Abstract
Aims
To assess the effect of a single dose of maraviroc on the QTc interval in healthy subjects and to evaluate the QTc interval–concentration relationship.
Methods
A single-dose, placebo- and active-controlled, five-way crossover study was conducted to investigate the effects of maraviroc (100, 300, 900 mg) on QTc in healthy subjects. Moxifloxacin (400 mg) was used as the active comparator. The study was double-blind with respect to maraviroc/placebo and open label for moxifloxacin. There was a 7-day wash-out period between each dose. QT interval measurements obtained directly from the electrocardiogram (ECG) recorder were corrected for heart rate using Fridericia's correction (QTcF). A placebo run-in day was conducted before period 3, when ECGs were collected at intervals while subjects were resting or during exercise. These ECGs plus other predose ECGs were used to evaluate the QT/RR relationship for each subject to enable calculation of an individual's heart rate correction for their QT measurements (QTcI). ECGs were taken at various intervals pre- and postdose in each study period. Pharmacokinetic parameters were determined for each maraviroc dose. The end-points that were evaluated were QTcF at median time to maximum concentration (Tmax) based on the machine readings and QTcI at median Tmax based on manual over-reads of the QT/RR data. A separate analysis of variance was used for each of the pair-wise comparisons for each end-point. The relationship between QTc interval and plasma concentration was also investigated using a mixed-effects modelling approach, as implemented by the NONMEM software system. A one-stage model was employed in which the relationship between QT and RR and the effects of maraviroc plasma concentration on QT were estimated simultaneously.
Results
The mean difference from placebo in machine-read QTcF at median Tmax for maraviroc 900 mg was 3.6 ms [90% confidence interval (CI) 1.5, 5.8]. For the active comparator, moxifloxacin, the mean difference from placebo in machine-read QTcF was 13.7 ms. The changes from placebo for each of the end-points were similar for men and women. No subjects receiving maraviroc or placebo had a QTcF ≥ 450 ms (men) or QTcF ≥ 470 ms (women), nor did any subject experience a QTcF increase ≥ 60 ms from baseline at any time point. Analysis based on the QTcI data obtained from the manual over-readings of the ECGs gave numerically very similar results. The QT:RR relationship was similar pre- and postdose and was not related to maraviroc concentration. The population estimate of the QT:RR correction factor was 0.324 (95% CI 0.309, 0.338). The population estimate of the slope describing the QT–concentration relationship was 0.97 μs ml ng−1 (95% CI −0.571, 2.48), equivalent to an increase of 0.97 ms in QT per 1000 ng maraviroc plasma concentration. Most adverse events were mild to moderate in severity.
Conclusions
Single doses of maraviroc, up to and including 900 mg, had no clinically relevant effect on QTcF or QTcI. At all maraviroc doses and for both end-points, the mean difference from placebo for QTc was <4 ms. There was no apparent relationship between QT interval and maraviroc plasma concentration up to 2363 ng ml−1. This conclusion held in both male and female subjects, and there was no evidence of a change in the QT/RR relationship with concentration.
Keywords: CCR5 inhibitor, maraviroc, moxifloxacin, pharmacokinetics, QTcF, QTcI, safety
Introduction
The QT interval is the summation of all the action potentials of individual cardiac cells, which occurs during ventricular depolarization and repolarization [1]. Prolongation of the QT interval is the most common reason that drugs in discovery are discontinued or drugs on the market are relabelled [2, 3]. A QT interval with values ≥ 450 ms for men and ≥ 470 ms for women is considered prolonged and may be predictive of the ability of a drug to induce cardiac arrhythmias and sudden death [4–6]. Patients who have prolonged QT intervals are considered to have long QT syndrome (LQTS), which can be congenital or acquired, and is associated with torsade de pointes, a life-threatening ventricular tachycardia [7, 8]. Congenital LQTS has been linked with mutations in a set of genes encoding ion channels expressed in cardiac myocytes [1]. One of these genes, the human ether-a-go-go gene (hERG), encodes the rapid component of the delayed rectifier potassium channel (Ikr) and has been suggested to be a marker for torsade de pointes [9]. Functionally, Ikr is a potassium channel that controls the K+ repolarizing current following ventricular depolarization [9], and interference with Ikr activity results in prolonged action potentials at the cellular level and a long QT interval [10, 11].
