Abstract
Change in diversity of fossil pollen through time is used as a surrogate for biodiversity history. However, there have been few studies to explore the sensitivity of the measured pollen diversity to vegetation changes and the relationship between pollen diversity and plant diversity. This paper presents results of a study to assess the relationship between pollen diversity and relative abundance of pollen from different altitudinal vegetation belts (subandean forest, Andean forest, subparamo and grassparamo) in three records from the tropical Andes in Colombia. The results indicated that plant diversity in the vegetation declined with altitude and pollen diversity is positively correlated to the abundance of pollen from lower altitude vegetation belts and negatively correlated to that from the grassparamo. These results, therefore, suggest that pollen diversity coarsely reflects the diversity of the surrounding vegetation. Using this interpretation, we were able to predict changes in plant diversity over the past 430 000 years in the Colombian Andes. Results indicated that under warmer climatic conditions, more species-diverse vegetation of low elevation moved upslope to contribute more pollen diversity to the study sites, and under colder conditions, species-poor grassparamo moved downslope and observed pollen diversity was lower. This study concludes that fossil pollen diversity may provide an important proxy to reconstruct the temporal changes in plant diversity.
Keywords: pollen diversity, plant diversity, altitudinal vegetation belts, pollen analysis, Andes, Colombia
1. Introduction
Fossil animal (e.g. bones and teeth) or plant remains (e.g. leaves, wood and seeds) have often been used to provide a history of long-term biodiversity (Valentine & Jablonski 1993; Courtillot & Gaudemer 1996; Benton 2000; Barnosky et al. 2005; Rohde & Muller 2005). This history may provide information about how past environment change has influenced biodiversity and how much variability occurs in response to change (Brown et al. 2001; Willis et al. 2005; Jaramillo et al. 2006). Such information is critical to determine how future change will affect biodiversity. However, this information is often restrained by the limited availability of fossil plant and/or animal datasets. A particularly useful proxy, therefore, is fossil pollen preserved in dated sedimentary sequences. Not only is this usually a more readily available source, but also it has the advantage of better temporal continuity than most other fossil datasets (e.g. Silvertown 1985; Odgaard 1999; Brown et al. 2001; Jaramillo et al. 2006). However, the relationship between fossil pollen data in sedimentary sequences and the vegetation producing the pollen is complicated (Davis 1963; Jackson 1994; Sugita 1994; Calcote 1995) and this makes the interpretation of pollen diversity as a surrogate for plant diversity more difficult (Weng et al. 2006).
Most of the difficulties arise from the variation in pollen productivity and dispersal ability from species to species (Faegri & Iversen 1989). Thus, the correlation between plant and pollen is not a one-to-one relationship. Most pollen grains are transported over a distance before they are deposited. Pollen taxa that are both rich pollen producers and good dispersers (e.g. most species of Pinus) may be always observed in study sites at a certain range (up to several hundred kilometres or more). In the opposite situation, taxa with poor pollen production and dispersal (e.g. most entomophilous taxa) may be almost always absent in records, but only occasionally recorded in nearby locations (e.g. forest hollows). Therefore, detected pollen diversity is a mixture of local and regional pollen. In most investigations, only a limited number of pollen grains (a few hundred grains) are counted, thus the detection of a rare taxon is often influenced by the dominant taxa. Whether or not such dominant taxa exist may make the detected number different even when the actual number of taxa in this area is the same (Weng et al. 2006). If all taxa deposited in a certain area during a certain time-interval (e.g. one year) are counted, the data from different sites or samples may be more comparable. However, this method may be limited by precision of time control (Weng et al. 2006). To register all taxa deposited in one site (i.e. to reach the saturation level) may be time-consuming (Weng et al. in review).
The correlation of the observed pollen diversity with plant diversity is also influenced by the size of the depositional basins, because the source range may be different (Jacobson & Bradshaw 1981; Prentice 1985; Sugita 1993; Jackson 1994; Calcote 1995; Jackson & Lyford 1999). To obtain a good estimate of plant diversity, it is ideal to have a network of pollen records from different types of basins at different locations (Weng et al. 2006). However, due to the need for of enough satisfactory sites (and enormous labour investment), such a network is difficult to develop.
In most records, the registered list of taxa is incomplete either due to lower identification level of pollen taxa (Faegri & Iversen 1989; Moore et al. 1991) or due to lack of detection of many rare taxa in a limited pollen count (Weng et al. 2006). Moreover, some rare and unidentified pollen taxa are often considered trivial, and therefore neglected by the investigators.
All these problems make biodiversity reconstruction based on pollen data complicated and challenging. Pollen diversity from different basins may be difficult to be compared directly because the different degrees of mixing of local and regional pollen (due to the differences in basin size) may make the interpretation of the detected pollen diversity different (unless there is evidence of source range). However, for a single site, the pollen source may be relatively comparable and the change of pollen diversity may reflect local or regional changes of vegetation composition.
There may be two ways to test the correspondence between pollen and plant diversity: (i) comparing pollen diversity and plant diversity along a diversity transect, and (ii) correlating the temporal changes of pollen diversity and plant diversity. For the former, measuring plant diversity along a transect is possible, but it is difficult to find a series of depositional basins with similar pollen source ranges along the transect. For the latter, it is even more difficult to have plant diversity data from different time periods corresponding to the observed pollen diversity.
