Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a clonal hematopoietic stem cell disorder resulting from mutations in an X-linked gene, PIG-A, that encodes an enzyme required for the first step in the biosynthesis of glycosylphosphatidylinositol (GPI) anchors. PIG-A mutations result in absent or decreased cell surface expression of all GPI-anchored proteins. Although many of the clinical manifestations (e.g., hemolytic anemia) of the disease can be explained by a deficiency of GPI-anchored complement regulatory proteins such as CD59 and CD55, it is unclear why the PNH clone dominates hematopoiesis and why it is prone to evolve into acute leukemia. We found that PIG-A mutations confer a survival advantage by making cells relatively resistant to apoptotic death. When placed in serum-free medium, granulocytes and affected CD34+ (CD59−) cells from PNH patients survived longer than their normal counterparts. PNH cells were also relatively resistant to apoptosis induced by ionizing irradiation. Replacement of the normal PIG-A gene in PNH cell lines reversed the cellular resistance to apoptosis. Inhibited apoptosis resulting from PIG-A mutations appears to be the principle mechanism by which PNH cells maintain a growth advantage over normal progenitors and could play a role in the propensity of this disease to transform into more aggressive hematologic disorders. These data also suggest that GPI anchors are important in regulating apoptosis.
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired, clonal disorder of hematopoietic stem cells manifested by intravascular hemolysis, peripheral blood cytopenias, and thrombosis; it has a propensity to transform into leukemia (1, 2). PNH results from the expansion of an abnormal clone that harbors a somatic mutation of the X-linked gene, termed PIG-A (3–5). The product of this gene is required for the first step of glycosylphosphatidylinositol (GPI) anchor biosynthesis; consequently, hematopoietic cells in PNH are characterized by an absence or deficiency of proteins affixed to cell membranes by a GPI anchor (6). Many of the clinical features of PNH can be explained by the absence of GPI-anchored proteins; failure to express several GPI-anchored complement inactivating proteins, such as membrane inhibitor of reactive lysis (CD59) and decay accelerating factor (CD55), accounts for the abnormal complement sensitivity of affected cells. However, it remains unclear why the defective PNH stem cell and its progeny displace normal hematopoietic cells, despite the fact that PNH cells are more susceptible to complement-mediated destruction (7).
PNH can arise de novo or evolve from aplastic anemia, suggesting a pathophysiologic link between the two disorders (2, 8, 9). It has been proposed that PIG-A mutations are a relatively common benign event and that the PNH clone achieves a growth advantage over normal stem cells only in the context of an immune attack (as appears to occur in aplastic anemia) directed against GPI-anchored proteins (10, 11). However, “immunologic escape” does not explain the clonal nature of the disease, the propensity of PNH patients to develop more aggressive clonal disorders, or the fact that immunosuppressive therapy is generally ineffective for treating PNH (12–14).
An alternative explanation for the clonal expansion of PNH cells is that PIG-A mutations may confer a growth advantage to the PNH clone similar to other genetic mutations that predispose to cancer. Recent in vitro (15) and in vivo (16, 17) data suggest that PNH cells have an intrinsic growth advantage over normal cells, although the nature of this growth advantage has not been characterized. Unregulated clonal growth may occur from signals that enhance cell proliferation or alternatively from signals that block apoptosis (i.e., programmed cell death) and enhance cell survival (18, 19). Overexpression of bcl-2 is responsible for the inhibited apoptosis observed in follicular lymphoma (20, 21). Similarly, clonal expansion in chronic myelogenous leukemia (CML) results from BCR-ABL-mediated inhibition of apoptosis (18, 22, 23). Like CML and follicular lymphoma, PNH is a chronic, clonal bone marrow disorder characterized by an initial indolent phase with uninterrupted differentiation of affected cells. Furthermore, in each of these disorders the affected clone is predisposed to more aggressive malignant phenotypes (blast crisis from CML, high-grade lymphoma from follicular lymphoma, and leukemia from PNH). In this study, we examined the possibility that PIG-A mutations contribute to clonal expansion by inducing cellular resistance to apoptosis.