Preclinical studies have shown that maraviroc, a novel CCR5 receptor antagonist, can interact with the hERG ion channel with low affinity (19 ± 3% inhibition at 10 μM) [12] and block repolarization through Ikr at unbound plasma concentrations > 3 μM, which is approximately 10 times higher than the expected plasma concentration from a clinically relevant 300-mg dose [13].
In a Phase I study enrolling healthy subjects, maraviroc was shown to increase the QT interval following a single 1200-mg dose, resulting in a mean increase in QTcP of 7.8 ms [14]. The QTcP values were calculated using a study-specific population heart rate correction factor of 0.28, QTcP = QT/RR0.28. Although a single dose of maraviroc 1200 mg appeared to be associated with a modest QTc prolongation, there was no evidence of a clinically significant effect of maraviroc on QTcP following multiple doses up to and including 300 mg twice daily (b.i.d.) and 600 mg once daily [14]. Dose-limiting adverse events (AEs) for maraviroc were postural hypotension [14], and given the potential for a tachycardia related to any fall in blood pressure, it is important to measure the effect of maraviroc on the heart-rate-corrected QT interval.
The objective of the current study was to examine the effect of single doses of maraviroc on QTc interval prolongation in healthy subjects. The analysis was based on QTcF (Fridericia's correction) derived from the machine-read QT/RR interval, and also on QTcI derived from manually overread QT/RR intervals. QTcI was calculated using the equation 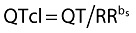 , in which bs is the slope of the regression line for loge(QT) against loge(RR) intervals for each individual in a mixed-effects model [15]. By comparison, for Fridericia's correction, bs = 0.333. For the statistical analysis, the effect on QTcI at median Tmax was considered the primary analysis. Use of a correction factor customized for each subject minimizes inconsistencies in QT interval calculations inherent in formulae, which standardize the length of one RR cycle to 60 bpm [16, 17]. This can be a concern when there are drug-induced changes in heart rate, particularly related to postural hypotension. In addition, any effect of maraviroc on the QT interval was explored using mixed-effects modelling to describe the QT–concentration relationship.
, in which bs is the slope of the regression line for loge(QT) against loge(RR) intervals for each individual in a mixed-effects model [15]. By comparison, for Fridericia's correction, bs = 0.333. For the statistical analysis, the effect on QTcI at median Tmax was considered the primary analysis. Use of a correction factor customized for each subject minimizes inconsistencies in QT interval calculations inherent in formulae, which standardize the length of one RR cycle to 60 bpm [16, 17]. This can be a concern when there are drug-induced changes in heart rate, particularly related to postural hypotension. In addition, any effect of maraviroc on the QT interval was explored using mixed-effects modelling to describe the QT–concentration relationship.
Methods
Subjects
The study enrolled healthy male and female subjects, 18–45 years of age, weighing between 60 and 90 kg (men) and 50 and 85 kg (women). Female subjects were postmenopausal, surgically sterile, or using an approved method of birth control. Subjects were excluded for clinical disease; allergies; hypersensitivity to quinolones; laboratory or physical examination abnormalities; investigational drug use; weekly alcohol use exceeding 21 units (1 unit = 285 ml of beer, 25 ml of spirits, or 125 ml of wine) for women or 28 units for men; tobacco use exceeding five cigarettes per day; blood donation in the previous 2 months; and positive serology for HIV, hepatitis B or hepatitis C. All subjects were required to have normal electrocardiogram (ECG) results. The study was conducted in accordance with the Declaration of Helsinki and approved by the Independent Ethics Committee at the study centre. Subjects were educated as to the risks and benefits of the study before enrolment and submitted written informed consent.
Study design
A randomized, single-dose, placebo- and active-controlled, five-way crossover study was designed to assess the effect of three oral doses of maraviroc (100, 300 and 900 mg) and an active comparator (oral moxifloxacin 400 mg) on the QT interval in healthy subjects. The study was double blind for maraviroc and placebo and open label for moxifloxacin. Subjects were randomized to one of 10 treatment sequences. A single-blind placebo run-in day was conducted during period 3 for all treatment sequences. Each treatment period lasted 2 days with the exception of period 3, which was extended to include the run-in day.
For each study period, subjects arrived at the study centre on the evening before dosing and fasted overnight. On day 1, subjects received either maraviroc, placebo or moxifloxacin according to the randomization schedule. ECG measurements were recorded immediately before blood collection. On day 2, subjects received a physical examination, along with supine and standing blood pressure, pulse rate, and 12-lead ECG assessment. A 7-day wash-out followed each treatment period.
