Abstract
The aim of this paper is to evaluate the respective roles of past changes in climate, geomorphology and human activities in shaping the present-day forest–savannah mosaic of the Bolivian Amazon, and consider how this palaeoecological perspective may help inform conservation strategies for the future. To this end, we review a suite of palaeoecological and archaeological data from two distinct forest–savannah environments in lowland Bolivia: Noel Kempff Mercado National Park (NKMNP) on the Precambrian Shield and the ‘Llanos de Moxos’ in the Beni basin. We show that they contain markedly contrasting legacies of past climatic, geomorphic and anthropogenic influences between the last glacial period and the Spanish Conquest. In NKMNP, increasing precipitation caused evergreen rainforest expansion, at the expense of semi-deciduous dry forest and savannahs, over the last three millennia. In contrast, pre-Hispanic indigenous cultures were instrumental in facilitating recent forest expansion in the Llanos de Moxos by building a vast network of earthworks. Insights from Mid-Holocene palaeodata, together with ecological observations and modelling studies, suggest that there will be progressive replacement of rainforest by dry forest and savannah in NKMNP over the twenty-first century in response to the increased drought predicted by general circulation models. Protection of the latitudinal landscape corridors may be needed to facilitate these future species reassortments. However, devising appropriate conservation strategies for the Llanos de Moxos will be more difficult due to its complex legacy of Palaeo-Indian impact. Without fully understanding the degree to which its current biota has been influenced by past native cultures, the type and intensity of human land use appropriate for this landscape in the future will be difficult to ascertain.
Keywords: conservation, Quaternary, palaeoecology, forest–savannah ecotone, Bolivia, Amazon
1. Introduction
Tropical forest–savannah ecotones are of considerable interest to biologists for several reasons. They typically exhibit high biodiversity, due to the mosaic of different ecosystems (i.e. habitat heterogeneity) resulting in high levels of beta diversity. They may play an important role in rainforest speciation (Bush 1994; Smith et al. 1997), whether via allopatric or sympatric mechanisms or both. Forest–savannah transitions are often influenced by numerous factors including nutrient supply, soil hydrology, natural disturbance and human activity, as well as direct climatic constraints, principally differences in seasonal and annual precipitation (Furley et al. 1992). A key uncertainty is the manner in which these vegetation boundaries will respond to future climatic change and anthropogenic influence (Houghton et al. 2001).
It is therefore unfortunate that vegetation transition zones, such as forest–savannah ecotones, have been neglected in recent conservation initiatives, as highlighted by Smith et al. (2001). For example, the ‘ecoregion’ (Dinerstein et al. 1995; Olson & Dinerstein 1998; Olson et al. 2001) and ‘biodiversity hotspot’ (Myers et al. 2000) schemes constitute two different approaches to prioritizing conservation efforts for the Earth's terrestrial biota, but both focus on species richness within a particular habitat type and neither consider the biological significance of biodiversity associated with transitions or boundaries between different kinds of habitat.
Hannah et al. (2002) cogently argue that these now traditional approaches to conservation are inappropriate for the twenty-first century, since they are predicated on the assumption that ecosystems are static and will not be affected by climate change. Hannah et al. (2002) advocate ‘climate change-integrated conservation strategies’ (CCS) that explicitly consider the likely ecosystem responses to climate change scenarios predicted for the next 100 years or more. In contrast to past conservation thinking, CCS would attach far greater importance to conserving forest–savannah ecotones, providing corridors spanning different vegetation types to allow species to shift their geographic ranges in response to a changing climate.
Although CCS take account of future climate change simulations (e.g. Malcolm et al. 2006), conservationists have rarely paid attention to proxy-based reconstructions of ecosystem responses to past changes in climate, whether over centuries, millennia or glacial–interglacial time-scales (Willis et al. 2005). This is most probably because conservation biologists are unfamiliar with palaeoecological techniques and literature and also because most palaeoecologists to date have not explored the implications of their findings for the conservation community, notwithstanding a few notable exceptions (e.g. Bush 2002).
