Abstract
ATP is a major chemical transmitter in purinergic signal transmission. Before secretion, ATP is stored in secretory vesicles found in purinergic cells. Although the presence of active transport mechanisms for ATP has been postulated for a long time, the proteins responsible for its vesicular accumulation remains unknown. The transporter encoded by the human and mouse SLC17A9 gene, a novel member of an anion transporter family, was predominantly expressed in the brain and adrenal gland. The mouse and bovine counterparts were associated with adrenal chromaffin granules. Proteoliposomes containing purified transporter actively took up ATP, ADP, and GTP by using membrane potential as the driving force. The uptake properties of the reconstituted transporter were similar to that of the ATP uptake by synaptic vesicles and chromaffin granules. Suppression of endogenous SLC17A9 expression in PC12 cells decreased exocytosis of ATP. These findings strongly suggest that SLC17A9 protein is a vesicular nucleotide transporter and should lead to the elucidation of the molecular mechanism of ATP secretion in purinergic signal transmission.
Keywords: ATP, chromaffin granule, purinergic signaling, storage and exocytosis, synaptic vesicle
Vesicular storage and subsequent exocytosis of neurotransmitters is essential for chemical transmission in neurons and endocrine cells. Thus far four distinct classes of transporters are known to participate in the uptake of neurotransmitters into neuronal synaptic vesicles and secretory granules in endocrine cells. These are vesicular monoamine transporters, vesicular acetylcholine transporters, vesicular inhibitory amino acid transporters, and vesicular glutamate transporters (VGLUTs) (1–7). These vesicular transporters mediate the active accumulation of their respective neurotransmitters through an electrochemical gradient of protons across the membrane generated by vacuolar proton-ATPase. ATP is stored in secretory vesicles and subsequently exocytosed, which leads to the various purinergic responses, such as central control of autonomic functions, pain and mechanosensory transduction, neural-glial interactions, control of vessel tone and angiogenesis, and platelet aggregation through purinoceptors, thus establishing its role as a chemical transmitter (8–12). Because the concentration of nucleotides in the vesicles was maintained at ≈0.1–1 M, an active transport mechanisms to accumulate nucleotides has been postulated (8–18). Although evidence increasingly supports the presence of a vesicular ATP transporters in secretory vesicles such as synaptic vesicles and adrenal chromaffin granules (13–19), the protein responsible for the ATP accumulation has not yet been identified.
Here, we report the expression and function of human and mouse SLC17A9, an isoform of the SLC17 phosphate transporter family. We present evidence that the SLC17A9 protein acts as a vesicular nucleotide transporter (VNUT) and that it plays an essential role in vesicular storage of ATP in the ATP-secreting cells.
Results and Discussion
Gene Organization and Expression of SLC17A9.
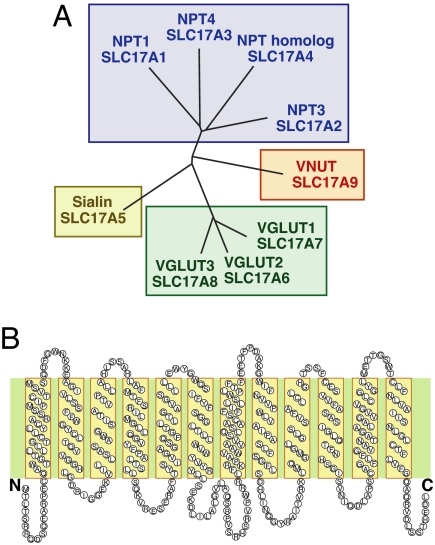
SLC17 is a type I phosphate transporter family consisting of eight genes that are classified into three distinct subfamilies: (i) SLC17A1–4 (NPT1, NPT3, NPT4, and Na+/PO42−cotransporter homologue), Na+ and inorganic phosphate cotransporters, (ii) SLC17A5 (sialin), a lysosomal H+/sialic acid cotransporter, and (iii) SLC17A6–8, VGLUT (5). We found that a fourth subfamily, tentatively designated SLC17A9, was identified in a genome data bank screening (Fig. 1A). SLC17A9 was located on chromosome 20 and contained 14 exons and 13 introns [supporting information (SI) Fig. S1]. The SLC17A9 protein was 430 aa residues long with 12 putative transmembrane helices with ≈23–29% identity and 41–48% similarity to that of other SLC17 members (Fig. 1B and Fig. S2). Orthologues of other mammalian species have been identified. Although amino acid sequences of the amino-terminal region in mammals exhibited species-specific variation, the remaining regions showed the relatively well conserved features (83% identity) (Fig. S3).