MATERIALS AND METHODS
Cell Lines and Transfections.
The human B lymphoblastoid cell line LD− was established from a patient with PNH by transformation with Epstein–Barr virus as described previously (8). The human B-lymphoblastic cell line JY-5 (24) is a GPI-anchor deficient line with a previously documented PIG-A mutation (25). An expression vector containing a 3.6-kb full-length PIG-A cDNA (25) (a gift from T. Kinoshita, Osaka University) was used to establish the GPI-anchor replete cell lines, LD− (PIG-A+) and JY-5 (PIG-A+). Liposomal transfection was performed by adding a solution containing 10 μg of pEBPIG-A and 50 ml of Lipofectamine (GIBCO/BRL) with 5 × 106 cells. After culture and selection with 200 μg/ml of hygromycin B (Boehringer Mannheim), the transfected cells were stained for CD59 with a fluorescein isothiocyanate-conjugated monoclonal antibody (Research Diagnostics, Flanders, NJ) and analyzed by flow cytometry (FACscan; Becton Dickinson). All cell lines were maintained in RPMI 1640 medium (GIBCO) with 10% heat inactivated fetal calf serum; after transfection and selection, the PIG-A+ lines were maintained without hygromycin.
Isolation of Hematopoietic Cells.
Bone marrow cells were obtained by posterior iliac crest aspiration from two patients with PNH, and peripheral blood was obtained from four PNH patients and five normal volunteers. All patients and volunteers gave informed consent approved by the Joint Committee on Clinical Investigation of the Johns Hopkins Medical Institutions. Peripheral blood granulocytes were isolated using Ficoll/Hypaque (specific gravity 1.077 followed by 1.119) as described previously (18, 26). Bone marrow mononuclear cells were recovered by Ficoll/Hypaque (density <1.077) centrifugation. CD34+ cells were separated using avidin-biotin immunoadsorption (CellPro, Bothell, WA). After isolation of CD34+ by immunoadsorption, the cells were stained with a fluorescein isothiocyanate-conjugated anti-CD59 and a phycoerythrin-conjugated anti-CD34 monoclonal antibody (Becton Dickinson). An EPICS 752 cell sorter (Coulter) was used to isolate CD34+59− and CD34+59+ populations.
Assessment of Cell Viability and Apoptosis.
The viability of cells was assessed by their ability to exclude trypan blue. Apoptotic cells were identified morphologically using standard criteria as described previously (22, 27); these criteria included cytoplasmic condensation and compaction of chromatin. For flow cytometric assessment of apoptosis, cells were fixed in 50% ethanol, permeabilized with 0.1% Triton X-100, treated with 5 μg/ml of RNase, and incubated at 37°C for 15 min before they were stained with 50 μg/ml of propidium iodide for 60 min at 4°C. The fraction of subdiploid cells with oligonucleosomal DNA degradation characteristic of apoptosis (18, 28) and the fraction of residual viable cells (excluding the subdiploid fraction) in the G0/G1 and S/G2M phases of the cell cycle were quantified by flow cytometric analysis (FACscan; Becton Dickinson). For detection of oligonucleosomal DNA fragments by gel electrophoresis, PNH and normal granulocytes (1 × 106 cells) were recovered after culture. DNA was isolated after sodium dodecyl sulfate (SDS) lysis and proteinase K digestion as described (29). DNA fragments were separated by agarose gel (2%) electrophoresis, and oligonucleosomal bands were visualized by staining with ethidium bromide. Control for size of DNA fragments were 1-kb DNA size markers (Invitrogen).
Radiation Susceptibility.
Cells were exposed to graded concentrations of ionizing radiation (0, 100, 250, and 500 cGy). The cells were then placed in serum-free medium (aMEM, GIBCO/BRL) for 16 hr. The sensitivity to radiation induced damage was determined by culturing the cells in methylcellulose clonogenic assays as described previously (18), and by flow cytometry for apoptosis.
Cell Synchronization.
