant alterations in the levels and distribution of free magnesium between cellular compartments that also influence cell phenotype (37, 38).
The lack of reduced cellular viability in IMR-90 populations in moderate magnesium deficiency was predicted from literature values. McKeehan and Ham (19) found that WI-38 human fibroblasts grew normally in reduced magnesium at or >0.2 mM extracellular magnesium for 14 days, and Sgambato et al. (21) found HC11 mouse epithelial cells grew normally in reduced magnesium ≥0.1 mM extracellular magnesium for 3 days. At lower magnesium levels, both studies found inhibition of cell cycle kinetics, activation of cyclin-dependent kinase inhibitor proteins, and reduced population growth rates. To avoid these complications, long-term exposure to less-severe magnesium depletion (≥0.1 mM) was studied. Although it was possible that low levels of cell loss still occurred, that alone could not account for the extent of PD loss observed in magnesium-deficient conditions. Theoretical population lifespan curves were constructed by using the PD equation log2(D/Do) and D and Do values measured in this study, but where D was reduced by a regular percentage at each passage. A reduction of 4–6 PD from control values required a 20–30% loss of cells at each passage (data not shown), yet this level of cell loss did not occur in this study. Therefore, an alternative explanation for the loss of PD was tested, namely that cellular senescence pathways were increasingly activated within cell populations maintained in magnesium deficiency.
Unlike primary cells, the growth of SV40-transformed, immortalized IMR-90 cells was not affected by moderate reductions in magnesium. SV40 transformation results in the inactivation of p53 and pRb (29, 30), suggesting that the site(s) at which magnesium deficiency affects cellular senescence occur(s) at or upstream of these cell-cycle checkpoints. However, SV40 transformation has also been reported to inhibit other transcriptional activators (e.g., p300 and CCAAT box-binding factor), so the effects of magnesium deficiency on other regulatory pathways cannot be ruled out (39, 40). Regardless, immortalization of IMR-90 cells resulted in tolerance to much lower magnesium concentrations than the originating primary cell type. This phenomenon has been shown in several different cells types. For example, McKeehan and Ham (19) found that SV40-transformed, immortalized WI-38 human fibroblasts could grow normally in media with <1% of standard magnesium levels, whereas primary WI-38 fibroblasts required at least 10% of standard magnesium levels for normal growth. Interestingly, the amount of calcium needed for normal proliferation was not different between the primary and immortalized cell types. Additionally, Ribeiro and Armelin (41) found reduced magnesium growth requirements in immortalized versus primary Swiss mouse 3T3 fibroblasts and Sgambato et al. (21) found a similar response in immortalized human MCF-7 versus primary mouse HC11 epithelial cells. Whether or not immortalized cells have a growth advantage during inadequate magnesium availability in vivo is completely unknown but may have clinical implications.
The molecular mechanisms that drive magnesium deficiency-induced cellular senescence are not clear at this time. One possible explanation is that exposure to magnesium-deficient conditions caused an increase in steady-state oxidative stress levels, because this is common among many micronutrient deficiencies (42, 43). Previous work showed that bovine endothelial cells cultured in magnesium-deficient media were more sensitive to oxidant challenge (20). Also, chicken hepatocytes cultured in magnesium-deficient media exhibited increased hydrogen peroxide and malondialdehyde levels and decreased catalase activity (24). Increased oxidative stress levels are known to promote accelerated cellular senescence. For example, we have previously shown that repeated administration of low-dose hydrogen peroxide shortened the population lifespan of IMR-90 cells and that antioxidants could reverse this effect (44, 45). However, any increase in oxidative stress resulting from magnesium deficiency would have to be modest, because no loss in cellular viability was observed.
