Abstract
The order Proboscidea includes extant elephants and their extinct relatives and is closely related to the aquatic sirenians (manatees and dugongs) and terrestrial hyracoids (hyraxes). Some analyses of embryological, morphological, and paleontological data suggest that proboscideans and sirenians shared an aquatic or semiaquatic common ancestor, but independent tests of this hypothesis have proven elusive. Here we test the hypothesis of an aquatic ancestry for advanced proboscideans by measuring δ18O in tooth enamel of two late Eocene proboscidean genera, Barytherium and Moeritherium, which are sister taxa of Oligocene-to-Recent proboscideans. The combination of low δ18O values and low δ18O standard deviations in Barytherium and Moeritherium matches the isotopic pattern seen in aquatic and semiaquatic mammals, and differs from that of terrestrial mammals. δ13C values of these early proboscideans suggest that both genera are likely to have consumed freshwater plants, although a component of C3 terrestrial vegetation cannot be ruled out. The simplest explanation for the combined evidence from isotopes, dental functional morphology, and depositional environments is that Barytherium and Moeritherium were at least semiaquatic and lived in freshwater swamp or riverine environments, where they grazed on freshwater vegetation. These results lend new support to the hypothesis that Oligocene-to-Recent proboscideans are derived from amphibious ancestors.
Keywords: Barytherium, Eocene, Fayum, Moeritherium, Proboscidea
The elephants Elephas and Loxodonta (order Proboscidea) are the only living remnants of a major adaptive radiation whose origin can now be traced back to the earliest Eocene (≈55 million years ago) in Africa (1). Genomic data place proboscideans within the placental mammalian superorder Afrotheria (2) and the more restricted supraordinal clade Paenungulata, which also contains the aquatic manatees and dugongs (order Sirenia) and terrestrial hyraxes (order Hyracoidea). Genetic evidence has thus far failed to resolve relationships among paenungulate orders (2, 3), but a recent analysis of genomic and morphological evidence provided weak support for a Proboscidea–Sirenia clade (Tethytheria) to the exclusion of Hyracoidea (4). A monophyletic Tethytheria has long been seen as the best explanation for available morphological evidence (5) and is key to the hypothesis [also based on developmental (6) and paleontological (5, 7) evidence] that the common ancestor of elephants and sea cows might have been at least semiaquatic.
Eocene proboscideans were radically different from living elephants in their size, skeletal and dental morphology, and presumably many aspects of their ecology and behavior as well (5, 7–9). Moeritherium was tapir-sized and possibly had a prehensile upper lip rather than a trunk (8). Moeritherium's lifestyle has been debated for over a century, with different lines of evidence supporting an aquatic, semiaquatic, or terrestrial mode of life (7, 9–13). Cranial features of Moeritherium that are seen in some aquatic or semiaquatic mammals, such as a long, tubular cranium and anteriorly positioned orbits, have previously been cited as evidence for an amphibious lifestyle, but they are not restricted to aquatic mammals (10). The auditory region of Moeritherium is more similar to that of extant elephants than to those of sirenians or older proboscideans, and it exhibits none of the characteristics that would facilitate underwater hearing (14). Moeritherium's postcranial morphology is not well known, but similarities to members of the extinct aquatic or semiaquatic clade Desmostylia have been noted (15). The pelvis associated with the most complete axial skeleton of Moeritherium has a very small hip socket (E.L.S., unpublished observation on Yale Moeritherium skeleton), suggesting hindlimb reduction as in aquatic mammals. It has been argued that many of the features Moeritherium shares with sea cows might be convergences resulting in part from adaptation to shared aquatic lifestyle (13, 16, 17).
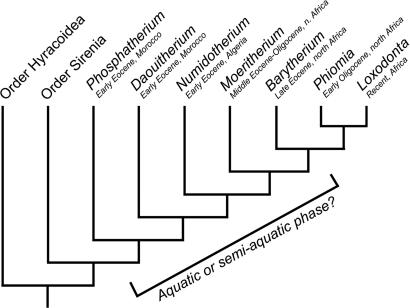
Much less is known about contemporaneous Barytherium. It is likely that the genus occupies a key position in proboscidean phylogeny, being either a sister group of Oligocene-to-Recent elephantiforms (ref. 4, Fig. 1), or perhaps a derived sister group of an older (early Eocene) genus such as Daouitherium or Numidotherium (1). Barytherium grave was first recovered from the Dir Abu Lifa Member of the Qasr el-Sagha Formation in the Fayum region of Egypt, which preserves both nearshore and alluvial deposits (18), and is estimated to have approached the size of modern Asian elephants (11). Barytherium remains from Libya derive from evaporite deposits that are probably diagenetic in origin (19). The genus was evidently more plantigrade than modern elephants (20).
Fig. 1.
Phylogenetic relationships of Paleogene proboscideans, from Seiffert (4).
Ideally, diverse lines of evidence, such as sedimentology, taphonomy, functional morphology, and analysis of coexisting fauna and flora could be used to determine the paleoecology and habitat of these early proboscideans, but in this case the situation is complicated by the large number of possible environments involved and a dearth of well-preserved postcranial skeletal material. This study employs stable isotopic evidence from tooth enamel to test the hypothesis that early proboscideans were amphibious, and it focuses on two late Eocene genera, Barytherium and Moeritherium, based on samples recently collected from early late Eocene (≈37 million-year-old) deposits of the Birket Qarun Formation in northern Egypt. The fossil-bearing sediments at the primary fossil locality in the area, Birket Qarun Locality 2 or BQ-2, were deposited during periodic flooding events of a landscape that otherwise might have regularly become ponded and stagnant; vertebrate fossils are predominantly of terrestrial animals, but the presence of shark and marine fish remains, and marine sediments above and below BQ-2, indicate that deposition occurred very close to the coast (21). Therefore the Birket Qarun proboscideans could have inhabited a wide range of habitats, including fluvial, coastal, lagoonal, or estuarine environments.