What can a palaeoecological perspective offer conservation scientists and practitioners with respect to tropical forest–savannah ecotones? Firstly, palaeoecological data can demonstrate whether a forest–savannah ecotonal shift observed over recent decades merely constitutes a minor short-term oscillation or instead is part of a much larger millennial-scale biome replacement forced by climate change. Palaeovegetation records, coupled with independent palaeoclimate data (e.g. palaeo lake-level changes) and palaeofire (charcoal) data can reveal how resilient (or sensitive) forest–savannah ecotones have been to past changes in climate–fire regimes and thereby inform conservationists of the kinds of vegetation responses to be expected in the future.
Secondly, combined palaeoecological and archaeological approaches can potentially inform conservationists whether or not a particular forest–savannah mosaic or transition zone is essentially natural or is instead largely anthropogenic in origin and a legacy of land-use practices and human intervention over past centuries/millennia (e.g. changes to microtopography, hydrology, soil conditions and burning frequency). This is important for conservation planners. For example, if the biodiversity and structure of current ecosystems is a function of past centuries or millennia of intensive land management, traditional attempts to conserve this biodiversity by establishing protected areas such as national parks, and excluding human land use from them, would be misguided and could unwittingly change the very species composition that is supposedly being conserved.
(a) Aim
This paper explores the biogeographical histories of two contrasting forest–savannah environments in lowland Bolivia (figure 1) in order to demonstrate the respective roles of Late Quaternary climate change, geomorphology and pre-Hispanic indigenous cultures in shaping the present-day forest–savannah mosaic in SW Amazonia. Noel Kempff Mercado National Park (NKMNP) in NE Bolivia was chosen as arguably the best example of a largely pristine forest–savannah ecotone anywhere in Amazonia, while the forest–savannah mosaic of the ‘Llanos de Moxos’ in the Beni Department, N Bolivia, was chosen because it contains instead a legacy of pre-Hispanic landscape modification, on a scale unequalled anywhere else in lowland Amazonia.
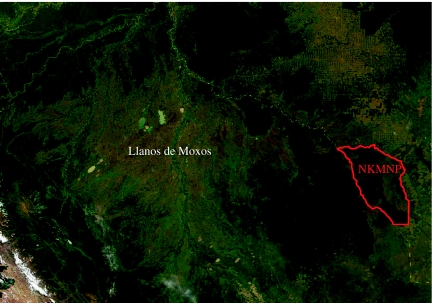
Figure 1.
MODIS image of the Bolivian Amazon and adjacent areas of Peru and Brazil, 28 July 2000, showing Noel Kempff Mercado National Park (NKMNP) and the Llanos de Moxos. Light areas with herringbone pattern to the north of NKMNP are zones deforested since the 1970s for agriculture in Rondônia, Brazil. Adapted from image processed by Jacques Descloitres, MODIS Land Rapid Response Team, NASA/Goddard Space Flight Center.
We consider the implications of these palaeovegetation and archaeological records for: (i) understanding the present forest–savannah mosaic and biodiversity patterns in these contrasting areas and (ii) adopting appropriate conservation strategies in the light of future climate change scenarios and ecological studies.
2. Noel Kempff Mercado National Park
NKMNP is a 15 230 km2 biological reserve in NE Bolivia on the Precambrian Shield near the SW margin of Amazonia, adjacent to the Brazilian States of Rondônia and Mato Grosso (figure 2). The following description summarizes detailed accounts by Killeen & Schulenberg (1998) and Killeen et al. (2003). NKMNP is of global importance, designated a UNESCO World Heritage Site in 2000, due to the diversity of pristine ecosystems that it contains: terra firme evergreen rainforest, riparian and seasonally flooded evergreen forest, seasonally flooded savannah wetlands, upland (cerrado) savannahs and semi-deciduous dry forest. In no other part of South America do such dramatically different ecosystems occur in such close proximity to one another (Killeen 1998). Although local (alpha) diversity (species richness) within any one ecosystem is not particularly high, the fact that these ecosystems are clearly distinct from each other (i.e. very high habitat heterogeneity or beta diversity), and well represented on the landscape, means that the total regional diversity for NKMNP is very high. These diversity patterns apply not only to the vegetation, but also to the birds, mammals and scarab beetles (Killeen & Schulenberg 1998).