Cells were synchronized in late G1/early S phases by incubation for 12 hr in 5% CO2 at 37°C in medium containing 2 μg/ml of aphidocolin. To release cells into S phase, cells were centrifuged, washed twice in prewarmed medium to remove aphidocolin, and resuspended in serum-free medium.
RESULTS
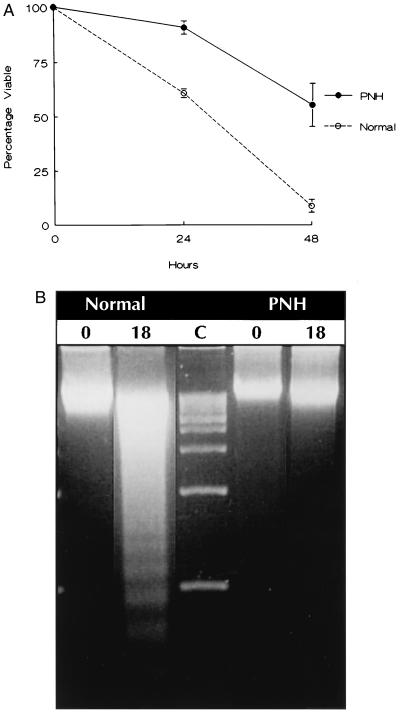
The effect of PIG-A mutations on survival, distinct from proliferation, was analyzed by comparing the survival of terminally differentiated peripheral blood granulocytes from four PNH patients and from five normal donors. CD59 phenotyping of the granulocytes from all four PNH patients demonstrated that at least 80% of the cells exhibited markedly reduced or absent expression of GPI-anchored proteins (data not shown). After 24 and 48 hr in serum-free medium, 41% and 92% of normal granulocytes were nonviable, respectively (Fig. 1A). In contrast, only 6% and 42% of PNH granulocytes were nonviable at 24 and 48 hr, respectively. Agarose gel electrophoresis (Fig. 1B) and morphologic examination of cells (data not shown) confirmed that the survival advantage of the PNH granulocytes was secondary to decreased apoptosis.
Figure 1.
(A) Survival of PNH and normal peripheral blood granulocytes in serum-free medium. Cell viability was determined by trypan blue dye exclusion. Each data point indicating percent survival represents the ratio of the absolute viable cell number at the time of assay and the number of viable cells at the start of each experiment. The data are the mean ± SEM of four patients with PNH and five normals. (B) Gel electrophoresis for detection of oligonucleosomal DNA fragments at time 0 and 18 hr after culture of normal and PNH granulocytes in serum-free medium. Lane C depicts 1-kb DNA size markers.
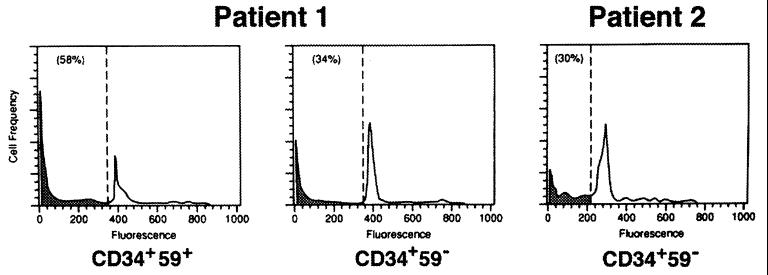
Using experimental conditions similar to those used for the granulocytes, PNH progenitors were also studied by isolating CD34+59+ and CD34+59− cells from two PNH patients. We showed previously that the majority of normal CD34+ cells undergo apoptosis by 72 hr in serum-free medium (18). After 72 hr in serum-free medium, 58% of unaffected CD34+59+ cells from one patient demonstrated subdiploid DNA characteristic of apoptosis (Fig. 2). In contrast, only 34% of affected CD34+59− cells were subdiploid (Fig. 2). In the second patient, only CD34+59− cells were available because they represented more than 90% of the patient’s CD34+ cells. Using the same experimental conditions, only 30% of the CD34+59− cells were subdiploid after 72 hr in serum-free medium, as found for the first patient (Fig. 2). At time 0, the percentage of subdiploid cells from all fractions (CD34+59+ and CD34+59−) was less than 5%.