The vegetation history of the Bogotá basin in the tropical Andes of Colombia has been well established (e.g. van der Hammen & González 1960; Van der Hammen 1974; Hooghiemstra 1984; Van't Veer & Hooghiemstra 2000; Torres 2006). During the past glacial/inter-glacial cycles, the altitudinal vegetation belts shifted altitudinally in response to climate change. The vegetation surrounding the study site changed with climate, while the change in pollen source range was relatively small. Modern vegetation surveys have shown that the plant diversity in these vegetation belts decreases with elevation (e.g. Cleef & Hooghiemstra 1984; Gentry 1988; Keizer 2000). We hypothesize that if the pollen diversity may essentially represent the relative plant diversity level of the surrounding vegetation, it may be considered as an indirect test of this idea. In this paper, we explore whether the correlation between vegetation change and pollen diversity exists, with the aim to evaluate pollen diversity as an indicator of plant diversity.
2. Study area
The Bogotá Basin is located in the Colombian Eastern Cordillera. This region is in the northern part of one of the 25 global biodiversity hotspots—the tropical Andes (Myers et al. 2000). The area is characterized by steep slopes and a very high level of biodiversity. The vegetation distribution is controlled by altitude, reflecting an altitudinal gradient in climatic parameters, mainly temperature. In the Eastern Cordillera, the vegetation may be classified into the following altitudinal belts (Van der Hammen 1974; Cleef & Hooghiemstra 1984; Van der Hammen & Cleef 1986):
Tropical lowland forest. Distributed below 1000 m altitude. Bombacaceae, Byrsonima, Iriartea, Mauritia and Spathiphyllum are frequent components of this zone. The average annual temperature is from 22°C at the lower altitudinal limit to 13°C at the upper altitudinal limit. The annual precipitation is about 1200–3000 mm.
The lower montane forest (subandean forest). Distributed from 1000 to 2300 m elevation. Acalypha, Alchornea, Cecropia, Palmae, Hieronima, Ficus and Malpighiaceae are common components. The average annual temperature is 19–23°C and annual precipitation is about 1500–1700 mm.
The upper montane forest (Andean forest zone). Ranges from 2300 (or 2500) to 3200 m altitude. Weinmannia and Quercus are frequent in this zone. Other important components include Alnus, Myrica, Styloceras, Podocarpus, Clusia, Rapanea, Juglans, Ilex and Hedyosmum. The average annual temperature is 9–16°C, and annual precipitation is 700–1400 mm in dry inter-Andean high plains and 1000–3000 mm on outer slopes of the Cordillera. The upper boundary of this zone reflects the upper forest line.
Subparamo. Occurs from 3200 to 3500 m. This is a transitional zone from forest to paramo. It is dominated by Compositae, Ericaceae, Polylepis, Escallonia and Hypericum. The average annual temperature is 6–9°C and the annual rainfall is 700–2500 mm.
Grassparamo. Occurs from 3500 to 4200 m. This zone is dominated by grasses, including genera of Agrostis, Calamagrostis, Festuca, Muehlenbergia and Swallenochloa. Other components include Espeletia, Gentiana, Halenia, Valeriana and Aragoa. The average annual temperature is 3–6°C and precipitation is 700–2500 mm.
Superparamo. Occurs from 4200 to 4800 m. The vegetation cover is scarce. Common components include species of Draba, Caryophyllaceae and Senecio niveo-aureus. The average annual temperature is 0–3°C. Above this zone is a permanent cover of snow.
Overall biodiversity levels in these belts are not well measured due to the difficulty of access and large amount of species numbers. Available data suggest that plant diversity is at its maximum in the subandean forest and from there on, it decreases with altitude. For example, family richness of vascular plants in the relevés of the eastern mountain side of the Sumapaz-transect (east of Bogotá basin) changes from less than 30 in the lowlands below 930 m elevation, to approximately 45 in the elevational range between 930 and 1300 m (lowland–subandean), then it decreases to 30–34 in the range from 1400 to 3100 m (subandean–Andean forest) and to less than 30 again at elevations between 3300 and 3500 m (subparamo). From 3500 to 4200 m, the family richness further decreases from 19 to 12 (grassparamo; Keizer 2000). In the relevés of the western mountain, the general trend is similar, but the family richness in the subandean (lower montane) and Andean (upper montane) forest belts is highest. Species level richness is not available in this region. However, family richness is considered to be highly correlated to species richness (e.g. Balmford et al. 1996a,b; Villaseñor et al. 2005). In similar montane forests of Costa Rica, a decreasing trend of woody plant species and family richness from lower to upper montane was clearly recorded by Kappelle & Zamora (1995), where species richness (family richness) decreases monotonously from 349 species (82 families) at 2000 m to 226 species (71 families) at 2300 m (both the areas are in the lower montane forest); further to 197 species (60 families) at 2600 m; 125 species (48 families) at 2900 m; and 74 species (34 families) at 3200 m (the latter three areas are in the upper montane forest). These data suggest that the plant diversity in the northern Andes decreases monotonically from subandean to Andean, subparamo and grassparamo. In Eastern Africa on Mt Kilimanjaro, Hemp (2006) also registered monotonically decreasing species numbers of vascular plants along an altitudinal gradient: from approximately 900 species at 1000 m to approximately 10 species at 4600 m.