Fig. 1.
Phylogenetic tree of SLC17 family (A) and putative secondary structure of SLC17A9 protein (B).
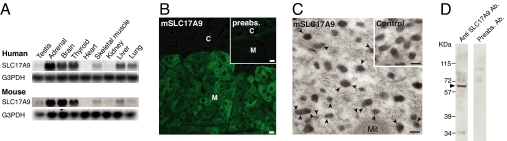
We cloned cDNA-encoded SLC17A9 (Fig. S2). Northern blot analysis indicated that the SLC17A9 and mouse counterpart (mSLC17A9) were widely expressed in various organs, but predominantly in the adrenal gland, brain, and thyroid gland (Fig. 2A). In mouse adrenal gland, the mSLC17A9 protein was specifically expressed in the medulla and was associated with chromaffin granules (Fig. 2 B and C). The presence of a SLC17A9 counterpart in bovine adrenal chromaffin granule membrane has been confirmed (Fig. 2D).
Fig. 2.
Expression of SLC17A9 and its association with chromaffin granules. (A) Northern blotting showing expression of SLC17A9 in human and mouse brain and adrenal gland. Expression of G3PDH is also shown as a control. (B) Immunohistochemical localization of SLC17A9 protein in mouse adrenal gland. (Inset) Shown is control staining with preabsorbed antibodies. C, cortex; M, medulla. (C) Immunoelectronmicroscopy showing that SLC17A9 proteins (gold particles) are associated with chromaffin granules. (Inset) Shown is background labeling with control serum. Mit, mitochondrion. (D) Western blot indicates the presence of a SLC17A9 counterpart in the membrane of bovine adrenal chromaffin granule with a relative mobility of 61 kDa. The preabsorbed antibodies did not bind to the protein. The positions of marker proteins are indicated on the left. (Scale bars: B, 10 μm; C, 100 nm.)
SLC17A9 Protein Is an ATP Transporter.
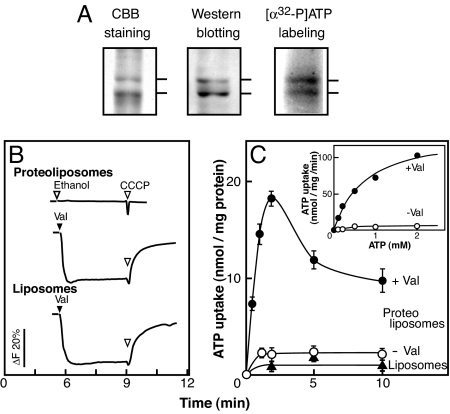
We hypothesized that SLC17A9 is responsible for the storage of nucleotides in chromaffin granules, in which high concentrations of ATP and other nucleotides (≈0.2 M) are accumulated in a membrane potential (Δψ)-dependent manner (8, 13, 16). To test this working hypothesis, the SLC17A9 protein was expressed in High Five cells, solubilized from the membranes, and purified by Ni-NTA column chromatography (Fig. 3A). The purified fraction showed two major protein bands (68 and 63 kDa) on SDS polyacrylamide gel, which corresponded to SLC17A9 protein because of their immunological reactivity to anti-SLC17A9 antibodies and ability to bind ATP upon UV light illumination (Fig. 3A). The observation of two SLC17A9 protein bands upon PAGE could have been caused by conformational differences or modification.
Fig. 3.