Figure 2.
Flow cytometric analysis for apoptosis of CD34+ cells from two PNH patients. Unaffected (CD59+) and affected (CD59−) cells from patient 1, and affected cells from patient 2. The number in parenthesis indicates the percentage of subdiploid DNA (shaded).
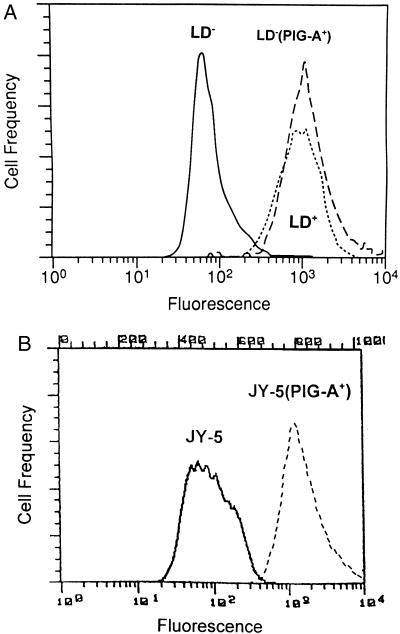
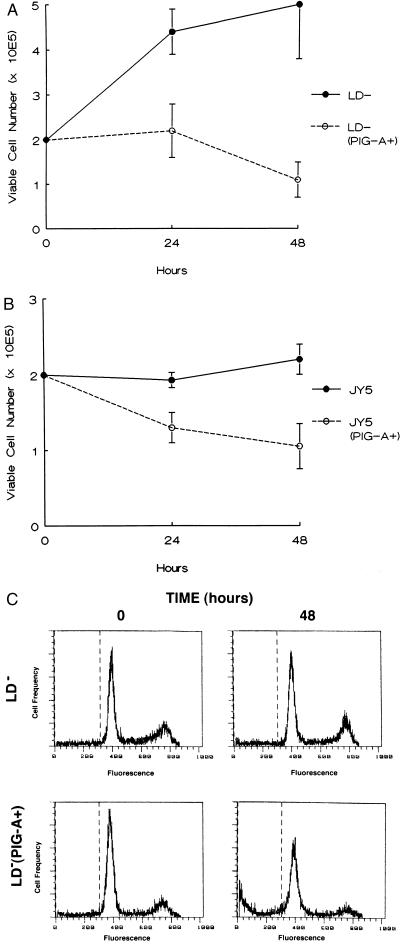
To determine if loss of PIG-A activity was directly responsible for the survival advantage of CD59− cells, we studied the effect of PIG-A gene mutations in a pair of lymphoblastoid B-cell lines (LD− and JY-5) that do not express GPI-anchored proteins. LD− was derived from a PNH patient with a previously characterized PIG-A mutation (8); JY-5 also harbors a PIG-A mutation but was derived experimentally (24). We stably transfected an expression vector containing the full-length PIG-A cDNA into the LD− and JY-5 cell lines to restore surface expression of GPI-anchored proteins (Fig. 3) and compared the growth and survival of the four cell lines in serum-free medium. Less than 50% of the LD− (PIG-A+) cells were viable after 48 hr in serum-free medium. In contrast, the LD− cell line demonstrated continued growth after 48 hr in serum-free medium with over 75% of the cells remaining viable (Fig. 4A). The JY-5 cell line also showed enhanced survival after serum-free culture compared with the JY-5 (PIG-A+) cells (Fig. 4B). Flow cytometric analysis confirmed that the survival advantage of the LD− (Fig. 4C) and JY-5 cell lines (data not shown) over the LD− (PIG-A+) and JY-5 (PIG-A+) cells, respectively, was the result of decreased apoptosis.
Figure 3.