If magnesium deficiency does result in increased oxidative stress, then telomeric DNA might be a sensitive target, because several reports have shown that telomeres are especially prone to oxidant damage (46, 47). We found that long-term culture in magnesium-deficient conditions did appear to increase telomere attrition in IMR-90 fibroblasts (Fig. 4). Although supplemental vitamin C, vitamin E, and nicotinamide have been shown to slow telomere shortening in cultured cell models (48–50), this report shows that a specific micronutrient deficiency resulted in shorter telomeres in human cells. However, the regulation of telomere attrition is likely more complex than simply responding to oxidative stress levels. Telomere attrition rates from IMR-90 cultures maintained in physiologic 5% oxygen (91 ± 10 bp/PD) were not significantly different from those maintained in ambient 20% oxygen (105 ± 17 bp/PD), which has a higher oxidant burden. A similar observation was reported by Wright et al. (51) in IMR-90 cultures. An alternative possibility is that magnesium deficiency directly affects telomere structure. One major function of intracellular magnesium is to stabilize the extensive negative charges within the chromatin for tight packing and correct secondary structure and to serve as protein cofactors for proper structural and catalytic activities (13). Alterations in magnesium levels might disrupt the DNA and/or proteins within the telomere supercomplex required for telomere capping, thereby promoting telomere attrition (52). However, no direct role for magnesium in maintaining telomere dynamics has yet been described. Moreover, it is unclear how magnesium deficiency affects intracellular magnesium homeostasis, especially the nuclear magnesium pool (13).
Reduced serum calcium levels (hypocalcemia) is one of the earliest clinical symptoms of magnesium inadequacy, which occurs even when dietary calcium is adequate and is unresponsive to calcium supplementation (1). Even moderate levels of magnesium inadequacy can significantly disrupt calcium homeostasis (38, 53). It is thought that secondary hypocalcemia results from the need to maintain a proper ratio of calcium to magnesium, which is critically important for homeostasis of both metal micronutrients (54). In fact, aberrant calcium/magnesium ratios are associated with disease states, including age-related neurodegeneration (55). The clinical reference ranges for human serum levels of calcium are 2.2–2.5 mM and for magnesium are 0.7–1.1 mM, making the normal calcium/magnesium ratio between 2 and 4. Standard DMEM/FBS-based media formulations provide calcium at 2.1 mM and magnesium at 0.8 mM, resulting in a calcium/magnesium ratio of 2.6, which is within the normal range for human serum. Because calcium levels were kept constant, the calcium/magnesium ratios were high in the magnesium-deficient conditions used in this study; the 50% magnesium condition (0.4 mM) had a ratio of 5.3 and the 13% magnesium condition (0.1 mM) had a ratio of 21.0. Therefore, the effects of magnesium deficiency on cellular senescence and telomere attrition in IMR-90 populations may be in part due to this abnormal calcium/magnesium ratio rather than reduced magnesium per se. Experiments are underway to evaluate this.
As previously described, a triage process has likely evolved within the cell to protect immediately essential functions during a micronutrient shortage at the expense of other processes (e.g., DNA repair) that are dispensable in the short term but lead to accelerated aging and late-onset disease (43). Accelerated senescence was reported in IMR-90 cells cultured in media deficient in other micronutrients including biotin (56). The results of this study suggest that the homeostatic response to magnesium deficiency may also carry negative consequences in IMR-90 cells. Magnesium is essential to a vast array of metabolic pathways. Because magnesium levels—like those of all micronutrients—are constantly in flux, homeostatic mechanisms must accommodate these variations in magnesium availability to maintain essential cell functions (e.g., ATP production) that are magnesium-dependent. The consequence of magnesium deficiency in these fibroblasts was accelerated senescence and increased telomere attrition rates. Although senescence and shortened telomeres are not immediately lethal, they do promote tissue remodeling that can lead to tissue aging and oncogenesis (26). Thus, given the prevalence of magnesium inadequacy in the population, the changes in cellular physiology demonstrated in this study may have significance.
Materials and Methods
Media and Materials.
Standard and custom-made magnesium-deficient DMEM were prepared and tested for osmolarity and sterility by University of California (San Francisco) Cell Culture Facility. The magnesium content in the magnesium-deficient DMEM was below detection, but preparation of complete medium by supplementation with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin resulted in adding magnesium to ≈13% of the level in standard DMEM. Complete full medium contained 0.8 ± 0.04 mM (n = 7) magnesium and the complete magnesium-deficient medium (called “13% Mg” condition) contained 0.1 ± 0.01 mM (n = 7) magnesium. For some conditions, magnesium was supplemented to 0.4 mM (“50% Mg” condition) or 0.8 mM (“100% Mg” condition) by using cell cultured-certified MgCl2 (Sigma–Aldrich) prepared in distilled water. The elemental content of each media batch was verified by elemental analysis before use (see below).
Cell Culture.