Purification and reconstitution of SLC17A9 protein. (A) Purification. (Left) Purified fraction (7 μg protein) was analyzed in an 11% PAGE in the presence of SDS and visualized by Coomassie brilliant blue staining. (Center) A duplicate gel was analyzed by Western blotting with anti-SLC17A9 antibodies. (Right) Labeling of SLC17A9 protein upon UV light illumination with [α-32P]ATP. (B) Formation of Δψ (positive inside) was measured by oxonol-V fluorescence quenching. Proteoliposomes (0.5 μg protein) or liposomes (20 μg lipid) containing trapped Na+ were suspended in buffer containing 0.15 M K-acetate plus 4 mM KCl and 1 μM oxonol-V, and fluorescence quenching was measured. Final concentration of valinomycin and carbonylcyanide m-chlorophenylhydrazone (CCCP) were added at 1 μM. (C) Time course of [α-32P]ATP uptake. Na+-trapped proteoliposomes or liposomes were suspended as above in the presence or absence of valinomycin. Upon the addition of [α-32P]ATP, samples were taken at the indicated times, and radioactivity taken up by the proteoliposomes was counted. (Inset) Dose dependence of ATP uptake.
We then reconstituted SLC17A9 protein into liposomes and investigated whether or not the proteoliposomes took up [α-32P]ATP. When an internal inside positive Δψ was established, such as a K+-diffusion potential through the addition of valinomycin, the proteoliposomes took up [α-32P]ATP in a time-dependent manner (Fig. 3 B and C and Table S1). ATP uptake was not observed in liposomes that did not contain SLC17A9 protein even when Δψ was already established (Fig. 3C). In contrast, artificially imposing pH gradient (ΔpH) did not evoke the ATP uptake (Table S1). The valinomycin-evoked ATP uptake exhibited dose dependence with Km and Vmax values of 0.8 mM, and 138 nmol/min of mg per protein, respectively (Fig. 3C). These results demonstrated that the SLC17A9 protein was an active transporter of ATP and used Δψ (positive inside) but not ΔpH (acidic inside) as the driving force.
Characterization of Nucleotide Transport.
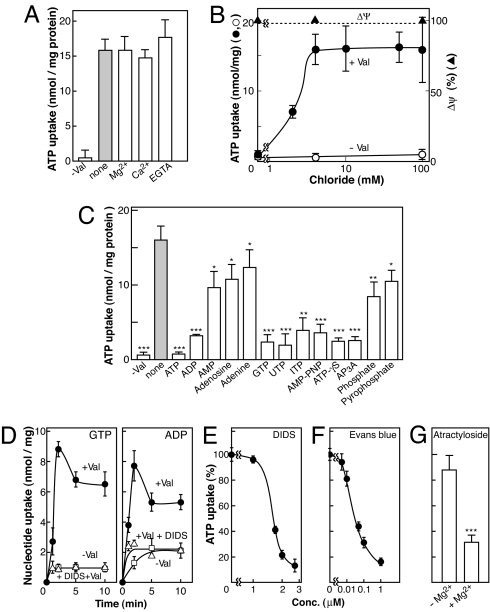
We further characterized the ATP uptake by SLC17A9 protein. Because ATP is a trivalent anion at the physiological pH and behaves as a monovalent anion upon chelation with divalent cations such as Mg2+, we asked which forms were recognized as transport substrates. We found that Mg2+ and Ca2+ did not affect the ATP uptake (Fig. 4A), suggesting that this transporter recognized ATP as a substrate irrespective of its charge status. Cl− dependence on the ATP uptake is of interest because VGLUT, another subfamily of SLC17, absolutely requires Cl− for transport activity (20), and Cl− stimulates ATP uptake in chromaffin granule membrane vesicles (16). As expected, the presence of Cl− was an absolute requirement for ATP transport activity in the SLC17A9 protein (Fig. 4B). The transport activity reached steady state at ≈4 mM Cl−, whereas the magnitude of Δψ was unchanged. Br− also activated ATP transport, whereas sulfate was not effective (Fig. S4). Various anions such as iodine and thiocyanate were rather inhibitory. The anion dependence of the SLC17A9 protein was similar to that of VGLUT (21, 22), suggesting the presence of a similar anion binding site and regulatory mechanism in this transporter family. Cis inhibition studies suggested that this transporter preferentially recognized ATP, GTP, and ADP as transport substrates. AMP-PNP and γS-ATP, nonhydrolysable ATP analogues, and diadenosine triphosphate (AP3A), one of the unique physiological ATP derivatives that accumulates in chromaffin granules and synaptic vesicles (9, 18), strongly inhibited the uptake. In contrast, adenosine and adenine were less effective inhibitors (Fig. 4C). The transporter-mediated uptake of GTP and ADP was confirmed by the direct measurement of ADP and GTP uptake (Fig. 4D). The ATP uptake was greatly inhibited by 4,4′-diisothiocyanatostilbene-2,2′-disulfonate (DIDS), an inhibitor of VGLUT (23). The concentration required for ID50 was 1.5 μM (Fig. 4E). DIDS also inhibited the uptake of GTP and ADP (Fig. 4D). Evans blue, a specific inhibitor of VGLUT (23), also potently inhibited the ATP uptake with an ID50 of 40 nM (Fig. 4F). Atractyloside, an inhibitor of the mitochondrial ATP/ADP exchanger, inhibited the ATP uptake of the SLC17A9 protein only in the presence of Mg2+ as reported for ATP uptake in chromaffin granules (16) (Fig. 4G). Thus, the SLC17A9 protein is a Cl−-dependent nucleotide transporter, whose properties are very similar, if not identical, to those observed for ATP uptake in chromaffin granules (8, 13, 15–17).