Inferring the Habitat and Dietary Preferences of Early Proboscideans Through Stable Isotope Analysis
The stable isotopes analyzed in this study are those of carbon (in the form of δ13C) and oxygen (as δ18O). Because the degree of isotope fractionation differs in different organic tissues, comparative analyses of fossil taxa should be based on one tissue type (22). Tooth enamel bioapatite is favored for isotopic studies of extinct taxa because of its durability and abundance in the fossil record. Enamel is also not easily altered during diagenesis, giving it an advantage over dentin and bone (23).
Tooth enamel grows in layers from the crown to the base at a rate of ≈30–60 mm/yr in large herbivores (24). Mixing of information from multiple layers during sample preparation provides a mean value of the isotopes incorporated into the enamel structure during amelogenesis; therefore the isotopic ratio of a finite volume of tooth enamel represents a time-averaged value of the individual's body water and diet over the period of enamel formation (24). Eocene mammalian enamel has previously been studied using these methods (25), which are suitable provided that diagenetic alteration has not changed the original in vivo isotopic signatures of the teeth.
Studies incorporating carbon and oxygen isotope analyses of tooth enamel hydroxyapatite have demonstrated that they are legitimate proxies for the habitat and diet of aquatic and terrestrial mammals in modern environments (22). Carbon isotope ratios recorded in mammalian tooth enamel can be used as a proxy for diet (26) because different types of vegetation have distinct δ13C values (27). Such differences result from isotopic fractionation during photosynthesis, productivity changes, and dissolved CO2 and bicarbonate levels in the surrounding environment (22). Carbon is integrated into mammalian body tissue in a ratio that is proportional to that in which it is taken up by the animal in the form of food (22). Therefore the carbon isotopes incorporated into body tissues record the isotopic signature of the food the mammal eats, and, by correcting for several fractionation steps, allow the δ13C values of the plants in an environment to be correlated with the δ13C of tooth enamel in their consumers all of the way up the food chain (22). There is considerable overlap between the δ13C ranges of certain vegetation types (Fig. 2B), but the use of other isotopic proxies (e.g., strontium) can potentially resolve such problems (28).
Fig. 2.
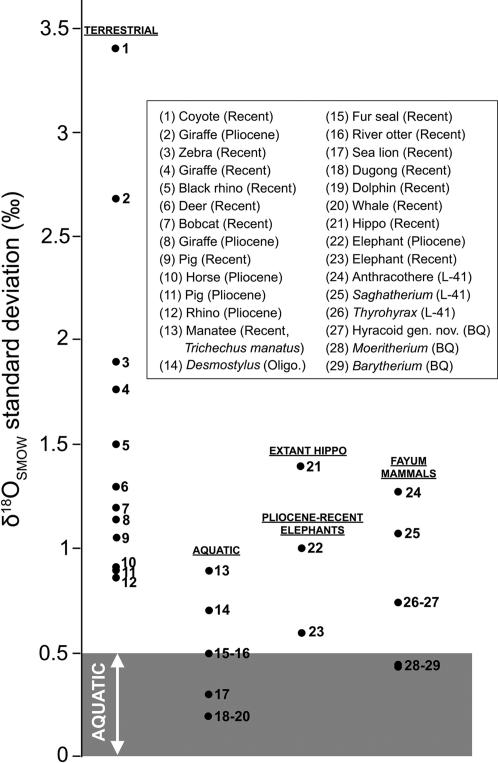
Carbon and oxygen isotype ratios in fossil mammalian teeth from the Fayum Depression, Egypt, compared with similar data from living and extinct mammals. (A) δ18O and δ13C data from late Eocene mammals from Egypt. (B) δ13C versus standard deviation of δ18O for a range of mammalian taxa. Each data point represents average values for one taxon. Taxa falling in the dark gray band (SD δ18O < 0.5‰), are considered aquatic, whereas taxa in the larger light-gray field (SD δ18O > 1.0‰) are likely to be terrestrial. Additional data are from Clementz et al. (25, 26) and Kingston and Harrison (39). All δ18O standard deviations are calculated from values normalized relative to standard mean ocean water (SMOW) (modern value). δ13C error bars are shown at 1σ for Fayum taxa only. PDB, Pee Dee Formation belemnite standard; POM, particulate organic matter.
The standard deviation of δ18O values in tooth enamel has been found to differ between terrestrial and aquatic mammals, with most terrestrial species showing markedly higher standard deviations than aquatic ones (22). The oxygen isotope ratio recorded in body tissues depends on three factors: the temperature at which the tissue/mineral is deposited, fractionation during metabolic and biological activity within the organism, and the fluxes and isotopic composition of the oxygen-containing matter entering the animal (29). The physiological fractionation of isotopes varies by species (30, 31), being determined by the animal's metabolism and the oxygen exchanges into and out of its body (32), which are, in turn, dependent on its size and mode of life. Because the fractionations for fossil taxa cannot be accurately established, this study does not consider physiological fractionation of oxygen isotopes in any calculations. Assuming that the mammals in this study are homeothermic [and therefore had a constant temperature-dependent fractionation between the enamel oxygen isotopes and body water ratios (33)] removes the temperature-dependent fractionation of δ18O. This assumption leaves the δ18O values of the oxygen sources and fluxes entering the body, and therefore the environmental surroundings, as the main factor influencing the isotopic values preserved in enamel. The sources of δ18O in the mammal's surroundings are in turn dominated by the oxygen isotope ratio of local meteoric water (33), which is predominantly dependent on latitude and amount of rainfall. Global marine δ18O values change because of global temperature or ice volume variations and are relatively unimportant in this context (34, 35).