Primary and immortalized human diploid lung fibroblasts were purchased from the Coriell Institute of Medical Research. Primary IMR-90 cultures were obtained at ≈20 PD. Immortalized cells were generated from SV40 virus-transformed IMR-90 cultures (SV-IMR-90) were obtained at ≈120 PD and were 100% T antigen-positive. Cultures were grown in incubators at 37°C, >95% humidity, 5% CO2, and either 20% or 5% oxygen. Adherent cells were detached by using 0.05% trypsin and 0.5 mM EDTA for 5 min at 37°C and then processed to single-cell suspension in PBS without calcium or magnesium. Replicate cells counts were measured on a Z2 Coulter Counter. Replicative age was determined in PD calculated as log2(D/Do) where D and Do are density at time of harvest and seeding, respectively (45).
Senescence-Associated β-Galactosidase Activity.
A cellular senescence marker assay was performed as described by Dimri and Campisi (57). Briefly, cells were fixed with 2% formaldehyde/0.2% glutaraldehyde in PBS and then exposed to 1 mg/ml X-Gal in an iron buffer at pH 6. Dark blue precipitate forms in older cells proportionally to approaching senescence. Replicate wells were also stained at pH 7 as a negative control to show specificity of activity, and care was taken to avoid artifacts caused by overconfluent or quiescent cultures (58). Positively staining cells were expressed as percentage of total cells in independent wells.
Elemental Analysis.
The magnesium content of media and cells was determined by inductively coupled plasma atomic-emission spectrometry (ICP) (59). Cell samples were washed with PBS and pelleted by centrifugation. Media samples and cell pellets were dissolved in OmniTrace 70% HNO3 (VWR Scientific) and then diluted with OmniTrace water (VWR Scientific) to 5% HNO3 before introduction via pneumatic nebulizer into a Vista Pro ICP (Varian). Elemental values were calibrated by using National Institute of Standards and Technology (NIST)-traceable standards and validated by using NIST bovine liver reference material 1577b. The minimum detection limit for magnesium was 5 μg/liter. The coefficient of variation (CV) of intraassay precision for magnesium was 4.2% (n = 10 in one run) and interassay precision for magnesium was 3.0% (seven independent runs) for the reference material. Cesium (50 mg/liter) was used for ionization suppression and yttrium (5 mg/liter) was used as an internal standard. All reagents and plasticware were certified or routinely tested for trace metal work. Data were collected and analyzed by using native software (ICP Expert).
Western Blot Analysis.
Protein expression was determined by Western blot analysis. Cell pellets were washed with PBS, homogenized in 50 mM Tris (pH 7.4) containing 1% SDS, 10 mM EDTA, 2 mM DTT, 1 mM PMSF, and protease inhibitor mixture at the manufacturer's recommended concentrations. The protein concentrations were measured by the bicinchoninic acid assay (Pierce) according to the manufacturer's instructions. Protein samples were separated by SDS/PAGE and then transferred to Immobilon-P membranes (Millipore). The Western blot analysis was performed with primary antibodies to p16INK4a and p21WAF1 (Lab Vision), β-actin (Sigma–Aldrich), and HRP-conjugated secondary antibodies (Santa Cruz Biotechnology). Protein levels were detected by chemiluminescence and exposure to film.
TRF Analysis.
Quantitation of telomere attrition was assessed by TRF analysis using the TeloTAGG kit according to the manufacturer's instructions (Roche). Briefly, genomic DNA was isolated by using the DNeasy Tissue kit (Qiagen) and quantitated by using UV spectrophotometry. One microgram of DNA was then restricted by using 20 units of HinfI and 20 units of RsaI for 2 h at 37°C and then separated by electrophoresis in 0.8% agarose at 5 vol/cm. DNA was transferred to nylon membrane by vacuum transfer and fixed by UV cross-linking. The membrane-bound DNA was then hybridized with an oligonucleotide probe complementary to human telomeric sequence and labeled with digoxigenin. The labeled DNA was hybridized with an anti-digoxigenin antibody coupled to alkaline phosphatase and subsequently incubated with appropriate chemiluminescent substrate. Chemiluminescence signal was detected by exposure to film. Mean TRF length was determined by using by least-squares method regression analysis using DNA size standards labeled with alkaline phosphatase.
Postprocessing and Statistics.
Images from microscopy were captured by digital camera, and images from film were scanned into digital files. Images were aligned and adjusted for brightness/contrast/γ-levels by using Photoshop software (Adobe Systems). Densitometric measurements of protein bands were quantitated from film by using Image J software. Densitometric measurements of mean TRF were quantitated from film by using Alpha Innotech Imager with AlphaEase FC software package. Graphing, regression, and statistical analysis were performed by using Prism software (GraphPad).