Maraviroc and placebo were supplied as 100- or 150-mg oral tablets. Moxifloxacin was supplied as a 400-mg oral tablet. A single dose of drug was administered in the morning with 250 ml water under fasted conditions. A follow-up examination was performed 7–14 days after completion of the study.
Pharmacokinetics and pharmacodynamics
To assess maraviroc plasma concentrations, blood samples were taken on day 1 of each treatment period and on the placebo run-in day (treatment period 3) at predose and postdose (1, 2, 3, 4, 8 and 12 h) intervals. To ensure the most stringent estimation of QT interval prolongation by maraviroc, data were collected at time points expected to be coincident with maximal exposure of maraviroc. Samples were prepared using solid-phase extraction and analysed using liquid chromatography/tandem mass spectrometry (Maxxam Analytics Inc., Mississauga, ON, Canada).
Resting ECG measurements were recorded at −75 and −45 min predose and then immediately before each pharmacokinetic blood sampling after subjects had been semirecumbent for at least 30 min. Subjects were restricted to room-temperature fluids until the 4-h postdose ECG was completed and for 1 h before each ECG recording. On the placebo run-in day, six additional ECGs were performed during an exercise period: three ECGs as heart rate was increasing and three ECGs as heart rate was decreasing. All measurements were taken at approximately equal intervals between the subject's resting heart rate and up to 90 bpm. QT, RR and QTcF intervals were calculated by the ECG recording machines (Hewlett Packard 709; Palo Alto, CA, USA). QT and RR intervals were also determined using a manual over-read by a third party (Spacelabs Healthcare, formerly Quantum Research, Welwyn Garden City, UK). Measurements were taken over three beats obtained from lead 2.
Safety
All observed or reported AEs were recorded for all subjects. AEs were classified as mild, moderate or severe, and the relationship to treatment was determined.
Statistical analysis
A sample size of 51 subjects was estimated to give 80% power of concluding non-inferiority between an active dose and placebo using a one-sided 5% level test in which non-inferiority was based on a ≤ 10-ms increase in QTc interval in the active group. This calculation assumed that the true difference from placebo was ≤ 5 ms. Sixty subjects were recruited to ensure that at least 51 subjects completed the study.
Maraviroc pharmacokinetic parameters were summarized by treatment group. Subjects fasted the evening before each study visit and up to 4 h post dose. No formal statistical analyses were performed on these data. The treatment means and the differences between active treatment and placebo were calculated along with 90% confidence intervals (CIs). Comparisons against placebo were carried out for the following end-points: (i) QTcF at median time to maximum concentration (Tmax) based on machine readings, and (ii) QTcI at median Tmax based on manually over-read data. Moxifloxacin was used as an internal positive control for the study. A separate analysis of variance (anova) was performed for each dose of maraviroc or moxifloxacin compared with placebo. The anova model included terms for sequence, subject within sequence (random effect), period, and treatment, with baseline as a covariate. Baseline was split into two variables: the average for subjects over the relevant two-study periods and the deviation of each study period baseline from this average. The Satterthwaite approximation for the denominator degrees of freedom was used. The treatment means and differences between active treatment and placebo were calculated along with 90% CIs.
For QTcI derivation, ECG data recorded predose and during exercise (day 3) were used to determine the QT/RR relationship for each subject. Data were analysed using a mixed-effects model such that each subject had a specific estimate for the slope (bs) of the QT/RR relationship. This slope was used to derive QTcI using the following equation: 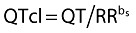 .
.
Concentration–QT modelling
An additional one-stage analysis using the QT interval as the dependent variable was employed, as described below. All postdose data for the maraviroc dose levels and placebo, and all predose and run-in-day data were used in the analysis.