Fig. 4.
Characterization of nucleotides transport by SLC17A9 protein. (A) The effect of divalent cations on the [α-32P]ATP uptake in the absence or presence of 2 mM Mg2+ or Ca2+ or 0.5 mM EGTA. (B) Dependence of [α-32P]ATP uptake on [Cl−]. The uptake was measured after 2 min. Part of the potassium acetate in the reaction mixture was replaced with the indicated concentration of KCl. The magnitude of Δψ (oxonol V-fluorescence quenching) was little affected under the assay conditions used. (C) [α-32P]ATP uptake in the presence of various nucleotides at 1 mM. (D) Uptake of radiolabeled GTP or ADP transport in the presence or absence of valinomycin. When indicated, DIDS at 2 μM was included. (E–G) The effect of DIDS, Evans blue, and atractyloside on [α-32P]ATP uptake in the presence or absence of Mg-acetate. Atractyloside was included at 200 μM. ∗, P <0.1; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Involvement of SLC17A9 Protein in Vesicular Storage and Exocytosis of ATP.
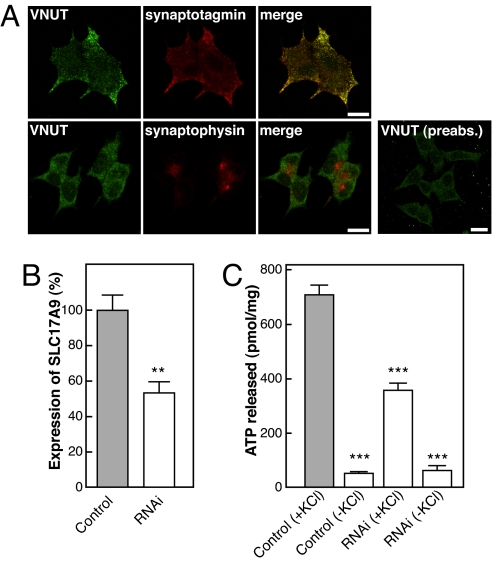
Finally, we investigated whether SLC17A9 is responsible for vesicular storage and subsequent exocytosis of ATP in purinergic cells. PC12 cells are known to store and secrete ATP through secretory granule-mediated exocytosis (24). We found that mSLC17A9 protein was associated with secretory granules (Fig. 5A) and that RNAi suppressed ≈53% of SLC17A9 expression and ≈50% of KCl-stimulated exocytosis of ATP from PC12 cells, whereas the control treated with scrambled siRNA did not show any inhibitory effect (Fig. 5 B and C). These results supported the participation of SLC17A9 protein in vesicular storage and exocytosis of ATP.
Fig. 5.