The average elevation of the surface of the Bogotá basin is approximately 2550 m and is currently covered with upper montane forest (Andean forest). During the Quaternary, the vegetation belts shifted altitudinally along the slopes. The upper forest line migrated between approximately 2000 and approximately 3400 m during the glacial/inter-glacial cycles: during the glacial periods this area was covered with grassparamo, and during the inter-glacial periods it was covered with Andean forest (e.g. Van der Hammen 1974; Hooghiemstra & Van der Hammen 2004; Torres 2006).
3. Materials and methods
Pollen samples in this study are from three well-studied sediment cores recovered from the Bogotá basin: Funza-2, Funza-2A and Fuquene-7C. Cores Funza-2 and Funza-2A were recovered from a palaeo-lake (approx. 48° N, approx. 74° W, 2550 m alt.), which dried out approximately 28 000 years ago (Hooghiemstra 1984; Torres 2006). Core Fuquene-7C was recovered at the border of Fuquene Lake (Mommersteeg 1998). The sediments from the last 430 000 years of Funza-2 core (top 120 m) were used in this study. Core Funza-2A is 38 m long and contains a record from ca 30 000 to ca 160 000 years before present (yr BP) (Mommersteeg 1998). Owing to the drying out of palaeo-lake Bogotá at ca 28 000 yr BP, the Holocene portion is not preserved in these two cores. The Fuquene-7C core is 20.7 m long and records the last ca 85 000 years.
Pollen data are from the papers mentioned above. Samples from Funza-2 core sediments were analysed at 20 cm intervals, representing 800–2000 years resolution. Samples from Funza-2A and Fuquene-7C were analysed at intervals of 5–10 cm. Further details of these cores are described by Mommersteeg (1998), Torres et al. (2005) and Torres (2006).
According to the major ecological requirements and altitudinal distribution ranges of the plant taxa, pollen taxa were assigned to one of the four altitudinal vegetations belts: subandean forest; Andean forest; subparamo; and grassparamo (table 1; Van der Hammen 1974; Cleef & Hooghiemstra 1984; Mommersteeg 1998). Other taxa may be from a wider range of altitudes and were not assigned to any one of these belts. The tropical lowland forest (less than 1000 m elevation) and superparamo (greater than 4200 m) were not included in this summary because they are at too great a distance from the study sites, and have little or no contribution to the studied pollen assemblages.
Table 1.
Lists of pollen taxa that are assigned to different altitudinal vegetation belts. The names follow Mabberley (1997).
| subandean forest | Andean forest | subparamo |
| Acalypha | Bocconia | Asteraceae |
| Alchornea | Daphnopsis | Ericaceae |
| Cecropia | Dodonaea | Hypericum |
| Urticaceae/ | Drimys | Polylepis/Aceana |
| Moraceae | Eugenia | |
| Gaiadendron | grassparamo | |
| Hedyosmum | Poaceae | |
| Ilex | Aragoa | |
| Juglans | Caryophyllaceae | |
| Melastomataceae | Gentiana | |
| Miconea | Geranium | |
| Myrica | Valeriana | |
| Myrsine | ||
| Myrtaceae | ||
| Podocarpus | ||
| Weinmannia | ||
| Pilea | ||
| Alnus | ||
| Quercus | ||
| Styloceras | ||
| Symplocos | ||
| Vallea |
In this study, pollen diversity was measured using both the observed number of taxa and the Shannon–Wiener index. A rarefaction method for a sum of 300 grains is applied to the former, so that the effect caused by various pollen counts in samples may be removed (Simberloff 1972; Birks & Line 1992). Samples with less than 300 grains are omitted for this analysis (i.e. 8, 8 and 15 samples for Funza-2, Funza-2A and Fuquene-7C, respectively). The Shannon–Wiener index is used as another measure of pollen diversity (Magurran 1988).
To reveal the influence of different vegetation belts on pollen diversity, correlations between pollen diversity (observed taxa, Shannon–Wiener indices) and the relative abundance of pollen from different vegetation belts were analysed. A linear regression was also conducted for data from the Funza-2 core. As the number of taxa assigned to the group of Andean forest is higher than those of other groups, the relationship between the measured pollen diversity and the pollen abundance from the Andean forest may be biased. To test if this is the case, a similar analysis was conducted after the taxa assigned to the Andean forest were excluded from the measures of the pollen diversity in the Funza-2 core. Because the remaining pollen grains are not many, a sum of 150 is chosen for the rarefaction analysis. Samples with less than 150 remaining grains are omitted in this analysis (32 out of 513 samples). These analyses were conducted using SPSS 11 software.
4. Results
In the top 120 m of the Funza-2 core, 112 terrestrial vascular pollen taxa were registered in 513 sediment samples. In the 38 m long Funza-2A core 106 taxa were registered in 603 samples, and in the Fuquene-7C core 93 terrestrial vascular pollen taxa were observed in 248 samples.
(a) Funza-2 core
The results from the correlation analysis indicate that the correlation between pollen diversity (both observed number of taxa and Shannon–Wiener index) and the relatively abundance (percentage) of pollen from different vegetation belts are significant at the 0.01 level except for the relationship between observed taxon number and pollen abundance from subparamo forest (figure 1, table 2).