Base model
A base model was estimated to describe the QT interval as a constant (intercept), allow for differences between male and female subjects, and adjust for heart rate by including RR intervals in the model. The base model with the heart rate correction, intercept and associated error is given below:
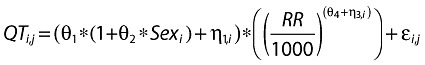 |
For the ith individual at the jth time, sex is a categorical variable equal to 0 for men and 1 for women, θ1 represents the population mean estimate of the intercept for male subjects, θ2 is provided to allow for a difference in the baseline QTc between male and female subjects, η1 represents random intersubject variability assumed to be independent and identically distributed with 0 mean and variance ω12(∼NIID(0, ω12)), and ε denotes the residual error that is assumed to be independent and identically distributed with 0 mean and variance σ2[∼NIID(0, σ2)]. θ4 represents the population correction factor and η3 its random intersubject variability assumed to be independent and identically distributed with 0 mean and variances, ω32. A correlation is assumed between the random intersubject variance components.
Model 1
A slope parameter describing the correlation between maraviroc concentration and QT was added to the base model. The null model with the slope and intercept for estimating QT is given below:
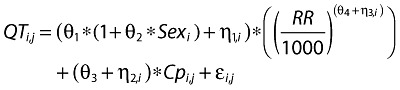 |
θ3, the population mean slope, is fixed to a value of 0, η2 represents random intersubject variability assumed to be independent and identically distributed with 0 mean and variance ω22, and a correlation is assumed between the random intersubject variance components. Cp is the observed maraviroc plasma concentration.
Model 2
A slope parameter describing the correlation between maraviroc concentration and QT was added to the base model. The drug–effect model with the slope and intercept for estimating QT is as described for Model 1 above, in which θ3 represents the population mean slope estimate.
Model 3
An additional parameter describing the correlation between the change in the slope of the QT/RR relationship and maraviroc plasma concentration was added to the model. The full drug–effect model for estimating QT is given below:
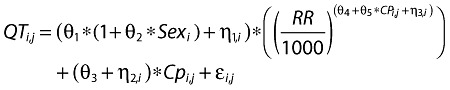 |
In this equation, θ5 represents the change in the slope of the QT/RR relationship in relation to maraviroc plasma concentration.
Results
The study enrolled 61 healthy subjects (men, n = 30; women, n = 31) between 19 and 44 years of age. The mean height and weight of the men was 175 cm and 72 kg, respectively; the group comprised one Asian and 29 White subjects. The mean height and weight of the women was 165 cm and 61 kg, respectively, and the group comprised one Asian, one Black and 29 White subjects. Of the 61 subjects enrolled in the study, four discontinued treatment. One subject defaulted, and three discontinued because of AEs that were not considered treatment related.
The pharmacokinetic profile of maraviroc at varying doses (100, 300 and 900 mg) is summarized in Table 1. Consistent with other Phase I studies [14], maraviroc was rapidly absorbed, with the mean Tmax of 2–3 h for all doses. The area under the plasma concentration–time curve up to the last measurable concentration (AUClast) and maximum observed plasma concentration (Cmax) increased in a nondose-proportional manner.
Table 1.
Mean maraviroc plasma pharmacokinetics
| Parameter, mean (SD) | Maraviroc 100 mg n = 60 | Maraviroc 300 mg n = 59 | Maraviroc 900 mg n = 58 |
|---|---|---|---|
| AUClast (ng h ml−1) mean (CV%) | 396 (45) | 1840 (34) | 5259 (29) |
| Cmax (ng ml−1) mean (CV%) | 111 (56) | 464 (38) | 1148 (36) |
| Tmax (h) mean (SD) | 2.8 (0.93) | 2.6 (1.00) | 2.2 (1.06) |
Means are geometric for AUClast and Cmax and arithmetic for Tmax.
The adjusted mean machine-derived QTcF values for each drug dose (maraviroc and moxifloxacin), the difference compared with placebo, and the 90% CIs are listed in Table 2. ECG data collected at the time point closest to the median Tmax (3 h for maraviroc 100 mg and 300 mg and 2 h for maraviroc 900 mg) were used to determine QTcF at the median Tmax for drug exposure at each dose. For maraviroc, the greatest observed mean difference from placebo occurred with the 900-mg dose, with an increase of 3.6 ms (90% CI 1.5, 5.8). Moxifloxacin (400 mg), the active comparator, elicited a 13.7-ms increase (90% CI 11.5, 15.8) over placebo at median Tmax. Note that for moxifloxacin, the Tmax was assumed to occur at 2 h postdose.
Table 2.