Flow cytometric analysis for CD59 expression. (A) The GPI anchor-deficient, LD− (solid line), and the GPI anchor-replete, LD+ (dotted line), Epstein–Barr virus-transformed B lymphoblastoid cell lines were derived from a patient with PNH. LD− (PIG-A+) (dashed line) was established after stable transfection of the PIG-A cDNA into the LD− cell line. (B) The GPI anchor-deficient JY-5 cells (solid line) and JY-5 (PIG-A+) cells (dashed line).
Figure 4.
Survival of PNH cells lines in serum-free medium. (A) LD− and LD− (PIG-A+). (B) JY-5 and JY-5 (PIG-A+). Cell viability was determined by trypan blue exclusion after incubation in serum-free medium for 48 hr. The data are the mean of ± SEM of three separate experiments. (C) Flow cytometric analysis for apoptosis of LD− and LD− (PIG-A+) cells after 48 hr in serum-free medium.
The growth advantage of the LD− and JY5 cells could result from a proliferative and a survival difference. However, all cell lines showed similar proliferative capacity. Flow cytometric cell cycle analysis demonstrated a similar cell cycle distribution for the LD− and JY-5 cells and their respective counterparts, LD− (PIG-A+) and JY-5 (PIG-A+). Furthermore, after synchronization in G1 with aphidocolin, all four cell lines demonstrated a similar cell cycle time (data not shown).
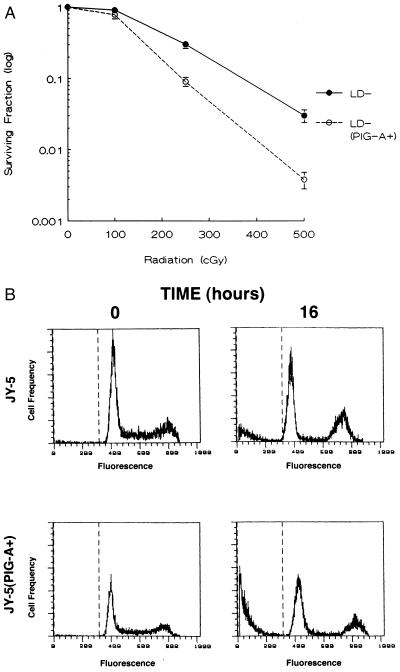
Signals that block apoptosis cause resistance to most DNA-damaging agents (23, 30–32). Thus, we measured the sensitivity of the cell lines to graded doses of ionizing radiation. Both PNH cell lines, LD− and JY-5, were significantly more resistant to ionizing radiation than the LD− (PIG-A+) and JY-5 (PIG-A+) lines, respectively (Fig. 5). Thus, PIG-A mutations, like other anti-apoptotic signals, are associated with resistance to agents that induce DNA damage.
Figure 5.
Relative sensitivity of PNH cell lines to irradiation. (A) The surviving fraction from LD− and LD− (PIG-A+) cells represents the number of clonogenic colonies after treatment compared with the corresponding untreated controls. Each data point represents the mean ± SEM of three separate experiments. (B) Since JY-5 cells were unable to be cloned in semi-solid medium, flow cytometric analysis was used to assess apoptosis after 500 cGy of ionizing radiation. Propidium iodide staining of the JY-5 cells for DNA content revealed 10.5% ± 2.3 subdiploid DNA 16 hr after radiation. In contrast, JY-5 (PIG-A+) cells revealed 31.3% ± 2.9 subdiploid DNA after identical conditions. A representative example from one of the three experiments is shown.
DISCUSSION
Although it is widely established that circulating PNH cells arise from the expansion of a mutant clone, it remains an enigma why this “diseased” clone has the ability to displace normal hematopoiesis. Recent reports suggest that PNH cells may exhibit an intrinsic growth advantage over their normal counterparts (15, 17). Iwamoto et al. (16) demonstrated that transplantation of CD34+ progenitors from PNH patients (showing a chimera of normal cells and the PNH clone) into severe combined immunodeficient mice yields multilineage hematopoiesis. Interestingly, the PNH clone continued to dominate hematopoiesis despite the absence of a functional immune system in the severe combined immunodeficient mouse; moreover, the PNH clone did not always require human cytokines. Nevertheless, these studies do not address the mechanism for the growth advantage of PNH cells.