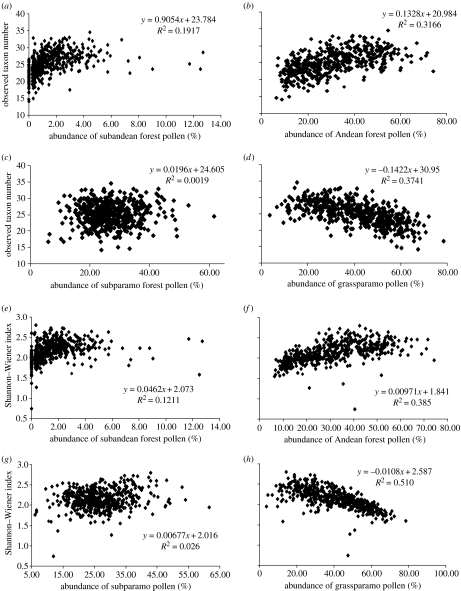
Figure 1.
Scatter diagrams showing the relationship between the observed number of pollen taxa (a)–(d) and the relative abundance (percentage) (e)–(h) of pollen from different altitudinal vegetation zones in the Funza-2 core (430 000–30 000 ka): (a)–(d) observed number of pollen taxa versus (a) subandean forest; (b) Andean forest; (c) subparamo; and (d) grassparamo. (e)–(h) Shannon–Wiener index versus (e) subandean forest; (f) Andean forest; (g) subparamo; and (h) grassparamo. The observed pollen taxon number is calibrated using a rarefaction method based on 300 pollen grains. The linear regression equations and R2 values are also shown.
Table 2.
Results from the correlation analysis (Pearson correlation) showing the relationship between pollen diversity and relative abundance of pollen from different vegetation belts. (**Significant at p=0.01 level.)
| sites | vegetation belts | observed number of pollen taxa in 300 grains | Shannon–Wiener index |
|---|---|---|---|
| Funza-2 | |||
| (with Andean forest taxa) | subandean forest | 0.438** | 0.348** |
| Andean forest | 0.563** | 0.580** | |
| subparamo | 0.043 | 0.180** | |
| grassparamo | −0.612** | −0.685** | |
| Funza-2 | |||
| (without Andean forest taxa) | subandean forest | 0.581** | 0.605** |
| Andean forest | 0.672** | 0.669** | |
| subparamo | 0.085 | 0.273** | |
| grassparamo | −0.735** | −0.838** | |
| Funza-2A | subandean forest | 0.173** | 0.311** |
| Andean forest | 0.033 | 0.162 | |
| subparamo | −0.000 | 0.060 | |
| grassparamo | −0.058 | −0.262** | |
| Fuquene-7C | subandean forest | 0.080 | 0.093 |
| Andean forest | 0.183** | 0.059 | |
| subparamo | 0.127 | 0.213 ** | |
| grassparamo | −0.294** | −0.221 ** | |
Results from the linear regression show that there is a positive relationship between the number of taxa and the pollen abundance of subandean forest (R2=0.19; figure 1a). In contrast, a much stronger positive relationship between taxon number and pollen abundance of Andean forest was estimated (R2=0.32; figure 1b). The relationship between the number of taxa and pollen abundance of subparamo vegetation is the weakest (R2=0.002). The relationship between taxon number and pollen abundance of grassparamo is strong, but negative (R2 =0.37; figure 1d).
When the pollen diversity is measured using the Shannon–Wiener index, the relationships between pollen diversity and pollen abundance from these vegetation belts are similar to those described above (figure 1, e–h). The relationship between Shannon–Wiener index value and the pollen abundance of the Andean forest, and between Shannon–Wiener index and the pollen abundance of the grassparamo are even closer (R2 =0.39 and 0.51, respectively; figure 1).
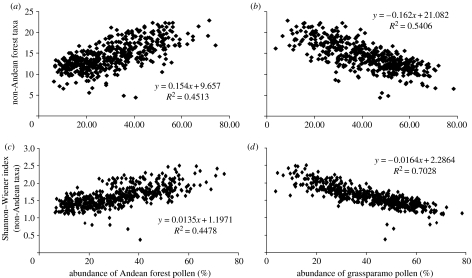
After the taxa assigned to Andean forest are excluded from the measures of the pollen diversity, the observed relationships between the pollen diversity and the pollen abundance of the Andean forest, and the abundance of the grassparamo still exist and are even stronger (figure 2).
Figure 2.
Scatter diagrams showing the relationship between the observed pollen diversity and the relative abundance (percentage) of pollen from Andean forest and grassparamo after the assigned Andean forest taxa are removed from the pollen diversity measurement: (a) number of observed non-Andean forest taxa versus pollen abundance from Andean forest; (b) number of observed non-Andean forest taxa versus pollen abundance from grassparamo; (c) Shannon–Wiener index of non-Andean forest taxa versus pollen abundance from Andean forest; and (d) Shannon–Wiener index of non-Andean forest taxa versus pollen abundance from grassparamo. The observed pollen taxon number is calibrated using a rarefaction method based on 150 pollen grains. The linear regression equations and R2 values are also shown.
(b) Funza-2A
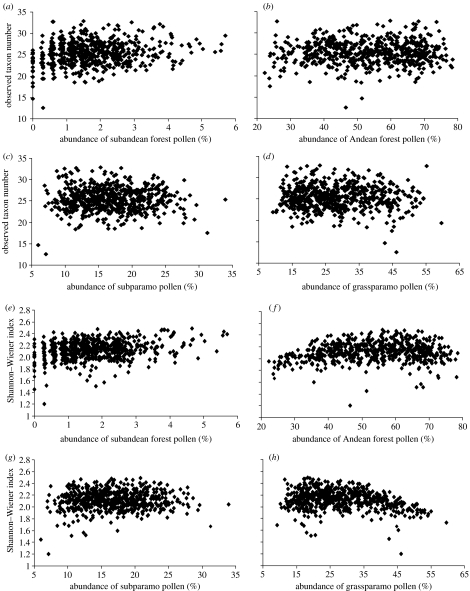
In the core Funza-2A, the observed number of taxa are positively related to the relative abundance of pollen from the subandean forest (figure 3). This relationship is significant at the 0.01 level (table 2). However, there is no significant relationship between pollen diversity and the pollen abundance from the subparamo. A negative relationship between pollen diversity and the pollen abundance from the grassparamo exists. However, these relationships are much weaker than observed in the Funza-2 core and are not significant (figure 3).