Difference from placebo in QTc at Tmax for maraviroc and moxifloxacin
| Adjusted means | ||||||
|---|---|---|---|---|---|---|
| End-point | Comparison | n | Active | Placebo | Mean diff. | 90% CI |
| Machine-read QTcF at median Tmax | Maraviroc 100 mg vs. placebo | 59 | 381.5 | 382.5 | −0.93 | (−2.8–0.98) |
| Maraviroc 300 mg vs. placebo | 58 | 381.1 | 382.4 | −1.3 | (−3.9–1.4) | |
| Maraviroc 900 mg vs. placebo | 58 | 383.6 | 380.0 | 3.6 | (1.5–5.8) | |
| Moxifloxacin 400 mg vs. placebo | 58 | 393.4 | 379.7 | 13.7 | (11.5–15.8) | |
| Manually read QTcI at median Tmax | Maraviroc 100 mg vs. placebo | 59 | 399.7 | 400.4 | −0.72 | (−3.0–1.6) |
| Maraviroc 300 mg vs. placebo | 58 | 400.8 | 400.6 | 0.24 | (−1.9–2.3) | |
| Maraviroc 900 mg vs. placebo | 58 | 402.8 | 399.2 | 3.6 | (1.0–6.2) | |
| Moxifloxacin 400 mg vs. placebo | 58 | 412.7 | 398.7 | 14.0 | (11.5–16.4) | |
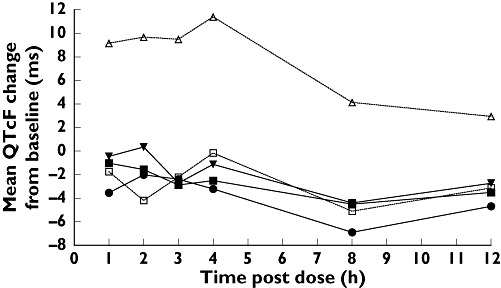
Overall, the largest machine-read mean QTcF changes from baseline with maraviroc occurred in subjects receiving the 900-mg dose (Figure 1) and occurred 1–2 h postdose, when the plasma concentration of maraviroc reached maximal levels (Figure 2).
Figure 1.
Mean machine-read QTcF changes from baseline over time following three oral doses of maraviroc (100, 300 and 900 mg); the active comparator, moxifloxacin (400 mg); or placebo. Baseline is defined as the average of the predose measurements taken on day 1 for the treatment period. *All n = 58, except for maraviroc 100 mg (n = 59). Maraviroc 100 mg (n = 59) ( ); Maraviroc 300 mg (n = 58) (▪); Maraviroc 900 mg (n = 58) (▾); Moxifloxacin 400 mg (n = 58) (▵); Placebo (n = 58/59*) (□)
); Maraviroc 300 mg (n = 58) (▪); Maraviroc 900 mg (n = 58) (▾); Moxifloxacin 400 mg (n = 58) (▵); Placebo (n = 58/59*) (□)
Figure 2.
Mean maraviroc plasma concentration of three oral doses of maraviroc (100, 300 and 900 mg) administered over 12 h. The lower limit of quantification was 0.500 mg ml−1. Maraviroc 100 mg ( ); Maraviroc 300 mg (▪); Maraviroc 900 mg (▾)
); Maraviroc 300 mg (▪); Maraviroc 900 mg (▾)
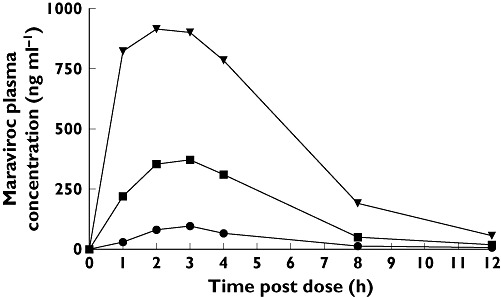
Figure 3 shows the manually read mean QTcI change from baseline over time for each treatment. By comparison with Figure 1, it can be seen that the machine readings give similar results to the manual readings, notwithstanding the different RR correction methods. The comparability of manual vs. machine readings has been discussed by Darpo et al., who suggest that there is little evidence that manual methods have advantages over automated methods in measuring QT, and that clinical interpretations remain the same [18].
Figure 3.