We found that PIG-A gene mutations block apoptosis, causing cells to have a survival advantage and to be relatively resistant to radiation. Signals that inhibit apoptosis, such as the oncogenes bcl-2, BCR-ABL, and mutant p53, result in clonal expansion and a proclivity for neoplastic progression (18, 21, 22, 33, 34). Thus, the clonal dominance, as well as the increased incidence of leukemic progression in PNH, can be explained by the ability of PIG-A mutations to block apoptosis.
Resistance to apoptosis secondary to PIG-A mutations may also explain the close relationship between PNH and aplastic anemia. Aplastic anemia is thought to result from damage to a hematopoietic stem cell that leads to an autoimmune response directed against the bone marrow (35). Sublethal damage to a stem cell may also produce genetic mutations (such as PIG-A mutations) that confer a growth advantage to the cell and its progeny. PNH cells that are resistant to apoptosis should have an even greater advantage in the setting of aplastic anemia, where depletion of normal hematopoietic cells results from increased apoptosis (36). Over time, an abnormal clone arising from a slowly proliferating, transformed stem cell could eventually become dominant (37).
PIG-A gene mutations affect hematopoietic cells in at least two ways. Decreased expression of GPI-anchored proteins accounts for many of the clinical manifestations of PNH, such as complement-mediated hemolytic anemia and possibly thrombosis. In addition, PIG-A gene mutations block apoptosis, leading to clonal expansion, and possibly the tumor progression that is sometimes observed in the disease. Although the mechanism by which PIG-A gene mutations block apoptosis remains unknown, our data suggest that GPI anchors are important in regulating apoptosis.
Acknowledgments
We thank Marie C. Moineau for assistance in manuscript preparation. This work was supported in part by National Institutes of Health Grants CA15396 and AI23598. R.J.J. is a Leukemia Society of America Scholar.
ABBREVIATIONS
- PNH
paroxysomal nocturnal hemoglobinuria
- GPI
glycosylphosphatidylinositol
- CML
chronic myelogenous leukemia
References
- 1.Hillmen P, Lewis S M, Bessler M, Luzzatto L, Dacie J V. N Engl J Med. 1995;333:1253–1258. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 2.Rotoli B, Luzzatto L. Bailliere’s Clin Haematol. 1989;2:113–138. doi: 10.1016/s0950-3536(89)80010-1. [DOI] [PubMed] [Google Scholar]
- 3.Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, Takahashi M, Kitani T, Kinoshita T. Cell. 1993;73:703–711. doi: 10.1016/0092-8674(93)90250-t. [DOI] [PubMed] [Google Scholar]
- 4.Miyata T, Yamada N, Iida Y, Nishimura J, Takeda J, Kitani T, Kinoshita T. N Engl J Med. 1994;330:249–255. doi: 10.1056/NEJM199401273300404. [DOI] [PubMed] [Google Scholar]
- 5.Bessler M, Mason P J, Hillmen P, Miyata T, Yamada N, Takeda J, Luzzatto L, Kinoshita T. EMBO J. 1994;13:110–117. doi: 10.1002/j.1460-2075.1994.tb06240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosse W F, Ware R E. Blood. 1995;86:3277–3286. [PubMed] [Google Scholar]
- 7.Tumen J, Kline L B, Fay J W, Scullin D C, Reisner E G, Rosse W F, Huang A T. Blood. 1980;55:1040–1046. [PubMed] [Google Scholar]
- 8.Nagarajan S, Brodsky R, Young N S, Medof M E. Blood. 1995;86:4656–4661. [PubMed] [Google Scholar]