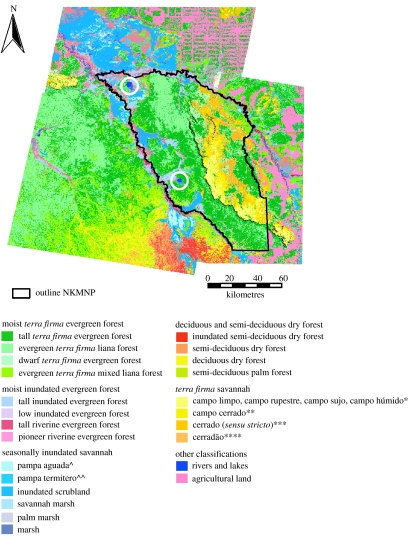
Figure 2.
Vegetation classification of Noel Kempff Mercado National Park (derived from Landsat TM data) (Modified from Killeen & Schulenberg 1998). Laguna Chaplin and Laguna Bella Vista are marked with circles. Translations of local Bolivian/Brazilian terms are as follows: *, open savannah; **, shrubby savannah; ***, open savannah woodland; ****, closed savannah woodland; ∧, seasonally inundated open savannah; ∧∧, seasonally inundated shrub savannah/termite savannah. (Modified from Gosling et al. 2005.)
The juxtaposition of these different ecosystems, and consequently high habitat diversity, is due to the ecotonal position of the park, between humid Amazon rainforests to the north, semi-deciduous Chiquitano dry forests to the south and upland cerrado savannahs to the east. The overriding control on this vegetation ecotone, at the regional scale, is climate; mean annual precipitation is approximately 1500 mm per annum (pa) and there is a four month dry season between June and September when mean monthly precipitation is less than 30 mm. Precipitation falls mainly in the austral summer, originating from a combination of deep-cell convective activity over the Amazon basin and southerly extension of the intertropical convergence zone (ITCZ) due to peak insolation in the austral summer (Bush & Silman 2004). Although the mean annual temperature is 25–26°C, temperatures frequently decrease to 10°C for several days at a time during the dry season (June–August) when cold dry Patagonian air masses (surazos) reach the area.
At finer spatial scales, geomorphology and disturbance become key controls upon the ecosystem mosaic. The most conspicuous geomorphologic feature is the Huanchaca Plateau, a table mountain between 600 and 900 m above sea level (a.s.l.), composed of Precambrian sandstone and quartzite, that dominates the eastern half of the park (Litherland & Power 1989). This is not only dominated by edaphically derived upland (cerrado) savannahs but also contains patches of evergreen and deciduous forests where soils are sufficiently deep and nutrient rich. The adjacent lowland peneplain to the west (200–250 m a.s.l.) is blanketed by Tertiary and Quaternary alluvial sediments, which are covered by humid evergreen rainforests that form an ecotone with semi-deciduous dry forests at the southern border of NKMNP. Two black-water rivers originating on the Precambrian Shield define the boundaries of the park: the Río Iténez to the north and east (forming the Brazilian border) and the Río Paraguá to the west (Killeen & Schulenberg 1998). The latter are bordered by seasonally flooded evergreen forests and savannah wetlands, the spatial arrangement of which is controlled by local edaphic, topographic and hydrological conditions.
Fires are a key feature of the study area, especially in the savannahs. Seasonally flooded savannah wetlands outside the park are often burned annually by cattle ranchers (Killeen 1991), although natural fires caused by lightning are common within the park, especially in the upland savannahs of the plateau escarpments, which typically burn once every 3 years on average (Killeen et al. 2003). Flooding is another important type of disturbance that creates distinct types of forest and savannah ecosystems, which vary in community composition according to the depth and duration of flooding. Changes in the seasonal distribution and intensity of precipitation can alter fire and flooding regimes which can in turn cause marked changes to the vegetation mosaic of NKMNP.
(a) Patterns of diversity
The terra firme humid evergreen rainforests have highest local species richness (alpha diversity) compared with the other forest and savannah ecosystems of NKMNP, but are depauperate compared with rainforest ecosystems further within the Amazon basin. This is true for tree species (Killeen 1998), birds (Bates et al. 1998) and mammals (Emmons 1998). Furthermore, these forests are quite distinct from those further west in Bolivia (i.e. pre-montane Andes) and instead belong to the Rondônia/Mato Grosso moist forest ‘ecoregion’ of Brazil (Dinerstein et al. 1995). It has been hypothesized (Killeen & Schulenberg 1998) that the lower alpha diversity of these rainforests may be because they are climatically stressed, at or close to the precipitation threshold of many Amazonian species in this ecotonal region. Alternatively, these rainforest ecosystems may have only recently become established in NKMNP, in which case they may still be undergoing succession, accumulating species.