The impact of rat SLC17A9 siRNA on the endogenous expression of SLC17A9 in in PC12 cells was compared with cells treated with scrambled siRNA (control). (A) Double-labeling immunohistochemistry indicated that SLC17A9 protein was colocalized at least in part with synaptotagmin, a marker of secretory granule, but not with synaptophysin, a marker of synaptic-like microvesicles in cultured PC12 cells. (B and C) Both SLC17A9 mRNA levels (B), as determined by real-time PCR, and the KCl-dependent secretion of ATP after 30 min (C) were assessed. n = 9 for three independent experiments. ∗∗, P < 0.01; ∗∗∗, P < 0.001.
SLC17A9 Protein as a VNUT.
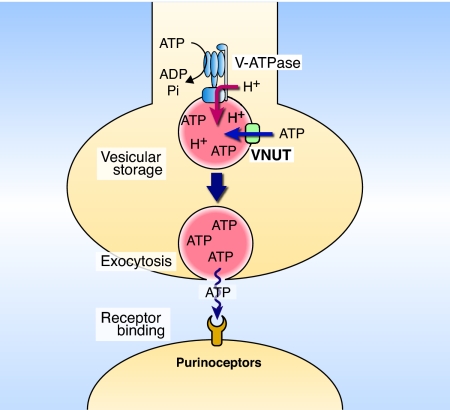
Based on this information, we concluded that SLC17A9 and its orthologues encode the VNUT. SLC17A9 protein may accumulate ATP and other nucleotides in the secretory vesicles such as adrenal chromaffin granules and synaptic vesicles. SLC17A9 protein is a long-searched nucleotide transporter and seems to be a missing link in purinergic chemical transmission (Fig. 6). Our immunohistochemical studies with anti-mSLC17A9 antibodies indicated that mSLC17A9 protein is expressed in a population of astrocytes, suggesting involvement of this transporter in storage of ATP in ATP-secreting astrocytes (25–27). Thus, identification of SLC17A9 protein as a VNUT may reveal the molecular mechanisms behind how ATP is secreted from purinergic cells and provide a novel molecular target for the pathophysiology of purinergic signaling and its therapeutic potential (9–11). Phylogenetic analysis indicates that the orthologues of SLC17A9 are widely distributed among various invertebrates and vertebrates (Fig. S5). Thus, our findings suggest the occurrence of common molecular mechanisms of the purinergic chemical transmission in animals.
Fig. 6.
Schematic presentation of purinergic chemical transmission. VNUT is present in secretory vesicles and is responsible for vesicular storage of nucleotides. The accumulated nucleotides, in particular ATP, are exocytosed upon stimulation and bind to purinoceptors at the surface of target cells, which then triggers intracellular signal transmission.
Materials and Methods
Expression and Purification of SLC17A9 Protein.
Recombinant baculovirus containing human SLC17A9 cDNA were constructed by using the Bac-to-Bac baculovirus expression systems (Invitrogen) according to the manufacturer's protocol. SLC17A9 cDNA was amplified by PCR using the primers (5′-CACCATGACCCTGACAAGCAGGCGCCAGGA-3′ and 5′-CTAGAGGTCCTCATGGGTAGAGCTC-3′). Sf9 or High Five cells were used for expression of SLC17A9 protein. Insect cells were infected by recombinant baculoviruses at a multiplicity of infection of 2 and cultured an additional 72 h for Sf9 cells and 48 h for High Five cells. The cell (≈1–2 × 108 cells) were suspended in a buffer containing 20 mM Tris·HCl (pH 8.0), 0.1 M potassium acetate, 10% glycerol, 0.5 mM DTT, 10 μg/ml pepstatin A, and 10 μg/ml leupeptin and disrupted by sonication with a TOMY UD200 tip sonifier. Cell lysates were centrifuged at 700 × g for 10 min to remove debris, and the resultant supernatant was centrifuged at 160,000 × g for 1 h. The pellet (membrane fraction) was suspended in buffer containing 20 mM Mops·Tris (pH 7.0), 10% glycerol, 10 μg/ml pepstatin A, and 10 μg/ml leupeptin at ≈6 mg protein/ml and was solubilized with 2% octyl glucoside. After centrifugation at 260,000 × g for 30 min, the supernatant was taken and applied to 1 ml of Ni-NTA Superflow resin (Qiagen). After incubation for 4 h at 4°C, the resin was washed with 10 ml of 20 mM Mops·Tris (pH 7.0), 5 mM imidazole, 20% glycerol, and 1% octyl glucoside. SLC17A9 protein was eluted from the resin with 3 ml of the same buffer containing 60 mM imidazole and could be stored at −80°C without loss of activity for at least a few months.