Supplementary Material
Acknowledgments.
We thank Benjamin Huang, Stella Sarmiento, and Anureet Tiwana for technical assistance. This work was supported by National Foundation for Cancer Research Grant M2661, National Center on Minority Health and Health Disparities Grant P60-MD00222-01, and the Bruce and Giovanna Ames Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712401105/DCSupplemental.
References
- 1.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: Natl Acad Press; 1997. pp. 190–249. [Google Scholar]
- 2.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133:2879–2882. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 3.Moshfegh A, Goldman J, Cleveland L. What We Eat in America, NHANES 2001–2002: Usual Nutrient Intakes from Food Compared to Dietary Reference Intakes. Washington, DC: US Department of Agriculture, Agriculture Research Service; 2005. [Google Scholar]
- 4.Bowman S. Low economic status is associated with suboptimal intakes of nutritious foods by adults in the National Health and Nutrition Examination Survey 1999–2002. Nutr Res. 2007;27:513–523. [Google Scholar]
- 5.Kant AK, Graubard BI. Ethnicity is an independent correlate of biomarkers of micronutrient intake and status in American adults. J Nutr. 2007;137:2456–2463. doi: 10.1093/jn/137.11.2456. [DOI] [PubMed] [Google Scholar]
- 6.Hartwig A. Role of magnesium in genomic stability. Mutat Res. 2001;475:113–121. doi: 10.1016/s0027-5107(01)00074-4. [DOI] [PubMed] [Google Scholar]
- 7.Larsson SC, Bergkvist L, Wolk A. Magnesium intake in relation to risk of colorectal cancer in women. J Am Med Assoc. 2005;293:86–89. doi: 10.1001/jama.293.1.86. [DOI] [PubMed] [Google Scholar]
- 8.Barbagallo M, Dominguez LJ, Resnick LM. Magnesium metabolism in hypertension and type 2 diabetes mellitus. Am J Ther. 2007;14:375–385. doi: 10.1097/01.mjt.0000209676.91582.46. [DOI] [PubMed] [Google Scholar]
- 9.Turlapaty PD, Altura BM. Extracellular magnesium ions control calcium exchange and content of vascular smooth muscle. Eur J Pharmacol. 1978;52:421–423. doi: 10.1016/0014-2999(78)90303-5. [DOI] [PubMed] [Google Scholar]
- 10.Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T. Magnesium deficiency and hypertension: correlation between magnesium-deficient diets and microcirculatory changes in situ. Science. 1984;223:1315–1317. doi: 10.1126/science.6701524. [DOI] [PubMed] [Google Scholar]
- 11.Goto Y, Nakamura M, Abe S, Kato M, Fukui M. Physiological correlates of abnormal behaviors in magnesium-deficient rats. Epilepsy Res. 1993;15:81–89. doi: 10.1016/0920-1211(93)90089-p. [DOI] [PubMed] [Google Scholar]
- 12.Altura BM, Altura BT. Magnesium and cardiovascular biology: An important link between cardiovascular risk factors and atherogenesis. Cell Mol Biol Res. 1995;41:347–359. [PubMed] [Google Scholar]
- 13.Wolf FI, Torsello A, Fasanella S, Cittadini A. Cell physiology of magnesium. Mol Aspects Med. 2003;24:11–26. doi: 10.1016/s0098-2997(02)00088-2. [DOI] [PubMed] [Google Scholar]
- 14.Rude RK, Gruber HE. Magnesium deficiency and osteoporosis: animal and human observations. J Nutr Biochem. 2004;15:710–716. doi: 10.1016/j.jnutbio.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Blache D, et al. Long-term moderate magnesium-deficient diet shows relationships between blood pressure, inflammation and oxidant stress defense in aging rats. Free Radic Biol Med. 2006;41:277–284. doi: 10.1016/j.freeradbiomed.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 16.He K, Song Y, Belin RJ, Chen Y. Magnesium intake and the metabolic syndrome: Epidemiologic evidence to date. J Cardiometab Syndr. 2006;1:351–355. doi: 10.1111/j.1559-4564.2006.05702.x. [DOI] [PubMed] [Google Scholar]
- 17.Rude RK, et al. Reduction of dietary magnesium by only 50% in the rat disrupts bone and mineral metabolism. Osteoporos Int. 2006;17:1022–1032. doi: 10.1007/s00198-006-0104-3. [DOI] [PubMed] [Google Scholar]
- 18.Mazur A, et al. Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys. 2007;458:48–56. doi: 10.1016/j.abb.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 19.McKeehan WL, Ham RG. Calcium and magnesium ions and the regulation of multiplication in normal and transformed cells. Nature. 1978;275:756–758. doi: 10.1038/275756a0. [DOI] [PubMed] [Google Scholar]