Because the body water δ18O of aquatic mammals is dominated by diffusion of water through the skin [up to 98% of total flux (36)], and aquatic mammals are often limited to just a few water sources during their lives, aquatic mammal populations tend to have low standard deviations for enamel δ18O measurements (22). When compared with aquatic mammals, terrestrial mammals have a greater variety of sources from which to obtain their oxygen (including the atmosphere, more varied water sources, and food material), and a terrestrial population will therefore have a relatively high δ18O standard deviation compared with strictly aquatic populations (22). By combining the δ13C ranges of vegetation types with the δ18O standard deviation fields for different habitats, an ecology diagram can be produced, from which the habitat and diet of fossil mammals can be determined (25) (Fig. 2B).
Results
The proboscidean samples from the Birket Qarun localities, and the control group (hyracoid and anthracothere) samples from BQ-2 and the terminal Eocene Quarry L-41, exhibit a well defined spread in their isotopic values [Fig. 2A, supporting information (SI) Table S1] and do not show any patterns that would suggest diagenetic or chemical alteration of isotopic ratios. Furthermore, the isotopic signature of the BQ-2 rock matrix plots well away from that of the teeth from BQ-2 (Table 1), suggesting that the values obtained from the BQ-2 mammals are reliable proxies for diet and habitat. Barytherium and Moeritherium are significantly different from all other taxa sampled in both δ18O mean and variance (Kruskal–Wallis and ANOVA, P < 0.01, Tukey post hoc test P < 0.01; Table s S2–S4). However, the proboscidean δ13C data are different only when compared with the hyracoids (Kruskal–Wallis and ANOVA, P < 0.01). When compared with each other, Barytherium and Moeritherium show a significant difference in carbon isotope values (ANOVA, Kruskal–Wallis, Mann–Whitney, P < 0.05, Table S5), but not oxygen isotope values (Kruskal–Wallis, P = 0.347, Tukey test P = 0.9765). The BQ-2 hyracoid's δ13C values are significantly different in mean and variance from those of all taxa sampled from both quarries (ANOVA, Kruskal–Wallis, Mann–Whitney all P < 0.02); its δ18O values are significantly different from those of all other taxa except for the L-41 anthracothere (ANOVA, P < 0.01; Mann–Whitney and Kruskal–Wallis, P < 0.05).
Table 1.
Mean values of carbon and oxygen isotope ratios for each taxon
| Taxon | n | Mean δ13C, ‰ PDB |
Mean (±1σ) δ18O,‰ PDB | Mean (±1σ) δ18O,‰ SMOW | |
|---|---|---|---|---|---|
| Enamel (±1σ) | Ecosystem | ||||
| Fossils | |||||
| Moeritherium sp. | 5 | −8.882 ± 0.36 | −22.882 | −3.887 ± 0.42 | 26.901 ± 0.44 |
| Barytherium sp. | 5 | −7.847 ± 0.56 | −21.847 | −4.255 ± 0.44 | 26.523 ± 0.45 |
| Anthracotheriid | 6 | −8.499 ± 1.16 | −22.499 | 0.747 ± 1.23 | 31.680 ± 1.27 |
| Thyrohyrax meyeri | 6 | −7.961 ± 0.70 | −21.961 | 1.336 ± 0.73 | 32.287 ± 0.75 |
| Saghatherium bowni | 9 | −8.276 ± 0.45 | −22.276 | 2.019 ± 1.04 | 32.991 ± 1.07 |
| Hyracoid (BQ-2) | 6 | −6.216 ± 0.44 | −20.216 | −0.079 ± 0.73 | 30.828 ± 0.75 |
| Rocks | |||||
| Rock from L-41 | 4 | −0.842 ± 0.69 | −1.734 ± 2.22 | 29.122 ± 2.29 | |
| Sample L-41a | 1 | −9.475 | 1.770 | 32.735 | |
| Rock from BQ-2 | 9 | −0.538 ± 0.31 | 6.975 ± 1.10 | 38.100 ± 1.13 | |
″Rock from L-41″ does not include sample L-41a, which is displayed separately to show the distinct difference between it and other L-41 results. n = number of specimens successfully sampled for each mammal group. Ecosystem δ13C values were obtained by subtracting an enamel-diet fractionation value of 14‰ from the enamel values.
The data from quarry L-41 (hyracoids and anthracotheres) show considerable overlap, and with one exception there are no significant differences between L-41 species in either mean or variance at the α = 0.05 level for either δ18O or δ13C (Kruskal–Wallis, ANOVA, Mann–Whitney, all P > 0.05). Only the δ18O values of Saghatherium and the L-41 anthracothere are significantly different (P < 0.1). The similarity of isotopic values from all L-41 taxa could be an indication that their isotopic compositions were homogenized by diagenetic processes, for instance by fluids that might have passed through the unit after burial. This hypothesis is weakly supported by the fact that one rock sample plots among the teeth (37). However, the molar teeth of hyracoids and anthracotheres are quite similar in their functional morphology, which may indicate that they were consuming similar foods; furthermore, anthracotheres and hyracoids previously sampled by Clementz et al. (25) from a slightly younger Fayum quarry also show very similar δ18O and δ13C values. As such, the isotopic overlap of L-41 hyracoids and anthracotheres need not be due to diagenesis, although the possibility should be explored through future sampling of additional, distantly related, mammalian species from the same quarry.