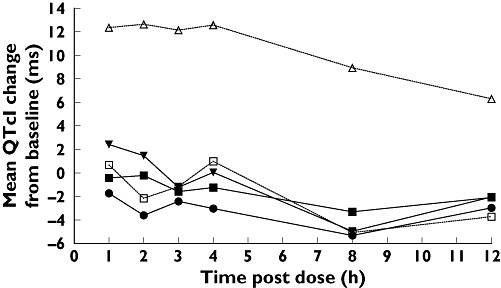
Mean manually read QTcI changes from baseline over time following three oral doses of maraviroc (100, 300 and 900 mg); the active comparator, moxifloxacin (400 mg); or placebo. Baseline is defined as the average of the predose measurements taken on day 1 of each treatment period. *All n = 58, except for maraviroc 100 mg (n = 59). Maraviroc 100 mg (n = 59) ( ); Maraviroc 300 mg (n = 58) (▪); Maraviroc 900 mg (n = 58); (▾); Moxifloxacin 400 mg (n = 58) (▵); Placebo (n = 58/59*) (□)
); Maraviroc 300 mg (n = 58) (▪); Maraviroc 900 mg (n = 58); (▾); Moxifloxacin 400 mg (n = 58) (▵); Placebo (n = 58/59*) (□)
Concentration–QT modelling
The estimate of the population correction factor from the one-stage analysis final model was 0.324 (95% CI 0.309, 0.338); this was similar to the estimate from the analysis of baseline data, indicating that the QT/RR relationship was similar pre- and postdose. The intersubject variability in the QT/RR slope (correction factor) was 0.00287 [coefficient of variation (CV) 16.5%].
The slope estimate from the final model was 0.970 μs ml ng−1 (95% CI −0.571, 2.48), within the concentration range studied. Given the imprecision in the estimate, it seems reasonable to conclude that there was no apparent relationship between QT interval and maraviroc plasma concentration up to 2363 ng ml−1. This conclusion held in both male and female subjects, and there was no evidence of a change in the QT/RR relationship with concentration. Interoccasion variability (IOV) was added to the model to account for the variability in QT across study periods. The intersubject variability in QT estimated from the final model was 178 (CV 3.35%), and the IOV was 13.4 (CV 0.92%). The residual intrasubject variability was 73.6 (CV 2.16%). Table 3 shows the parameter estimates for the model, and Figure 4 shows the population predicted values of QTcI interval vs. concentration for male and female subjects.
Table 3.
Parameter estimates for final QT–concentration model
| Model parameter | Estimate | 95% CI* |
|---|---|---|
| Structural model | ||
| Intercept (ms) | 398 | 391–403 |
| Sex (women) | 0.0166 | −0.000489–0.0364 |
| QT correction factor | 0.324 | 0.309–0.338 |
| Slope (concentration, μs ml ng−1) | 0.970 | −0.571–2.48 |
| Statistical model | Estimate | CV% |
| Intersubject variability (intercept) | 178 | 3.35 |
| Intersubject variability (correction factor) | 0.00287 | 16.5 |
| Intersubject variability (slope) | 5.41 | 240 |
| Interoccasion variability (intercept) | 13.4 | 0.920 |
| Residual intrasubject variability | 73.6 | 2.16 |
CIs derived from nonparametric bootstrap methods.
Figure 4.
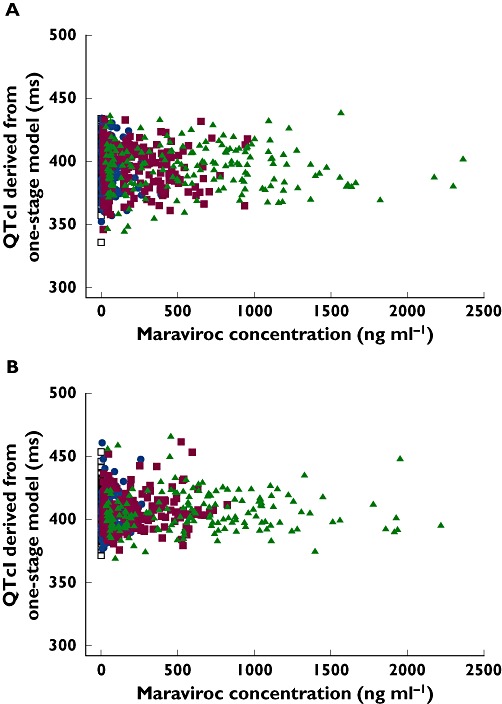
Population predicted values of QTcI vs. concentration for (A) male and (B) female subjects. Maraviroc 100 mg ( ); Maraviroc 300 mg (▪); Maraviroc 900 mg (▴); Placebo (□)
); Maraviroc 300 mg (▪); Maraviroc 900 mg (▴); Placebo (□)
Safety
Neither maraviroc nor placebo induced QTcI elevations ≥ 450 ms for men or ≥ 470 ms for women, whereas moxifloxacin treatment elicited maximum QTcI values above these limits in two subjects. There were no reported QTcI increases ≥ 60 ms from baseline for any subject at any time point. There were no serious AEs or discontinuations due to treatment-related AEs. Three subjects discontinued owing to nontreatment-related AEs (miscarriage, tonsil abscess, and pyelonephritis). Most AEs were mild to moderate in severity. The number of AEs was highest in subjects taking maraviroc 900 mg. The most frequent treatment-related AE was dizziness, followed by headache, nausea and postural hypotension. A similar number of subjects in all treatment groups, including placebo, experienced laboratory abnormalities, but none was considered clinically significant.