- 20.Dickens BF, Weglicki WB, Li YS, Mak IT. Magnesium deficiency in vitro enhances free radical-induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS Lett. 1992;311:187–191. doi: 10.1016/0014-5793(92)81098-7. [DOI] [PubMed] [Google Scholar]
- 21.Sgambato A, Wolf FI, Faraglia B, Cittadini A. Magnesium depletion causes growth inhibition, reduced expression of cyclin D1, and increased expression of P27Kip1 in normal but not in transformed mammary epithelial cells. J Cell Physiol. 1999;180:245–254. doi: 10.1002/(SICI)1097-4652(199908)180:2<245::AID-JCP12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Maier JA, Malpuech-Brugere C, Zimowska W, Rayssiguier Y, Mazur A. Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta. 2004;1689:13–21. doi: 10.1016/j.bbadis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Martin H, et al. N-acetylcysteine partially reverses oxidative stress and apoptosis exacerbated by Mg-deficiency culturing conditions in primary cultures of rat and human hepatocytes. J Am Coll Nutr. 2006;25:363–369. doi: 10.1080/07315724.2006.10719547. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, et al. Magnesium deficiency enhances hydrogen peroxide production and oxidative damage in chick embryo hepatocyte in vitro. Biometals. 2006;19:71–81. doi: 10.1007/s10534-005-6898-1. [DOI] [PubMed] [Google Scholar]
- 25.Altura BM, et al. Expression of the nuclear factor-kappaB and proto-oncogenes c-fos and c-jun are induced by low extracellular Mg2+ in aortic and cerebral vascular smooth muscle cells: possible links to hypertension, atherogenesis, and stroke. Am J Hypertens. 2003;16:701–707. doi: 10.1016/s0895-7061(03)00987-7. [DOI] [PubMed] [Google Scholar]
- 26.Campisi J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Guyton A, Hall J. Textbook of Medical Physiology. Philadelphia, PA: Saunders; 1966. pp. 513–523. [Google Scholar]
- 28.Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci USA. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- 30.Hara E, Tsurui H, Shinozaki A, Nakada S, Oda K. Cooperative effect of antisense-Rb and antisense-p53 oligomers on the extension of life span in human diploid fibroblasts, TIG-1. Biochem Biophys Res Commun. 1991;179:528–534. doi: 10.1016/0006-291x(91)91403-y. [DOI] [PubMed] [Google Scholar]
- 31.Itahana K, Dimri G, Campisi J. Regulation of cellular senescence by p53. Eur J Biochem. 2001;268:2784–2791. doi: 10.1046/j.1432-1327.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 32.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcorta DA, et al. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McConnell BB, Starborg M, Brookes S, Peters G. Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr Biol. 1998;8:351–354. doi: 10.1016/s0960-9822(98)70137-x. [DOI] [PubMed] [Google Scholar]
- 35.Stein GH, Drullinger LF, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romani A, Marfella C, Scarpa A. Regulation of magnesium uptake and release in the heart and in isolated ventricular myocytes. Circ Res. 1993;72:1139–1148. doi: 10.1161/01.res.72.6.1139. [DOI] [PubMed] [Google Scholar]
- 37.Altura BM, Zhang A, Altura BT. Magnesium, hypertensive vascular diseases, atherogenesis, subcellular compartmentation of Ca2+ and Mg2+ and vascular contractility. Miner Electrolyte Metab. 1993;19:323–336. [PubMed] [Google Scholar]
- 38.Altura BM, Zhang A, Cheng TP, Altura BT. Extracellular magnesium regulates nuclear and perinuclear free ionized calcium in cerebral vascular smooth muscle cells: Possible relation to alcohol and central nervous system injury. Alcohol. 2001;23:83–90. doi: 10.1016/s0741-8329(00)00131-2. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Campisi J, Padmanabhan R. SV40 large T antigen transactivates the human cdc2 promoter by inducing a CCAAT box binding factor. J Biol Chem. 1996;271:13959–13967. [PubMed] [Google Scholar]
- 40.Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- 41.Ribeiro SM, Armelin HA. Ca2+ and Mg2+ requirements for growth are not concomitantly reduced during cell transformation. Mol Cell Biochem. 1984;59:173–181. doi: 10.1007/BF00231313. [DOI] [PubMed] [Google Scholar]