The mean variability between isotope values within individual proboscidean teeth was ±0.11‰ for carbon, and ±0.17‰ for oxygen, far lower than the population variability (approximately ±0.4‰). Where sufficient data were available, intratooth variability in the control taxa was approximately ±0.2‰ for carbon and approximately ±0.3‰ for oxygen. These values are slightly higher than the variability of the standard, which never exceeded ± 0.17‰ for either isotope.
The standard deviations of δ18O values were calculated after conversion of all values (from the current study and the literature) to the SMOW standard. Available data from extant mammals show a clear trend, with terrestrial mammals having δ18O standard deviations of >0.8‰ and aquatic mammals having deviations of <0.8‰. Barytherium and Moeritherium's δ18O standard deviations (0.45‰ and 0.44‰, respectively) are lower than the 0.5‰ boundary below which only obligatorily aquatic taxa fall (Fig. 3). The anthracotheres and Saghatherium bowni plot within the range of definitively terrestrial taxa. Interestingly, Thyrohyrax meyeri and the new hyracoid genus from BQ-2 both plot in the intermediate zone between definitively aquatic and definitively terrestrial taxa (along with taxa as diverse as extant sirenians and elephants; Pliocene pigs, rhinos, and horses, and Oligocene Desmostylus). These data unexpectedly raise the possibility that, like their Paleogene paenungulate relatives, some early hyracoids might have also been, to some extent, semiaquatic.
Fig. 3.
δ18O standard deviations for a variety of aquatic and terrestrial taxa and late Eocene mammals from Egypt. Note the sharp break at ≈0.5‰, below which are definitively aquatic extant mammals. Additional data are from Clementz et al. (25, 26), Kingston and Harrison (39), and MacFadden et al. (41).
Discussion
The new species of Barytherium from the Birket Qarun localities has a low oxygen standard deviation (0.45‰) and primary-producer carbon isotope values of −21.847 ± 0.56‰. These data suggest that Barytherium was an aquatic or semiaquatic mammal that consumed freshwater plants, near-shore phytoplankton, or offshore particulate organic matter. The molars of Barytherium are strongly bilophodont (double-crested) (9), which, by analogy with living bilophodont mammals, are likely to have been used to shear through tough, resistant plant matter. Therefore it seems highly unlikely, based on functional morphology, that Barytherium would have been a filter feeder, or consumed large amounts of phytoplankton (if any). Based on the combined isotopic and dental evidence, we suggest that Barytherium consumed large amounts of freshwater vegetation.
In interpreting Barytherium's oxygen isotope data, one possible caveat is that extant mammals that weigh over 1,000 kg obtain a larger proportion of their oxygen intake from drinking water than do smaller mammals (38), and if large mammals drink from a water source that is isotopically homogenous, their δ18O values will be less variable and may falsely indicate an aquatic habitat (26). This may explain the low δ18O standard deviations of modern elephants, despite their largely terrestrial existence (26). The Barytherium species analyzed here was certainly a large mammal, although it was smaller than B. grave and extant elephants; body mass estimates for the new species are, unfortunately, currently unavailable. However, unlike extant elephants, Barytherium not only shows a very low δ18O standard deviation but also is relatively depleted in δ18O (by ≈4‰) compared with the contemporaneous and younger (and presumably largely terrestrial) hyracoids from BQ-2 and L-41. This is another important source of information, because many extant aquatic herbivores are similarly depleted in 18O compared with contemporaneous terrestrial taxa (25, 39). Barytherium's depleted 18O values increase the likelihood that its restricted δ18O values are due to at least semiaquatic lifestyle and not body mass alone.
Another concern is that mammals inhabiting humid climates may show lower δ18O variability because they lose little water through evaporation (26). Judging from the diverse arboreal primate fauna preserved at BQ-2 (40), it is quite likely that the Fayum region was covered by lush tropical rain forest during the earliest part of the late Eocene, and so high humidity could conceivably have restricted mammalian δ18O variability. However in the same region, the hyracoid Saghatherium and the anthracotheres from L-41 and younger localities show δ18O variability typical of terrestrial mammals, suggesting that humidity did not restrict δ18O variability of Fayum mammals in the late Eocene unless it had a nonuniform effect across different taxa. We do not see high humidity as being a likely cause for Barytherium or Moeritherium's low δ18O standard deviations.
Moeritherium samples show low δ18O standard deviations (0.44‰) and dietary δ13C values of −22.882 ± 0.36‰. Again, as with Barytherium, this indicates that Moeritherium was probably a largely aquatic animal that fed on freshwater plants or offshore particulate organic matter, although a semiaquatic existence with reliance on both freshwater and terrestrial C3 vegetation cannot be ruled out by the data for either proboscidean genus. As with Barytherium, we consider it unlikely that nearshore or offshore particulate organic matter was a major component of Moeritherium's diet because of dental functional morphology, as well as the fluvial nature of the sediment in which the remains were found. The body mass-related caveat detailed above for Barytherium could not explain Moeritherium's low δ18O variability, because members of the latter genus probably fell within the size range of living tapirs. Given the combined evidence from morphology and isotopes, Moeritherium probably spent a considerable amount of time in the water, and fed largely on freshwater vegetation. When compared with Barytherium, Moeritherium's lower, and significantly different, δ13C values suggest that the two proboscideans were eating different freshwater plants that were available in the Fayum ecosystem in the earliest late Eocene.