Reconstitution.
Reconstitution of purified SLC17A9 protein into liposomes was carried out by the freeze-thaw method as described (20). In brief, 10 μg of SLC17A9 protein was mixed with asolectin liposomes (0.5 mg of lipid), frozen at −80°C, and left at this temperature for at least 5 min. The mixture was thawed quickly by holding the samples tube in hands and diluted 60-fold with reconstitution buffer containing 20 mM Mops·Tris (pH 7.0), 0.5 mM DTT, 0.15 M sodium acetate, and 2 mM magnesium acetate. Reconstituted proteoliposomes were sedimented by centrifugation at 200,000 × g for 1 h at 4°C, suspended in 0.2 ml of 20 mM Mops·Tris (pH 7.0) containing 0.15 M sodium acetate and 2 mM magnesium acetate, and used within 1 day of preparation. Asolectin liposomes were prepared as follows. Soybean lecithin (20 mg; Sigma type IIS) was suspended in 2 ml of 20 mM Mops·NaOH (pH 7.0) containing 0.5 mM DTT. The mixture was sonicated in a bath-type sonicator until clear, divided into small aliquots, and stored at −80°C until use.
Nucleotide Transport.
Reconstituted proteoliposomes (0.5 μg protein per assay) were suspended in 500 μl of 20 mM Mops·Tris (pH 7.0), 5 mM magnesium acetate, 4 mM KCl, and 0.15 M potassium acetate and incubated for 3 min at 27°C. Valinomycin was added to give a final concentration of 2 μM, and the mixture was incubated for an additional 3 min. The assay was initiated by addition of 0.1 mM [α-32P]ATP (3.7 GBq/mmol), and 130-μl aliquots were taken at the times indicated and centrifuged through a Sephadex G-50 (fine) spin column at 760 × g for 2 min (20). Radioactivity and protein concentration of the eluate were measured. In the case of GTP or ADP transport, [α-32P]GTP (3.7 GBq/mmol) or [2,8-3H]ADP (0.37 GBq/mmol) were used for the substrates instead of ATP.
Cell Culture and RNAi.
PC12 cells were cultured as described (24). HiPerFect transfection reagent (Qiagen) was used for transfection of 25 nM AllStars negative control siRNA or rat SLC17A9 siRNA: UAUUCGAGAGAAUGUCACG. KCl-stimulated ATP secretion was assayed as described 3 days later (24).
Data Analysis.
All numerical values are shown as the mean ± SEM for three to six experiments. Statistical significance was determined by Student's t test with ∗, <0.1; ∗∗, < 0.01; ∗∗∗, < 0.001.
Northern blot analysis, cDNA cloning, immunohistochemistry, and other detailed methods are provided in SI Text.
Supplementary Material
Acknowledgments.
N.E. was supported by a Research Fellowship from the Japan Society for the Promotion of Science for Young Scientists. This work was supported in part by a Grant-in-Aid from the Japanese Ministry of Education, Science, Sport, and Culture (to Y.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.N. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800141105/DCSupplemental.