In contrast, the cerrado savannahs of the Huanchaca plateau are highly diverse, especially with respect to their avifauna (Bates et al. 1998; Da Silva & Bates 2002), comparable with any other savannah region in South America. Killeen & Schulenberg (1998) suggest this high biodiversity likely reflects the long history of isolation of this edaphic table mountain savannah, possibly spanning millions of years. The seasonally inundated savannahs have markedly lower plant diversity than the upland cerrado savannahs, although this is probably unrelated to their history but due to their seasonal flood and drought regime, which is no doubt inhospitable for many species.
Palaeoecological data may help explain some of these biodiversity patterns and address some of these hypotheses/questions. In particular, why do the rainforests of NKMNP exhibit lower alpha diversity than those in more central parts of Amazonia? Are observed forest–savannah shifts merely minor short-term oscillations, or instead part of a long-term, millennial-scale trend? If the latter, then which is the predominant process—forest encroachment into savannah or savannah encroachment into forest? To what extent have Palaeo-Indians contributed to the current forest–savannah mosaic and species assemblages? How are the ecosystems of NKMNP likely to be affected by future climate change scenarios?
(b) Late Quaternary history of Noel Kempff Mercado National Park
(i) Fossil pollen and charcoal data
Two large lakes occur in NKMNP below the plateau, Laguna Bella Vista (13°37′ S, 61°33′ W) to the north, and Laguna Chaplin (14°28′ S, 61°04′ W) 100 km further south, approximately 30 km from the transition to semi-deciduous dry forest (figure 2). Pollen and charcoal records of both sites, spanning the past 50 000 years, are discussed in detail by Mayle et al. (2000) and Burbridge et al. (2004) and summarized here. The key finding is that humid evergreen rainforest communities have only recently expanded to dominate the park below the plateau, progressively spreading southward over the last two millennia. Prior to this time, over at least the previous 50 000 years, a mosaic of semi-deciduous dry forest and savannah communities dominated the landscape under a drier climate. The following discussion centres on the Laguna Chaplin pollen and charcoal records (figures 3 and 4), as the Laguna Bella Vista record has a significant hiatus, although the broad trends in the pollen changes of the two sites are similar.
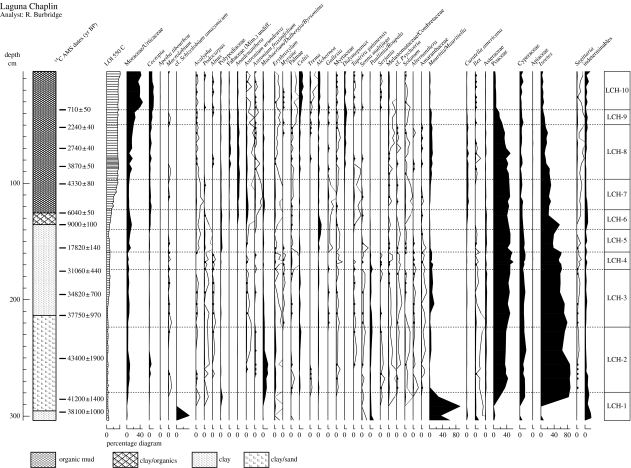
Figure 3.
Laguna Chaplin summary pollen percentage diagram, showing the most common taxa from the full complement of 290 pollen types. Dots on the curves denote less than 0.5%. Curves showing ×10 exaggeration are depicted for selected taxa which have low percentages. ‘Total land pollen’ (TLP) sums are greater than 300 grains per sample, except for the 4 pollen spectra in zone LCH-1 which had lower TLP sums of 100–160, due to low pollen concentrations. (After Burbridge et al. 2004.)
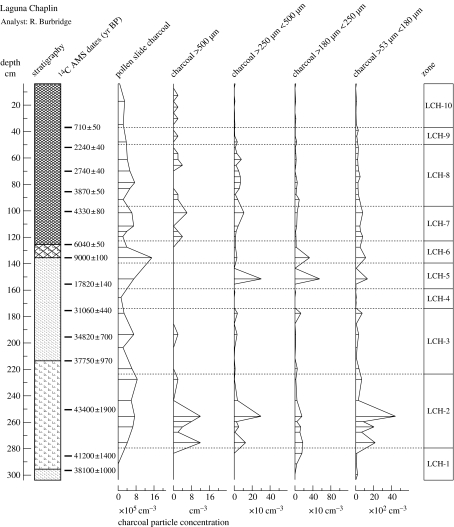
Figure 4.