Discussion
In response to a number of serious cardiac problems resulting from the use of noncardiovascular drugs, the European Agency for Evaluation of Medicinal Products of the Committee for Proprietary Medicinal Products adopted guidelines in 1997 that detailed preclinical and clinical testing procedures that should be performed on new drugs coming to market [6]. These guidelines, as well as general safety concerns, dictated careful scrutiny of any potential effect of maraviroc on QT interval prolongation. Subsequently, and after the current study was complete, the Expert Working Group (Efficacy) of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use issued the E14 guidance on the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs, which has been adopted by national/regional regulatory bodies [19]. The E14 guidelines give further advice on the assessment of QT in a ‘thorough QT study’.
This study has demonstrated that maraviroc did not induce clinically significant changes in QTcF at single doses up to and including 900 mg. To assess robustly possible effects of maraviroc, QTcF was assessed at a number of different time points based on established pharmacokinetics. The end-points analysed were QTcF at the median Tmax for maraviroc based on machine-read data and QTcI at the median Tmax based on manually read data. The assessments were performed from 1 to 4 h postdose when systemic maraviroc exposure was the highest and background biases were minimized (i.e. subjects were fasted and mainly confined to their beds). The largest mean increase in QTcF with maraviroc across the end-points was observed following a single 900-mg dose, resulting in a mean increase from placebo of 3.6 ms (90% CI 1.5, 5.8). This was not considered to be clinically relevant because the 90% CI excluded 10 ms. The clinical dose of maraviroc is 300 mg b.i.d. in the absence of potent inhibitors or inducers of cytochrome P450 3A4. At this dose, QTcF prolongation was not apparent as determined by a mean increase of −1.3 ms (90% CI −3.9, 1.4). Based on these data, b.i.d. administration of maraviroc 300 mg would not be expected to cause a clinically significant prolongation of the QT interval. For the active comparator, moxifloxacin, the estimated QTcF difference from placebo was about 14 ms. This increase is in accordance with the increase in QTc for moxifloxacin 400 mg observed in large study populations and other Phase I studies [20–22].
QT interval prolongation is sensitive to many intrinsic and extrinsic factors, including sex. Women have a significantly greater risk of prolonged QT interval compared with men and also a greater response to drugs that prolong QT [11, 23]. Mechanisms responsible for this sex difference are unknown, although variability in the regulation of genes controlling cardiac physiology has been proposed [10]. There was no evidence of a gender difference in the QTc interval–concentration relationship.
Conclusions
Single doses of maraviroc up to and including 900 mg had no clinically relevant effect on QTcF or QTcI. At all maraviroc doses and for both end-points, the mean difference from placebo for QTc was <4 ms. Moxifloxacin produced mean differences from placebo of 14 ms under the same conditions. There was no apparent relationship between QT interval and maraviroc plasma concentration. Overall, maraviroc was well tolerated at the doses studied.
Competing interests
J.D.D., F.H., G.L., T.H., D.S. and G.W. were employees of Pfizer Ltd at the time of this research.
This study was sponsored by Pfizer Global Research and Development. Editorial assistance was provided by Susan DeRocco, PhD, Nancy E. Price, PhD and Janet E. Matsuura, PhD at Complete Healthcare Communications, Inc., and was funded by Pfizer Inc., New York, NY, USA.