Figure 3.
Scatter diagrams showing the relationship between the observed pollen diversity and the relative abundance (percentage) of pollen from different altitudinal vegetation zones in the Funza-2A core (130 000–30 000 ka): (a)–(d) observed number of pollen taxa versus (a) subandean forest; (b) Andean forest; (c) subparamo; and (d) grassparamo. (e)–(h) Shannon–Wiener index versus (e) subandean forest; (f) Andean forest; (g) subparamo; and (h) grassparamo. The observed pollen taxon number is calibrated using a rarefaction method based on 300 pollen grains.
Similar relationships are also found when the Shannon–Wiener index was used to represent pollen diversity. The positive relationship between the Shannon–Wiener index value and the pollen abundance from the subandean forest and the negative relationship between Shannon–Wiener index value and the pollen abundance from grassparamo are significant at the 0.01 level. The relationship between the Shannon–Wiener index and the pollen abundance from the subparamo is not significant.
(c) Fuquene-7C
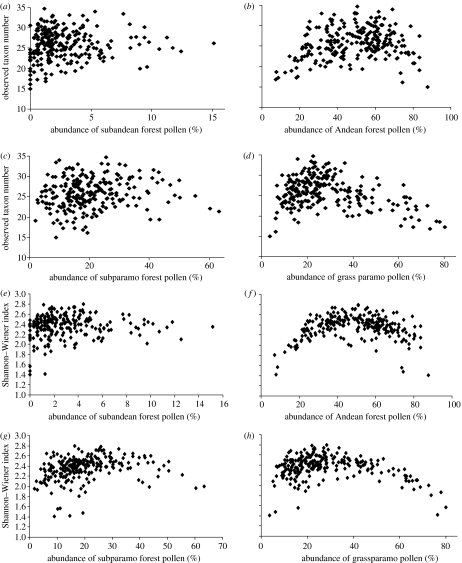
In the Fuquene-7C core, as in the other cores, pollen diversity has a positive relationship with the pollen abundance from the Andes forest and is negatively related to that of pollen from the grassparamo (figure 4). However, it shows an arch-shape in the scatter plots for the Andean and grassparamo data, with the maximum at the mid-level of the pollen abundance, which is due to the heavy influence of overrepresented Alnus pollen (which is a heavy pollen producer) in these samples with very high pollen abundance from the Andean forest belts. However, the clear trends of increasing pollen diversity with Andean Forest pollen abundance are noticeable. The same effects are seen in the scatter diagram of the pollen from the grassparamo.
Figure 4.
Scatter diagrams showing the relationship between the observed pollen diversity and the relative abundance (percentage) of pollen from different altitudinal vegetation zones in the Fuquene-7C core (85 000–0 ka): (a)–(d) observed number of pollen taxa versus (a) subandean forest; (b) Andean forest; (c) subparamo; and (d) grassparamo. (e)–(h) Shannon–Wiener index versus (e) subandean forest; (f) Andean forest; (g) subparamo; and (h) grassparamo. The observed pollen taxon number is calibrated using a rarefaction method based on 300 pollen grains.
5. Discussion
In spite of differences among the results from the three cores, the analyses from the three selected study sites show similar patterns in the relationship between the observed pollen diversity and relative pollen abundance of the different vegetation belts. The observed pollen diversity is positively related to the relative pollen abundances of the subandean forest and Andean forest belts, and negatively related to the pollen abundance from the grassparamo vegetation.
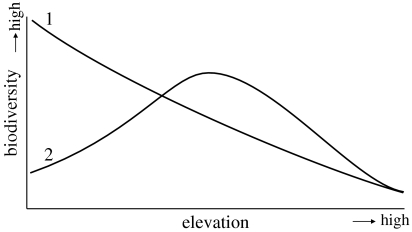
Altitudinal distribution of biodiversity is considered to follow one of the two patterns (figure 5): (i) a monotonic decline with altitude, the highest diversity is at the lowest elevation and decreases at higher elevations (Gentry 1988; Hamilton 1989; Rahbek 1995; Kessler 2001), and (ii) ‘hump-shaped’, the diversity is at maximum at mid-altitudes (1000–2000 m) and decreases at very high and very low altitudes (Rahbek 1995, 1997; Keizer 2000). As we have mentioned earlier, no matter which of the two patterns is true, the available data suggest that in our study region, the plant diversity decreases monotonically from subandean to Andean, subparamo and grassparamo (Kappelle & Zamora 1995; Keizer 2000). Gentry (1988) also found that in the tropical Andes (Colombia, Ecuador and Peru), from about 1500 m to near the upper forest line above 3000 m, the plant diversity decreased linearly and steeply.
Figure 5.
Schematic diagram showing the two most accepted altitudinal diversity distribution models. Model 1 shows that the biodiversity levels decrease with elevation and the maximum is at low elevations. Model 2 indicates that most diversity zones are in the mid-elevation.