Did these early proboscideans ever move into marine waters? Extant manatee populations have higher δ18O standard deviations than other aquatic mammals (Fig. 3), presumably because they migrate between freshwater and marine settings that have different isotopic compositions (41). The relatively low δ18O variability of Barytherium and Moeritherium suggests that these proboscideans were relatively restricted in their habitat preferences. MacFadden et al. (41) suggested that, among living and extinct fully aquatic sirenians, δ18O SMOW values of ≈25 are indicative of freshwater lifestyle, whereas values of ≈30 are indicative of marine habitat. Moeritherium's δ18O SMOW mean of 26.9, and Barytherium's mean of 26.5, provide additional evidence that these early proboscideans are more likely to have inhabited freshwater, and not marine, habitats. Given that remains of the two proboscidean genera are now documented at Locality BQ-2 in a purely fluvial setting, we consider it likely that they did not venture far from purely freshwater, riverine, or swampy habitats.
Fig. 2B shows a summary of all of the data from Fayum mammals, plotted alongside modern and fossil mammals from the literature on an ecology diagram (25). The control taxa (hyracoids and anthracotheres) plot as being terrestrial, or in the intermediate zone between terrestrial and aquatic ecosystems, with diets more enriched in 13C than would be expected from a purely C3 diet. Possible reasons include (i) the diet–enamel isotope fractionations have been calculated by using figures that are inaccurate; (ii) there is another source of food supplementing the diets of these mammals—either freshwater plants or some very early C4 vegetation [which is unlikely but not impossible (42)]; or (iii) the higher than expected δ13C values from late Eocene mammals represent a background global δ13C record that was 13C enriched compared with the present. A global signal makes sense, given that data from anthracotheres and Saghatherium sampled from slightly younger beds in the Fayum succession by Clementz et al. (25) [and considered to be early Oligocene in age by Seiffert (43)] show markedly depleted 13C values compared with L-41 taxa, suggesting that both creatures were terrestrial with diets composed of pure C3 vegetation (25). However, the δ13C differences between latest Eocene (L-41) and early Oligocene (Clementz et al. samples) lineages might also be explained by the major negative δ13C excursion that occurred after the Eocene–Oligocene boundary (44).
Finally, the anthracothere results are of interest given that recent phylogenetic studies have placed these artiodactyls as stem hippopotamids (45). All but one of the data points for the L-41 anthracothere are clustered close together, with one seemingly anomalous data point falling far away from the other samples (indeed, far away from all of the other L-41 samples) (Fig. 2A). If this outlier is excluded, the δ18O standard deviation for the L-41 anthracothere falls to ±0.6‰, and thus close to habitually aquatic mammals. This is unlike extant Hippopotamus amphibius, however, which has a δ18O variability of ±1.4‰ despite being semiaquatic (26) (Fig. 3), perhaps because of a varied diet of terrestrial C4 grasses, freshwater plants, leaves, and fruits (which all contain water with different isotopic ratios), or perhaps because female and young male hippos move between water bodies. H. amphibius is similar to aquatic mammals, and unlike terrestrial mammals (and the L-41 anthracothere), however, in having depleted 18O values (26). These data suggest that the L-41 anthracothere is unlikely to have been particularly similar in its ecology to extant hippos.
Conclusions and Prospects
The combined evidence from Barytherium and Moeritherium's low δ18O standard deviations, depleted 18O values, dental functional morphology, dietary δ13C “ecosystem” values, and occurrence in fluvial deposits suggests that these early proboscideans were largely aquatic mammals that fed on freshwater vegetation in riverine or swampy settings. Because Moeritherium and Barytherium are placed as consecutive sister taxa of Oligocene-to-Recent proboscideans in the most comprehensive available analysis of early paenungulate relationships (4), these data provide new evidence for a semiaquatic phase in early proboscidean evolution. This hypothesis can be further tested through similar isotopic analyses of demonstrably more primitive proboscideans, such as early Eocene Phosphatherium, Daouitherium, and Numidotherium (5). Unfortunately, at present nothing is known of the postcranial morphology of Phosphatherium aside from a single phalanx (1), but Court's analysis of a more extensive collection of Numidotherium postcrania (46) led him to speculate that this genus might have been semiaquatic. All of these early proboscideans had bilophodont molars that were similar in morphology to those of Barytherium (1), suggesting exploitation of food resources with similar material properties. An additional test of the hypothesis presented here could be provided by similar stable isotopic analyses of other Barytherium and Moeritherium populations of different ages and geographical locations, for instance those from Dor el-Talha in Libya (47).
Methods
Sample preparation followed that outlined in Clementz and Koch (22) with the sample pretreatment methods of Koch et al. (23). Methods are described in greater detail in SI Materials and Methods. Control taxa include an unnamed new hyracoid genus from Locality BQ-2, the younger hyracoids Saghatherium bowni and Thyrohyrax meyeri from the ≈34-million-year-old Quarry L-41, and an unnamed anthracotheriid from L-41 (43). The L-41 fossils were extracted from a well-indurated green mudstone that could represent an ancient swamp or lake (48).