REFERENCES
- 1.Crumb W, Cavero II. QT interval prolongation by non-cardiovascular drugs: issues and solutions for novel drug development. Pharm Sci Technol Today. 1999;2:270–80. doi: 10.1016/s1461-5347(99)00172-8. [DOI] [PubMed] [Google Scholar]
- 2.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 3.Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:2215–20. doi: 10.1001/jama.287.17.2215. [DOI] [PubMed] [Google Scholar]
- 4.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–94. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 5.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–7. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 6.Committee for Proprietary Medicinal Products. 1997. Points to Consider: the Assessment of the Potential for QT Interval Prolongation by Non-cardiovascular Medicinal Products. Report no. CPMP/986/96. London. [DOI] [PMC free article] [PubMed]
- 7.Dessertenne F. [Ventricular tachycardia with 2 variable opposing foci] Arch Mal Coeur Vaiss. 1966;59:263–72. [PubMed] [Google Scholar]
- 8.Schwartz PJ, Periti M, Malliani A. The long Q-T syndrome. Am Heart J. 1975;89:378–90. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- 9.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 10.Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996;94:1471–4. doi: 10.1161/01.cir.94.6.1471. [DOI] [PubMed] [Google Scholar]
- 11.Pham TV, Sosunov EA, Gainullin RZ, Danilo P, Rosen MR. Impact of sex and gonadal steroids on prolongation of ventricular repolarization and arrhythmias induced by I (K)-blocking drugs. Circulation. 2001;103:2207–12. doi: 10.1161/01.cir.103.17.2207. [DOI] [PubMed] [Google Scholar]
- 12.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49:4721–32. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansfield RW, Brunton NS, Sutton MR, Leishman D. Pre-clinical assessment of the potential of UK-427,857, a CCR5 antagonist, to effect cardiac QT intervals. 15th International AIDS Conference; 2004 July 11–16; Bangkok, Thailand. 2004. [Google Scholar]
- 14.Abel S, van der Ryst E, Rosario MC, Ridgway CE, Medhurst CG, Taylor-Worth RJ, Muirhead GJ. Assessment of the pharmacokinetics, safety and tolerability of maraviroc, a novel CCR5 antagonist, in healthy volunteers. Brit J Clin Pharm. 2008;65(1):5–18. doi: 10.1111/j.1365-2125.2008.03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah A, Hajian G. A maximum likelihood approach for estimating the QT correction factor using mixed effects model. Stat Med. 2003;22:1901–9. doi: 10.1002/sim.1434. [DOI] [PubMed] [Google Scholar]
- 16.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353. [Google Scholar]
- 17.Fredericia LS. Die Systolendauer im Elektrokardiogramm bei normalen Menschen und bei Herzkranken. Acta Med Scand. 1920;52:469. [Google Scholar]
- 18.Darpo B, Agin M, Kazierad DJ, Layton G, Muirhead G, Gray P, Jorkasky DK. Man versus machine: is there an optimal method for QT measurements in thorough QT studies? J Clin Pharmacol. 2006;46:598–612. doi: 10.1177/0091270006286900. [DOI] [PubMed] [Google Scholar]
- 19.Darpo B, Nebout T, Sager PT. Clinical evaluation of QT/QTc prolongation and proarrhythmic potential for nonantiarrhythmic drugs: the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use E14 guideline. J Clin Pharmacol. 2006;46:498–507. doi: 10.1177/0091270006286436. [DOI] [PubMed] [Google Scholar]
- 20.Bednar MM, Harrigan EP, Anziano RJ, Camm AJ, Ruskin JN. The QT interval. Prog Cardiovasc Dis. 2001;43(5 Suppl. 1):1–45. doi: 10.1053/pcad.2001.21469. [DOI] [PubMed] [Google Scholar]
- 21.Demolis JL, Kubitza D, Tenneze L, Funck-Brentano C. Effect of a single oral dose of moxifloxacin (400 mg and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68:658–66. doi: 10.1067/mcp.2000.111482. [DOI] [PubMed] [Google Scholar]
- 22.Noel GJ, Natarajan J, Chien S, Hunt TL, Goodman DB, Abels R. Effects of three fluoroquinolones on QT interval in healthy adults after single doses. Clin Pharmacol Ther. 2003;73:292–303. doi: 10.1016/s0009-9236(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Anderson ME. Reduced repolarization reserve in ventricular myocytes from female mice. Cardiovasc Res. 2002;53:763–9. doi: 10.1016/s0008-6363(01)00387-x. [DOI] [PubMed] [Google Scholar]