The variation of the relative abundance of pollen from different vegetation belts indicates the relative distance and pollen supply of the vegetation belts to the study site (e.g. Van der Hammen 1974; Mommersteeg 1998; Torres 2006). Paleoecological studies suggest that during the Quaternary interglacial/glacial (warm/cold) cycles, the upper forest line oscillated between approximately 2000 and 3400 m in the study area (Hooghiemstra & Van der Hammen 2004; Torres 2006). The vegetation cover around the study sites (2550 m) shifted between Andean forest and grassparamo. During the warm interglacial times, the study site was surrounded by the Andean forest. Even during the warmest Eemian time (MIS 5e) and the MIS 7 stage, in which the upper forest line was up to approximately 3400 m, the study site was close to the lower boundary of Andean forest, but still within the Andean forest zone (Van't Veer & Hooghiemstra 2000; Torres 2006). During the glacial times, the upper forest line dropped to between 2000 and 2200 m, well below the study site, and the surrounding vegetation was composed of less diverse grassparamo, which supplied most of the pollen in the basin.
The positive relationship between the pollen diversity and relative pollen abundance from more species-diverse lower vegetation belts and the negative relationship between the pollen diversity and relative pollen abundance from the less diverse grassparamo indicate that the measured pollen diversity is in agreement with the diversity distribution pattern. The less influence from the subandean forest than that for the Andean forest is probably due to the longer distance of the vegetation from the study site. Subparamo is a narrow transitional zone between the Andean forest and grassparamo, and therefore its components may overlap widely with zones both below and above it. That may be the reason why this zone does not show a clear relationship with pollen diversity.
However, much of the plant diversity in the grassparamo originates from a few large families, such as Poaceae, Asteraceae and Ericaceae (Luteyn 1999; Rangel-Ch 2006). Genera or species are not identifiable in pollen for these families. This may significantly reduce the pollen diversity from this belt. A similar effect occurred to other belts too, such as Melastomataceae and Urticaceae/Moraceae at lower elevations. Further work is required to evaluate the effect of this on the results.
We also must realize that the quality of the datasets varies. The observed pattern of the relationship between pollen diversity and the pollen abundance from the vegetation belts is weak for the data from Funza-2A core and strong for the Funza-2 core. Two factors may explain this difference. First, the dataset for the former contains approximately 10–46 unidentified pollen grains in each sample. Most of these grains may be rare taxa and near the ‘tail’ of the abundance distribution curve (Hubbell 2001); therefore, these grains may represent various numbers of different species. Neglecting these grains in pollen counting may have significantly reduced the quality of pollen diversity measurement and also reduced the accuracy of biodiversity reconstruction. Second, the latter dataset is from a longer time-scale, which covered more extreme vegetation changes caused by the glacial/inter-glacial climatic cycles and displayed a better relationship between the pollen diversity and vegetation changes. However, for all of these three sites, we may only identify the coarse trend of diversity changes. This suggests that the pollen datasets with incomplete pollen taxa may only be used to infer general patterns of diversity, such as the examples in this paper, in which only a few diversity groups could be identified. When more accurate diversity reconstructions are required, the quality of data must be better measured and standardized. Measurement of the palynological richness in a pollen count proportional to the accumulation rate, or the estimation of total pollen diversity may be suitable options, but would require more careful management of pollen analyses and more effort (Weng et al. 2006, in review).
6. Conclusions
The recorded pollen diversity in the Funza 2, Funza-2A and Fuquene-7C cores is in agreement with the plant diversity pattern of the altitudinal vegetation belts. Its change may reflect the diversity dynamics caused by climate change. In the past, when climate was warmer, the vegetation belts moved upslope and low elevation belts were closer to the study site. More pollen taxa originated from the species-rich low elevation belts. The higher contribution from the low elevation belts may have enriched the diversity registered in the pollen assemblages. This suggests that the observed pollen diversity in our study sites may be correlated to the plant diversity. This result is important for the development of methods of using pollen data to reconstruct changes in plant diversity history driven by climate variability. However, this result may be only considered as preliminary, because there are more factors that may affect the observed results and their effects vary for different study sites. Moreover, the observed relationship is weak in these sites, which suggests that the application of incomplete pollen data to biodiversity reconstruction may only be valid for large changes in biodiversity. Large datasets including all observed pollen types may be required for more detailed reconstructions.
Acknowledgments
This research was supported by the Netherlands Foundation for Scientific Research (grant ALW-852.00.030). Professor A. M. Cleef kindly provided the information of modern vegetation. We also thank Dan Yeloff for correcting the English.
Footnotes
One contribution of 14 to a Theme Issue ‘Biodiversity hotspots through time: using the past to manage the future’.