For carbon, a fractionation of approximately +14.0–14.3‰ in tooth enamel compared with diet is cited for herbivores, with larger mammals having the greater offset, although a value of +9.5‰ is given for carnivores (25, 27, 39). However a +12‰ diet–apatite fractionation is calculated for herbivores from South Africa (49). The values used here are +14‰ for herbivores (all of our taxa), and +9.5‰ for carnivores, in line with Clementz et al. (25). This was followed by a 0.8‰ fractionation for each successive trophic level an organism inhabits (22, 25, 50). Herbivores were assigned a trophic level of zero, terrestrial carnivores one, and aquatic carnivores two (consistent with ref. 25). Also, ambient δ13C during the Eocene should ideally be corrected for, but as the exact ages of quarries L-41 and BQ-2 are not known, and the global isotopic curve is very variable near the time periods considered here, it is not possible to make this correction without risking large errors of ±0.5‰ (44). The suggestion that δ13C values need to be shifted in fossils with respect to modern values to take into account the effect of anthropogenic CO2 emissions cannot be investigated for similar reasons (27).
SO2 in the CO2 released from the teeth during analysis can offset δ13C values by as much as 4‰, although this study has no way of quantifying this effect (27). Cerling et al. (27) found that water-stress and high light levels can both lead to enrichments in 13C in C3 plants. These fractionations are assumed to have affected all of the Fayum taxa equally because of their close spatial and temporal distribution, and they can therefore be corrected for by comparison with results from the control taxa.
In several mammal species, enamel formation begins in the womb, so isotopic ratios will include influences and fractionations related to growth and weaning, for example the isotopic signature of the mother's milk (22), offsetting isotope ratios substantially (25). It has also been noted that teeth forming later in life contain more 13C and less 18O than those formed earlier (51). Therefore it is important to consistently use teeth known to have formed later in life for isotopic studies wherever possible, e.g., M3 and P4 (25), and this has been done here, although the position (i.e., M1, M2, M3) of Barytherium molars could not be determined with confidence. Differences in isotope ratios have been found both within and between jaws and teeth of mammals (32, 52, 53).
In enamel bioapatite, up to 10% of the oxygen is in the form of carbonate, while the rest is present as phosphate and hydroxyl ions (53). Of this carbonate, 90% is in phosphorus sites of apatite (resistant to alteration), while 10% is in hydroxyl sites (54). If the hydroxyl-site oxygen has been replaced, or the isotope ratios have been affected by alteration, δ18O ratios could have an error of ±1‰ (54). It has not been possible to determine whether this has affected these samples. The isotopic ratios of the carbonate (CO3) fraction of apatite are considered by many to be liable to diagenetic alteration, whereas the hydroxyl component can be extremely modified by chemical alteration (54). The amount of diagenetic alteration is lower in the enamel phosphate component than in the carbonate component, so oxygen values should be taken from the phosphate sites where possible (37), a recommendation supported by enamel carbonate δ18O values having a larger range than their respective phosphate δ18O values (32). However, the carbonate fraction has retained a usable oxygen signal in some fossil specimens (29), and this study considers these carbonate values to give reliable and useful information. δ13C values are more reliable than δ18O from apatite structural carbonate, because oxygen is more prone to diagenetic alteration than carbon (55). Diagenesis has also been hypothesized to cause δ18O values to equilibrate with those of the diagenetic fluid (37), but teeth may retain original oxygen isotope values if the diagenetic system is of both low temperature and water/biomineral ratio (55). If diagenesis has altered the samples significantly, it would be expected to have homogenized all δ18O values of the vertebrate remains in the rock unit (56). Therefore more than one mammal type has been analyzed from the two main localities, and samples of bulk rock from the localities were tested to determine the extent of any diagenetic overprint.
Supplementary Material
Acknowledgments.
Dr. P. Ditchfield provided advice on isotope methodology and analysis, and the staff at the University of Oxford Research Laboratory for Archaeology and the History of Art provided access to equipment. A. Currant provided access to collections. N. Charnley ran the stable isotope analyses. B. Beatty and W. Sanders provided useful comments. Fieldwork in Egypt has been supported by National Science Foundation Grants BCS-0114856 and BCS-0416164 and by The Leakey Foundation; facilitated by the Egyptian Mineral Resources Authority and Egyptian Geological Museum; directed by E.L.S; and managed by P. Chatrath. A.G.S.C.L. thanks St Peter's College, the Oxford Earth Sciences Department, and the Burdett-Coutts Fund (Oxford) for funding. This research was undertaken as part of a Master of Earth Science thesis by A.G.S.C.L. during the 2006–2007 academic year in the Department of Earth Sciences, University of Oxford.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800884105/DCSupplemental.