- 9.Griscelli-Bennaceur A, Gluckman E, Scrobohaci M L, Jonveaux P, Vu T, Bazarbachi A, Carosella E D, Sigaux F, Socie G. Blood. 1995;85:1354–1363. [PubMed] [Google Scholar]
- 10.Young N S. Blood. 1992;79:1385–1392. [PubMed] [Google Scholar]
- 11.Hertenstein B, Wagner B, Bunjes D, Duncker C, Raghavachar A, Arnold R, Heimpel H, Schrezenmeier H. Blood. 1995;86:1487–1492. [PubMed] [Google Scholar]
- 12.Rosse W F. Blood. 1982;60:20–23. [PubMed] [Google Scholar]
- 13.Nakao S, Yamaguchi M, Takamatsu H, Shiobara S, Matusda T. Blood. 1992;80:2943–2950. [PubMed] [Google Scholar]
- 14.Schrezenmeier H, Hertenstein B, Wagner B, Raghavachar A, Heimpel H. Exp Hematol. 1995;23:81–87. [PubMed] [Google Scholar]
- 15.Tseng J E, Hall S E, Howard T A, Ware R E. Am J Hematol. 1995;50:244–253. doi: 10.1002/ajh.2830500405. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto N, Kawaguchi T, Horikawa K, Nagakura S, Kagimoto T, Suda T, Takatsuki K, Nakakuma H. Blood. 1996;87:4944–4948. [PubMed] [Google Scholar]
- 17.Endo M, Beatty P G, Vreeke T M, Wittwer C T, Singh S P, Parker C J. Blood. 1996;88:742–750. [PubMed] [Google Scholar]
- 18.Bedi A, Zehnbauer B A, Barber J P, Sharkis S J, Jones R J. Blood. 1994;83:2038–2044. [PubMed] [Google Scholar]
- 19.Korsmeyer S J. Blood. 1992;80:879–886. [PubMed] [Google Scholar]
- 20.McDonnell T, Deane N, Platt F M, Nunez G, Jaeger U, McKearn J P, Korsmeyer S J. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 21.Vaux D L, Cory S, Adams J M. Nature (London) 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 22.McGahon A, Bissonnette R, Schmitt M, Cotter K M, Green D R, Cotter T G. Blood. 1994;83:1179–1187. [PubMed] [Google Scholar]
- 23.Bedi A, Barber J P, Bedi G C, El-Deiry W S, Sidransky D, Vala M S, Akhtar A J, Hilton J, Jones R J. Blood. 1995;86:1148–1158. [PubMed] [Google Scholar]
- 24.Hollander N, Selvaraj P, Springer T A. J Immunol. 1988;141:4283–4290. [PubMed] [Google Scholar]
- 25.Miyata T, Takeda J, Iida Y, Yamada N, Inoue N, Takahashi M, Maeda K, Kitani T, Kinoshita T. Science. 1993;259:1318–1320. doi: 10.1126/science.7680492. [DOI] [PubMed] [Google Scholar]
- 26.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 27.Wyllie A H, Kerr J F, Currie A R. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 28.Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz M A, Lassota P, Traganos F. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 29.Bedi A, Zehnbauer B A, Collector M I, Barber J P, Zicha M S, Sharkis S J, Jones R J. Blood. 1993;81:2898–2902. [PubMed] [Google Scholar]
- 30.Miyashita T, Reed J C. Blood. 1993;81:151–157. [PubMed] [Google Scholar]
- 31.Lotem J, Sachs L. Blood. 1993;82:1092–1096. [PubMed] [Google Scholar]
- 32.Fisher D E. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 33.Williams G T. Cell. 1991;65:1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- 34.Bedi A, Pasricha P J, Akhtar A J, Barber J P, Bedi G C, Giardiello F M, Zehnbauer B A, Hamilton S R, Jones R J. Cancer Res. 1995;55:1811–1816. [PubMed] [Google Scholar]
- 35.Thomas E D, Storb R. Blood. 1984;64:325–328. [PubMed] [Google Scholar]
- 36.Maciejewski J P, Selleri C, Sato T, Anderson S, Young N S. Br J Haematol. 1995;91:245–252. doi: 10.1111/j.1365-2141.1995.tb05277.x. [DOI] [PubMed] [Google Scholar]
- 37.Brodsky R A, Sensenbrenner L L, Jones R J. Blood. 1996;87:491–494. [PubMed] [Google Scholar]