References
- Balmford A, Green M.J.B, Murray M.G. Using higher-taxon richness as a surrogate for species richness 1: regional tests. Proc. R. Soc. B. 1996a;263:1267–1274. [Google Scholar]
- Balmford A, Jayasuriya A.H.M, Green M.J.B. Using higher-taxon richness as a surrogate for species richness 2: local applications. Proc. R. Soc. B. 1996b;263:1571–1575. [Google Scholar]
- Barnosky A.D, Carrasco M.A, Davis E.B. The impact of the species–area relationship on estimates of paleodiveristy. PLoS Biol. 2005;8:1356–1361. doi: 10.1371/journal.pbio.0030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton M.J. Quality of the fossil record through time. Nature. 2000;403:534–537. doi: 10.1038/35000558. doi:10.1038/35000558 [DOI] [PubMed] [Google Scholar]
- Birks H.J.B, Line J.M. The use of rarefaction analysis for estimating palynological richness from Quaternary pollen-analytical data. The Holocene. 1992;2:1–10. [Google Scholar]
- Brown J.H, Ernest S.K.M, Parody J.M, Haskell J.P. Regulation of diversity: maintenance of species richness in changing environments. Oecologia. 2001;126:321–332. doi: 10.1007/s004420000536. doi:10.1007/s004420000536 [DOI] [PubMed] [Google Scholar]
- Calcote R. Pollen source area and pollen productivity—evidence from forest hollows. J. Ecol. 1995;83:591–602. doi:10.2307/2261627 [Google Scholar]
- Cleef A.M, Hooghiemstra H. Present vegetation of the area of the high plain of Bogotá. In: Hooghiemstra H, editor. Vegetational and climatic history of the high plain of Bogota. Dissertationes Botanicae. vol. 79. J. Cramer; Vaduz: 1984. pp. 42–66. [Google Scholar]
- Courtillot V, Gaudemer Y. Effects of mass extinctions on biodiversity. Nature. 1996;381:146–148. doi:10.1038/381146a0 [Google Scholar]
- Davis M.B. On the theory of pollen analysis. Am. J. Sci. 1963;261:897–912. [Google Scholar]
- Faegri K, Iversen J. 4th edn. Wiley; Chichester, UK: 1989. Textbook of pollen analysis. [Google Scholar]
- Gentry A.H. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann. Miss. Bot. Gard. 1988;75:1–34. doi:10.2307/2399464 [Google Scholar]
- Hamilton A.C. African forests. In: Leith H, Werger M.J.A, editors. Ecosystems of the World series. Tropical rainforest ecosystems, biogeographical and ecological studies. Elsevier; Amsterdam, The Netherlands: 1989. pp. 155–182. [Google Scholar]
- Hemp A. The impact of fire on diversity, structure, and composition of the vegetation on Mt. Kilimanjaro. In: Spehn E.M, Liberman M, Körner C, editors. Land use change and mountain biodiversity. Taylor & Francis; Boca Raton; London, UK: 2006. pp. 51–68. [Google Scholar]
- Hooghiemstra H. Dissertationes Botanicae. vol. 79. Gantner Verlag; Vaduz, Liechtenstein: 1984. Vegetational and climatic history of the high plain of Bogota, Colombia; p. 368. [Google Scholar]
- Hooghiemstra H, Van der Hammen T. Quaternary Ice-Age dynamics in the Colombian Andes: developing an understanding of our legacy. Phil. Trans. R. Soc. B. 2004;359:173–181. doi: 10.1098/rstb.2003.1420. doi:10.1098/rstb.2003.1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell S.P. Princeton University Press; Princeton, NJ: 2001. The unified neutral theory of biodiversity and biogeography. [DOI] [PubMed] [Google Scholar]
- Jackson S.T. Pollen and spores in Quaternary lake sediments as sensors of vegetation composition: theoretical models and empirical evidence. In: Traverse A, editor. Sedimentation of organic particles. Cambridge University Press; Cambridge, UK: 1994. pp. 253–286. [Google Scholar]
- Jackson S.T, Lyford M.E. Pollen dispersal models in Quaternary plant ecology: assumptions, parameters, and prescriptions. Bot. Rev. 1999;65:39–75. [Google Scholar]
- Jacobson G.L, Bradshaw R.H.W. The selection of sites for paleovegetational studies. Quatern. Res. 1981;16:80–96. doi:10.1016/0033-5894(81)90129-0 [Google Scholar]
- Jaramillo C, Rueda M.J, Mora G. Cenozoic plant diversity in the Neotropics. Science. 2006;311:1893–1896. doi: 10.1126/science.1121380. doi:10.1126/science.1121380 [DOI] [PubMed] [Google Scholar]
- Kappelle M, Zamora N. Changes in woody species richness along an altitudinal gradient in Talamancan montane Quercus forests, Costa Rica. In: Churchill S.P, Balslev H, Forero E, Luteyn J.L, editors. Biodiversity and conservation of Neotropical Montane forests. The New York Botanical Garden. Bronx; New York, NY: 1995. pp. 135–148. [Google Scholar]
- Keizer, J. J. 2000 Vascular plant family composition of the Eastern Cordillera of Colombia: Diversity patterns, classification and ordination. Ph.D. thesis, University of Amsterdam, The Quaternary of Colombia 26, p. 170.
- Kessler M. Patterns of diversity and range size of selected plant groups along an elevational transect in the Bolivian Andes. Biodiv. Conserv. 2001;10:1897–1921. doi:10.1023/A:1013130902993 [Google Scholar]
- Luteyn J.L. Páramos; a checklist of plant diversity, geographical distribution, and botanical literature. Mem. NY Bot. Gard. 1999;84:278. [Google Scholar]
- Mabberley D.J. 2nd edn. Cambridge University Press; Cambridge, UK: 1997. The plant-book: a portable dictionary of the vascular plants. [Google Scholar]
- Magurran A.E. Croom Helm; London, UK: 1988. Ecological diversity and its measurement. [Google Scholar]
- Mommersteeg, H. 1998 Vegetation development and cyclic and abrupt climatic changes during the late Quaternary: palynological evidence from the Colombian Eastern Cordillera. Ph.D. thesis. University of Amsterdam, Amsterdam.