References
- 1.Gheerbrant E, et al. New data on Phosphatherium escuilliei (Mammalia, Proboscidea) from the early Eocene of Morocco, and its impact on the phylogeny of Proboscidea and lophodont ungulates (Translated from French) Geodiversitas. 2005;27:239–333. [Google Scholar]
- 2.Amrine-Madsen H, Koepfli K-P, Wayne RK, Springer MS. A new phylogenetic marker, apolipoprotein B, provides compelling evidence for eutherian relationships. Mol Phylogenet Evol. 2003;28:225–240. doi: 10.1016/s1055-7903(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 3.Rokas A, Carroll SB. Bushes in the tree of life. PLoS Biol. 2006;4:1899–1904. doi: 10.1371/journal.pbio.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seiffert ER. A new estimate of afrotherian phylogeny based on simultaneous analysis of genomic, morphological, and fossil evidence. BMC Evol Biol. 2007;7:224. doi: 10.1186/1471-2148-7-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gheerbrant E, Domning DP, Tassy P. In: The Rise of Placental Mammals: Origins and Relationships of the Major Extant Clades. Rose KD, Archibald JD, editors. Baltimore: Johns Hopkins Univ Press; 2005. pp. 84–105. [Google Scholar]
- 6.Gaeth AP, Short RV, Renfree MB. The developing renal, reproductive, and respiratory systems of the African elephant suggest an aquatic ancestry. Proc Natl Acad Sci USA. 1999;96:5555–5558. doi: 10.1073/pnas.96.10.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domning D, Ray CE, McKenna MC. Two new Oligocene desmostylians and a discussion of tethytherian systematics. Smithson Contrib Paleobiol. 1986;59:1–56. [Google Scholar]
- 8.Shoshani J. Understanding proboscidean evolution: A formidable task. Trends Ecol Evol. 1998;13:480–487. doi: 10.1016/s0169-5347(98)01491-8. [DOI] [PubMed] [Google Scholar]
- 9.Shoshani J, et al. In: The Proboscidea: Evolution and Palaeoecology of Elephants and Their Relatives. Shoshani J, Tassy P, editors. Oxford: Oxford Univ Press; 1996. pp. 57–75. [Google Scholar]
- 10.Matsumoto H. A contribution to the knowledge of Moeritherium. Bull Am Mus Nat Hist. 1923;48:97–139. [Google Scholar]
- 11.Andrews CW. London: Trustees of the British Museum (Natural History); 1906. A descriptive catalogue of the Tertiary Vertebrata of the Fayum, Egypt; based on the collection of the Egyptian Government in the Geological Museum, Cairo, and on the collection in the British Museum (Natural History) [Google Scholar]
- 12.Coppens Y, Beden M. In: Evolution of African Mammals. Maglio VJ, Cooke HBS, editors. Cambridge, MA: Harvard Univ Press; 1978. pp. 333–335. [Google Scholar]
- 13.Osborn HF. The feeding habits of Moeritherium and Palaeomastodon. Nature. 1909;81:139–140. [Google Scholar]
- 14.Court N. The periotic of Moeritherium (Mammalia, Proboscidea): Homology or homoplasy in the ear region of Tethytheria McKenna, 1975? J Zool Linn Soc. 1994;112:13–28. [Google Scholar]
- 15.Simons EL. Early Cenozoic mammalian faunas, Fayum Province, Egypt. Part I. African Oligocene mammals: Introduction, history of study, and faunal succession. Bull Peabody Mus Nat Hist. 1968;28:1–21. [Google Scholar]
- 16.Court N. A new species of Numidotherium (Mammalia: Proboscidea) from the Eocene of Libya and the early phylogeny of the Proboscidea. J Vertebr Paleontol. 1995;15:650–671. [Google Scholar]
- 17.Osborn HF. Proboscidea. Vol 1. New York: Am Mus Nat Hist Press; 1936. [Google Scholar]
- 18.Holroyd PA, et al. New records of terrestrial mammals from the upper Eocene Qasr el Sagha Formation, Fayum Depression, Egypt. Palaeovertebrata. 1996;25:175–192. [Google Scholar]
- 19.Rasmussen DT, Tshakreen SO, Abugares MM, Smith JB. In: Elwyn L. Simons: A Search for Origins. Fleagle JG, Gilbert CC, editors. New York: Springer; 2008. pp. 181–196. [Google Scholar]
- 20.Delmer C. Paris: Mus Natl d'Histoire Naturelle; 2005. The first stages in the differentiation of proboscideans: The role of Barytherium grave from Libya (Translated from French) PhD dissertation. [Google Scholar]
- 21.Seiffert ER, Bown TM, Clyde WC, Simons EL. In: Elwyn L. Simons: A Search for Origins. Fleagle JG, Gilbert CC, editors. New York: Springer; 2008. pp. 71–86. [Google Scholar]
- 22.Clementz MT, Koch PL. Differentiating aquatic mammal habitat and foraging ecology with stable isotopes in tooth enamel. Oecologia. 2001;129:461–472. doi: 10.1007/s004420100745. [DOI] [PubMed] [Google Scholar]
- 23.Koch PL, Tuross N, Fogel ML. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. J Archaeol Sci. 1997;24:417–429. [Google Scholar]
- 24.Passey BH, Cerling TE. Tooth enamel mineralization in ungulates: Implications for recovering a primary isotopic time-series. Geochim Cosmochim Acta. 2002;66:3225–3234. [Google Scholar]
- 25.Clementz MT, Goswami A, Gingerich PD, Koch PL. Isotopic records from early whales and sea cows: Contrasting patterns of ecological transition. J Vertebr Paleontol. 2006;26:355–370. [Google Scholar]
- 26.Clementz MT, Hoppe KA, Koch PL. A paleoecological paradox: The habitat and dietary preferences of the extinct tethythere Desmostylus, inferred from stable isotope analysis. Paleobiology. 2003;29:506–519. [Google Scholar]
- 27.Cerling TE, et al. Global vegetation change through the Miocene/Pliocene boundary. Nature. 1997;389:153–158. [Google Scholar]
- 28.Capo RC, Stewart BW, Chadwick OA. Strontium isotopes as tracers of ecosystem processes: Theory and methods. Geoderma. 1998;82:197–225. [Google Scholar]