Microscopic and macroscopic charcoal concentrations for Laguna Chaplin. Pollen assemblage zones are the same as in figure 3. (After Burbridge et al. 2004.)
Throughout the glacial period, between ca 50 000 and 11 400 calendar years before present (yr BP), the dominance of Poaceae (more than 40%) and Cyperaceae pollen (approx. 15%), together with Mauritia/Mauritiella palm pollen, suggests that low-lying areas which are today covered by seasonally flooded rainforest were instead covered by seasonally flooded termite savannahs and savannah marsh. Consistent presence of Paullinia/Roupala, Celtis, Machaerium-type and Erythroxylum pollen suggests that semi-deciduous dry forests may have occupied higher ground presently covered by terra firme evergreen rainforest. However, absence or negligible abundance of pollen of Curatella americana and Anadenanthera, which are important elements in present-day termite savannahs and dry forests, respectively, shows that these glacial-age ecosystems were floristically distinct from those that occupy the region today. The charcoal record shows that fires were more prevalent than today around these lakes through most of the glacial sequence, as would be expected in landscapes dominated by savannah and dry forest. These Pleistocene (glacial-age) plant communities are consistent with reduced precipitation (e.g. longer or more severe dry season) and lowered atmospheric CO2 levels, both of which would have favoured expansion of C4 grasses and sedges and drought-adapted savannah and dry forest tree species. Continuous presence of pollen of the predominantly Andean genus Podocarpus throughout the glacial sediments corroborates other palaeoclimatic evidence (e.g. Stute et al. 1995) for an Amazon-wide cooling of approximately 5°C at the Last Glacial Maximum (LGM; approx. 21 000 yr BP).
A mosaic of seasonally flooded savannahs and semi-deciduous dry forest communities also occupies the park throughout most of the Holocene (present interglacial period, 11 500 yr BP to present), but presence of C. americana, Anadenanthera, Astronium spp. and Gallesia pollen shows that, in contrast to the savannah and dry forest communities of the Pleistocene, these Holocene communities are floristically similar to those growing in the region today. Abundance of charcoal demonstrates the continued importance of fire in the park. Although it is unwise to attach too much significance to the high abundance of grass and sedge pollen that dominates most of the record, as it could conceivably have originated from shoreline aquatic vegetation rather than neighbouring savannahs, our interpretation of seasonally flooded termite savannahs is corroborated by grass and sedge cuticle analyses (Metcalfe 2005).
Humid evergreen rainforest, characterized principally by high percentages of Moraceae pollen (more than 40%) and low percentages of herbaceous pollen, only expanded southward from more central parts of Amazonia to dominate the park within the last two millennia, surrounding Laguna Bella Vista ca 1500 yr BP and Laguna Chaplin ca 660 yr BP. The initial onset of this rainforest spread probably began ca 3000 yr BP at Laguna Bella Vista and 2000 yr BP at Laguna Chaplin, when Moraceae pollen percentages began to rise and herbaceous pollen percentages began to fall. These dates suggest that rainforest spread southward between Laguna Bella Vista and Laguna Chaplin at a rate of ca 100–120 m per year. As expected, this rainforest expansion was accompanied by a reduction in fires, as reflected in the charcoal record. Although Moraceae pollen is continuously present throughout the entire sequence, modern pollen rain trap samples collected from 1 ha permanent vegetation study plots in each of the different ecosystems in NKMNP demonstrate that it only signifies local closed canopy rainforest when above 40% (Gosling et al. 2005) and that values of 25% or below are consistent with local semi-deciduous dry forest, savannah or long-distance transport from distant rainforest populations (Gosling 2004).