References
- 1.Maycox PR, Hell JW, Jahn R. Amino acid neurotransmission: Spotlight on synaptic vesicles. Trends Neurosci. 1990;13:83–87. doi: 10.1016/0166-2236(90)90178-d. [DOI] [PubMed] [Google Scholar]
- 2.Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: From bacteria to humans. Physiol Rev. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- 3.Parsons SM. Transport mechanisms in acetylcholine and monoamine storage. FASEB J. 2000;14:2423–2434. doi: 10.1096/fj.00-0203rev. [DOI] [PubMed] [Google Scholar]
- 4.Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflügers Arch. 2004;447:756–759. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- 5.Reimer RJ, Edwards RH. Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflügers Arch. 2004;447:629–635. doi: 10.1007/s00424-003-1087-y. [DOI] [PubMed] [Google Scholar]
- 6.Fremeau RT, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Moriyama Y, Yamamoto A. Glutamatergic chemical transmission: Look! Here, there, and anywhere. J Biochem. 2004;135:155–163. doi: 10.1093/jb/mvh018. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RG. Accumulation of biological amines into chromaffin granules: A model for hormone and neurotransmitter transport. Physiol Rev. 1988;68:232–307. doi: 10.1152/physrev.1988.68.1.232. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann H. ATP and acetylcholine, equal brethren. Neurochem Int. 2008;52:634–648. doi: 10.1016/j.neuint.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Lazarowski E. Regulated release of nucleotides and UDP sugars from astrocytoma cells. Novartis Found Symp. 2006;276:73–84. discussion 84–90. [PubMed] [Google Scholar]
- 11.Pankratov Y, Lalo U, Verkhrasky A, North RA. Vesicular release of ATP at central synapses. Pflügers Arch. 2006;452:589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- 12.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 13.Aberer W, Kostron H, Huber E, Winkler H. A characterization of the nucleotide uptake of chromaffin granules of bovine adrenal medulla. Biochem J. 1978;172:353–360. doi: 10.1042/bj1720353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luqmani YA. Nucleotide uptake by isolated cholinergic synaptic vesicles: Evidence for a carrier of adenosine 5′-triphosphate. Neuroscience. 1981;6:1011–1021. doi: 10.1016/0306-4522(81)90067-1. [DOI] [PubMed] [Google Scholar]
- 15.Weber A, Winkler H. Specificity and mechanism of nucleotide uptake by adrenal chromaffin granules. Neuroscience. 1981;6:2269–2276. doi: 10.1016/0306-4522(81)90016-6. [DOI] [PubMed] [Google Scholar]
- 16.Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts: Synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem. 1996;271:17132–17138. doi: 10.1074/jbc.271.29.17132. [DOI] [PubMed] [Google Scholar]
- 17.Gualix J, Abal M, Pintor J, Garcia-Carmona F, Miras-Portugal MT. Nucleotide vesicular transporter of bovine chromaffin granules: Evidence for mnemonic regulation. J Biol Chem. 1996;271:1957–1965. doi: 10.1074/jbc.271.4.1957. [DOI] [PubMed] [Google Scholar]
- 18.Gualix J, Pintor J, Miras-Portugal MT. Characterization of nucleotide transport into rat brain synaptic vesicles. J Neurochem. 1999;73:1098–1104. doi: 10.1046/j.1471-4159.1999.0731098.x. [DOI] [PubMed] [Google Scholar]
- 19.Zalk R, Shoshan-Barmatz V. Characterization of DIDS-sensitive ATP accumulation in brain synaptic vesicles. FEBS Lett. 2006;580:5894–5898. doi: 10.1016/j.febslet.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 20.Juge N, Yoshida Y, Yatsushiro S, Omote H, Moriyama Y. Vesicular glutamate transporter contains two independent transport machineries. J Biol Chem. 2006;281:39499–39506. doi: 10.1074/jbc.M607670200. [DOI] [PubMed] [Google Scholar]
- 21.Hartinger J, Jahn R. An anion binding site that regulates the glutamate transporter of synaptic vesicles. J Biol Chem. 1993;268:23122–23127. [PubMed] [Google Scholar]
- 22.Moriyama Y, Yamamoto A. Vesicular l-glutamate transporter in microvesicles from bovine pineal glands: Driving force, mechanism of chloride anion activation, and substrate specificity. J Biol Chem. 1995;270:22314–22320. doi: 10.1074/jbc.270.38.22314. [DOI] [PubMed] [Google Scholar]
- 23.Thompson CM, et al. Inhibitor of the glutamate vesicular transporter (VGLUT) Curr Med Chem. 2005;12:2041–2056. doi: 10.2174/0929867054637635. [DOI] [PubMed] [Google Scholar]
- 24.Fabbro A, Skorinsva E, Grandolfo M, Nistri A, Giniatullin R. Quantal release of ATP from clusters of PC12 cells. J Physiol (London) 2004;560:505–517. doi: 10.1113/jphysiol.2004.068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coco S, et al. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 26.Pangrsic T, et al. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, et al. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.