- 29.Koch PL, Fisher DC, Dettman D. Oxygen isotope variation in the tusks of extinct proboscideans—a measure of season of death and seasonality. Geology. 1989;17:515–519. [Google Scholar]
- 30.Luz B, Kolodny Y. Oxygen isotope variations in phosphate of biogenic apatites, 4. Mammal teeth and bones. Earth Planet Sci Lett. 1985;75:29–36. [Google Scholar]
- 31.Huertas AD, et al. Oxygen-isotope variations of phosphate in mammalian bone and tooth enamel. Geochim Cosmochim Acta. 1995;59:4299–4305. [Google Scholar]
- 32.Fricke HC, Clyde WC, O'Neil JR, Gingerich PD. Evidence for rapid climate change in North America during the latest Paleocene thermal maximum: Oxygen isotope compositions of biogenic phosphate from the Bighorn Basin (Wyoming) Earth Planet Sci Lett. 1998;160:193–208. [Google Scholar]
- 33.Longinelli A. Oxygen isotopes in mammal bone phosphate—a new tool for paleohydrological and paleoclimatological research. Geochim Cosmochim Acta. 1984;48:385–390. [Google Scholar]
- 34.Dansgaard W. Stable isotopes in precipitation. Tellus. 1964;16:436–468. [Google Scholar]
- 35.Fricke HC, Wing SL. Oxygen isotope and paleobotanical estimates of temperature and δ18O–latitude gradients over North America during the early Eocene. Am J Sci. 2004;304:612–635. [Google Scholar]
- 36.Hui CA. Sea-water consumption and water flux in the common dolphin Delphinus delphis. Physiol Zool. 1981;54:430–440. [Google Scholar]
- 37.Grimes ST, Mattey DP, Hooker JJ, Collinson ME. Paleogene paleoclimate reconstruction using oxygen isotopes from land and freshwater organisms: The use of multiple paleoproxies. Geochim Cosmochim Acta. 2003;67:4033–4047. [Google Scholar]
- 38.Bryant JD, Froelich PN. A model of oxygen-isotope fractionation in body-water of large mammals. Geochim Cosmochim Acta. 1995;59:4523–4537. [Google Scholar]
- 39.Kingston JD, Harrison T. Isotopic dietary reconstructions of Pliocene herbivores at Laetoli: Implications for early hominin paleoecology. Palaeogeogr Palaeocl. 2007;243:272–306. [Google Scholar]
- 40.Seiffert ER. Evolution and extinction of Afro-Arabian primates near the Eocene–Oligocene boundary. Folia Primatol. 2007;78:314–327. doi: 10.1159/000105147. [DOI] [PubMed] [Google Scholar]
- 41.MacFadden BJ, Higgins P, Clementz MT, Jones DS. Diets, habitat preferences, and niche differentiation of Cenozoic sirenians from Florida: Evidence from stable isotopes. Paleobiology. 2004;30:297–324. [Google Scholar]
- 42.Sage RF. The evolution of C-4 photosynthesis. New Phytol. 2004;161:341–370. doi: 10.1111/j.1469-8137.2004.00974.x. [DOI] [PubMed] [Google Scholar]
- 43.Seiffert ER. Revised age estimates for the later Paleogene mammal faunas of Egypt and Oman. Proc Natl Acad Sci USA. 2006;103:5000–5005. doi: 10.1073/pnas.0600689103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zachos J, et al. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 45.Boisserie JR, Lihoreau F, Brunet M. The position of Hippopotamidae within Cetartiodactyla. Proc Natl Acad Sci USA. 2005;102:1537–1541. doi: 10.1073/pnas.0409518102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Court N. Limb posture and gait in Numidotherium koholense, a primitive proboscidean from the Eocene of Algeria. J Zool Linn Soc. 1994;111:297–338. [Google Scholar]
- 47.Savage RJG. Early Tertiary mammal locality in southern Libya. Proc Geol Soc London. 1969;1657:167–171. [Google Scholar]
- 48.Simons EL, Cornero S, Bown TM. The taphonomy of fossil vertebrate Quarry L-41, Upper Eocene, Fayum Province, Egypt. Geological Survey of Egypt Special Publication. 1998;75:785–791. [Google Scholar]
- 49.Lee-Thorp J, Sealy JC, van der Merwe N. Stable carbon isotope ratio differences between bone-collagen and bone apatite, and their relationship to diet. J Archaeol Sci. 1989;16:585–599. [Google Scholar]
- 50.Kelly JF. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool. 2000;78:1–27. [Google Scholar]
- 51.Wright LE, Schwarcz HP. Stable carbon and oxygen isotopes in human tooth enamel: Identifying breastfeeding and weaning in prehistory. Am J Phys Anthropol. 1998;106:1–18. doi: 10.1002/(SICI)1096-8644(199805)106:1<1::AID-AJPA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 52.Fricke HC, O'Neil JR. Inter- and intra-tooth variation in the oxygen isotope composition of mammalian tooth enamel phosphate: Implications for palaeoclimatological and palaeobiological research. Palaeogeogr Palaeocl. 1996;126:91–99. [Google Scholar]
- 53.Lindars ES, et al. Phosphate δ18O determination of modern rodent teeth by direct laser fluorination: An appraisal of methodology and potential application to palaeoclimate reconstruction. Geochim Cosmochim Acta. 2001;65:2535–2548. [Google Scholar]
- 54.Kohn MJ, Schoeninger MJ, Barker WW. Altered states: Effects of diagenesis on fossil tooth chemistry. Geochim Cosmochim Acta. 1999;63:2737–2747. [Google Scholar]
- 55.Wang Y, Cerling TE. A model of fossil tooth and bone diagenesis—implications for paleodiet reconstruction from stable isotopes. Palaeogeogr Palaeocl. 1994;107:281–289. [Google Scholar]
- 56.Lécuyer C, et al. Stable isotope composition and rare earth element content of vertebrate remains from the Late Cretaceous of northern Spain (Lano): Did the environmental record survive? Palaeogeogr Palaeocl. 2003;193:457–471. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.