- 42.Ames BN, Atamna H, Killilea DW. Mineral and vitamin deficiencies can accelerate the mitochondrial decay of aging. Mol Aspects Med. 2005;26:363–378. doi: 10.1016/j.mam.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci USA. 2006;103:17589–17594. doi: 10.1073/pnas.0608757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen QM, et al. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J. 1998;332:43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Killilea DW, Atamna H, Liao C, Ames BN. Iron accumulation during cellular senescence in human fibroblasts in vitro. Antioxid Redox Signal. 2003;5:507–516. doi: 10.1089/152308603770310158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Zglinicki T, Pilger R, Sitte N. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic Biol Med. 2000;28:64–74. doi: 10.1016/s0891-5849(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 47.Rubio MA, Davalos AR, Campisi J. Telomere length mediates the effects of telomerase on the cellular response to genotoxic stress. Exp Cell Res. 2004;298:17–27. doi: 10.1016/j.yexcr.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Furumoto K, Inoue E, Nagao N, Hiyama E, Miwa N. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 1998;63:935–948. doi: 10.1016/s0024-3205(98)00351-8. [DOI] [PubMed] [Google Scholar]
- 49.Kang HT, Lee HI, Hwang ES. Nicotinamide extends replicative lifespan of human cells. Aging Cell. 2006;5:423–436. doi: 10.1111/j.1474-9726.2006.00234.x. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka Y, Moritoh Y, Miwa N. Age-dependent telomere-shortening is repressed by phosphorylated alpha-tocopherol together with cellular longevity and intracellular oxidative-stress reduction in human brain microvascular endotheliocytes. J Cell Biochem. 2007;102:689–703. doi: 10.1002/jcb.21322. [DOI] [PubMed] [Google Scholar]
- 51.Forsyth N, Evans A, Shay J, Wright W. Developmental differences in the immortalization of lung fibroblasts by telomerase. Aging Cell. 2003;2:235–243. doi: 10.1046/j.1474-9728.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 52.Gilley D, Tanaka H, Herbert BS. Telomere dysfunction in aging and cancer. Int J Biochem Cell Biol. 2005;37:1000–1013. doi: 10.1016/j.biocel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Nielsen FH, Milne DB, Gallagher S, Johnson L, Hoverson B. Moderate magnesium deprivation results in calcium retention and altered potassium and phosphorus excretion by postmenopausal women. Magnes Res. 2007;20:19–31. [PubMed] [Google Scholar]
- 54.George GA, Heaton FW. Changes in cellular composition during magnesium deficiency. Biochem J. 1975;152:609–615. doi: 10.1042/bj1520609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemke MR. Plasma magnesium decrease and altered calcium/magnesium ratio in severe dementia of the Alzheimer type. Biol Psychiatry. 1995;37:341–343. doi: 10.1016/0006-3223(94)00241-T. [DOI] [PubMed] [Google Scholar]
- 56.Atamna H, Newberry J, Erlitzki R, Schultz CS, Ames BN. Biotin deficiency inhibits heme synthesis and impairs mitochondria in human lung fibroblasts. J Nutr. 2007;137:25–30. doi: 10.1093/jn/137.1.25. [DOI] [PubMed] [Google Scholar]
- 57.Dimri GP, Campisi J. Molecular and cell biology of replicative senescence. Cold Spring Harb Symp Quant Biol. 1994;59:67–73. doi: 10.1101/sqb.1994.059.01.010. [DOI] [PubMed] [Google Scholar]
- 58.Yang NC, Hu ML. The limitations and validities of senescence associated-beta-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp Gerontol. 2005;40:813–819. doi: 10.1016/j.exger.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 59.Killilea AN, Downing KH, Killilea DW. Zinc deficiency reduces paclitaxel efficacy in LNCaP prostate cancer cells. Cancer Lett. 2007;258:70–79. doi: 10.1016/j.canlet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 60.Ferre S, Mazur A, Maier JAM. Low magnesium induces senescent features in cultured human endothelial cells. Magnes Res. 2007;20:66–71. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.