- Moore P.D, Webb J.A, Collinson M.E. Blackwell Scientific; Oxford, UK: 1991. Pollen analysis. [Google Scholar]
- Myers N, Mittermeier R.A, Mittermeier C.G, da Fonseca G.A.B, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. doi:10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Odgaard B.V. Fossil pollen as a record of past biodiversity. J. Biogeogr. 1999;26:7–17. doi:10.1046/j.1365-2699.1999.00280.x [Google Scholar]
- Prentice I.C. Pollen representation, source area, and basin size: Toward a unified theory of pollen analysis. Quatern. Res. 1985;23:76–86. doi:10.1016/0033-5894(85)90073-0 [Google Scholar]
- Rahbek C. The elevational gradient of species richness: a uniform pattern? Ecography. 1995;18:200–205. doi:10.1111/j.1600-0587.1995.tb00341.x [Google Scholar]
- Rahbek C. The relationship among area, elevation, and regional species richness in Neotropical birds. Am. Nat. 1997;149:875–902. doi: 10.1086/286028. doi:10.1086/286028 [DOI] [PubMed] [Google Scholar]
- Rangel-Ch J.O. The biodiversity of the Colombian paramo and its relation to anthropogenic impact. In: Spehn E.M, Liberman M, Korner C, editors. Land use change and mountain biodiversity. Taylor & Francis; London, UK: 2006. pp. 103–117. [Google Scholar]
- Rohde R.A, Muller R.A. Cycles in fossil diversity. Nature. 2005;434:208–210. doi: 10.1038/nature03339. doi:10.1038/nature03339 [DOI] [PubMed] [Google Scholar]
- Silvertown J. History of a latitudinal diversity gradient: woody plants in Europe 13,000–1000 years B.P. J. Biogeogr. 1985;12:519–525. doi:10.2307/2844907 [Google Scholar]
- Simberloff D.S. Properties of the rarefaction diversity measurement. Am. Nat. 1972;106:414–418. doi:10.1086/282781 [Google Scholar]
- Sugita S. A model of pollen source area for an entire lake surface. Quatern. Res. 1993;39:239–244. doi:10.1006/qres.1993.1027 [Google Scholar]
- Sugita S. Pollen representation of vegetation in Quaternary sediments theory and method in patchy vegetation. J. Ecol. 1994;82:881–897. doi:10.2307/2261452 [Google Scholar]
- Torres, V. 2006 Pliocene–Pleistocene evolution of flora, vegetation and climate: a palynological and sedimentological study of a 586-m core from the Bogotá Basin, Colombia. Ph.D. thesis, University of Amsterdam, Amsterdam, Netherlands.
- Torres V, Vandenberghe J, Hooghiemstra H. An environmental reconstruction of the sediment infill of the Bogotá basin (Colombia) during the last 3 million years from abiotic and biotic proxies. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005;226:127–148. doi:10.1016/j.palaeo.2005.05.005 [Google Scholar]
- Valentine J.W, Jablonski D. Fossil communities: compositional variation at many time scales. In: Ricklefs R.E, Schluter D, editors. Species diversity in ecological communities. The University of Chicago Press; Chicago, IL: 1993. pp. 341–349. [Google Scholar]
- Van der Hammen T. The Pleistocene changes of vegetation and climate in tropical South America. J. Biogeogr. 1974;1:3–26. doi:10.2307/3038066 [Google Scholar]
- Van der Hammen T, Cleef A.M. Development of the high Andean páramo flora and vegetation. In: Vuilleumier F, Monasterio M, editors. High altitude tropical biogeography. Oxford University Press; Oxford, UK: 1986. pp. 153–201. [Google Scholar]
- van der Hammen T, González E. Upper Pleistocene and Holocene climate and vegetation of the Sabana de Bogotá (Colombia, South America) Leidse Geol. Meded. 1960;25:261–315. [Google Scholar]
- Van't Veer R, Hooghiemstra H. Montane forest evolution during the last 650 000 yr in Colombia: a multivariate approach based on pollen record Funza-I. J. Quatern. Sci. 2000;15:329–346. doi:10.1002/1099-1417(200005)15:4<329::AID-JQS538>3.0.CO;2-3 [Google Scholar]
- Villaseñor J.L, Ibarra-Manríquez G, Meave J.A, Ortíz E. Higher taxa as surrogates of plant biodiversity in a megadiverse country. Conserv. Biol. 2005;19:232–238. doi:10.1111/j.1523-1739.2005.00264.x [Google Scholar]
- Weng C, Hooghiemstra H, Duivenvoorden J.F. Challenges in estimating past plant diversity from fossil pollen data: statistical assessment, problems, and possible solutions. Divers. Distrib. 2006;12:310–318. doi:10.1111/j.1366-9516.2006.00230.x [Google Scholar]
- Weng, C., Hooghiemstra, H. & Duivenvoorden, J. F. In review. Influence of pollen count number on measurement of pollen diversity observed from two samples from the tropical Colombian Andes. Rev. Palaeobot. Palynol.
- Willis K.J, Gillson L, Brncic T.M, Figueroa-Rangel B.L. Providing baselines for biodiversity measurement. Trends Ecol. Evol. 2005;20:107–108. doi: 10.1016/j.tree.2004.12.003. doi:10.1016/j.tree.2004.12.003 [DOI] [PubMed] [Google Scholar]