Although the relative contributions of atmospheric CO2 concentrations, precipitation, temperature and geomorphology as controls of glacial-age vegetation in NKMNP are difficult to discern, it is clear that a precipitation increase was the primary driver of this late Holocene rainforest expansion, as CO2 concentrations and temperature remained relatively constant through this period. Although rainforests reached northern NKMNP (Laguna Bella Vista) by 3000 yr BP, the absence of fossil pollen data further north means that the timing and geographic origin of the onset of this rainforest expansion beyond NKMNP is uncertain. However, we can be confident that this advance began not earlier than 5000 yr BP because Lake Titicaca (which lies at a roughly similar latitude to NKMNP on the Bolivian/Peruvian Altiplano) experienced a Mid-Holocene lowstand (approx. 90 m below present) between 8000 and 5000 yr BP (its lowest water level of the past 25 000 years) and rose to modern levels by 3000 yr BP (Cross et al. 2000; Baker et al. 2001; D'Agostino et al. 2002). It is possible, though, that some of this forest expansion in the lowlands of NKMNP was only indirectly driven by climate and that local geomorphologic changes played a more direct role. For example, increased precipitation may have led to higher rates of deposition and aggradation in the flood plains, thereby changing the hydrological conditions to facilitate expansion of riparian forest in the floodplains and on the levees.
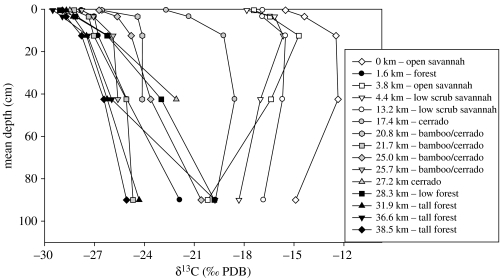
(ii) Soil stable carbon isotope data from the Huanchaca plateau
The forest–savannah mosaic of the Huanchaca plateau is largely governed by differences in edaphic conditions. Deep fertile soils support forest, while heavily weathered sandstone rocks with only a thin veneer of soil can only sustain open savannah (Killeen & Schulenberg 1998). However, stable carbon isotope studies have shown that this forest–savannah mosaic is far from static. Killeen et al. (2003) examined the δ13C profiles of 15 soil pits (each 1 m deep) dug along a 60 km transect in the centre of the plateau spanning open savannah (campo cerrado), savannah woodland (cerrado and cerradão) and high closed-canopy evergreen forest (figure 5). Changes in the δ13C profiles reflect changes in the relative proportions of C4 savannah grasses versus C3 woody vegetation, with progressively lighter isotopic values indicative of increasing abundance of trees. δ13C values of high forest soil-surface samples range between −29.5 and −28.5‰, while those from savannah are heavier but cover a wide range across a gradient of tree density and C4 grass cover, that is, −26.5 (dense savannah woodland, cerradão) to −15.5‰ (open savannah grassland). The humin fraction of the soil samples at the base of the pits (100 cm depth) dates to ca 3000 yr BP (S. Panfil 2002, unpublished data). Although detailed chronologies are not yet available, precluding reliable correlations between different profiles, some clear trends are nevertheless apparent. The savannah soil profiles reveal continuous savannah cover over at least the past 3000 years, although they appear to have been more open in the middle of the profiles. The soil profiles for the forest sites show progressively heavier δ13C values with increased depth, suggesting that a more open forest (e.g. cerradão) or savannah woodland (e.g. cerrado) existed ca 3000 yr BP, which was gradually invaded by forest tree species, eventually resulting in the closed-canopy tall forest that surrounds these soil pits today. Consequently, there appears to have been progressive forest encroachment into savannah in ecotonal areas on the plateau over the last few millennia, consistent with the pollen and charcoal data from the lakes below the plateau. It therefore appears that the late Holocene precipitation increase not only forced ecotonal changes in the lowlands of NKMNP, but also on the plateau as well, at least in areas where edaphic conditions permitted such responses.
Figure 5.
The δ13C values of soil profiles of 15 soil pits dug along an ecotonal transect between open savannah (grassland) and tall forest in the central part of the Huanchaca plateau, NKMNP. (Modified from Killeen et al. 2003.)
(iii) Past anthropogenic impacts
The vegetation in NKMNP is essentially pristine today. The only obvious human impact over the twentieth century, aside from minimal logging activity in the 1980s, is the removal of mahogany and rubber tapping. The park is unpopulated, with a few small villages at the perimeter. Ceramics recovered from a soil pit in an interfluve approximately 25 km northwest of Laguna Chaplin (T. J. Killeen 2002, personal communication) provide evidence of past indigenous communities in the park, although absence of anthropogenic indicators in the pollen record (e.g. maize pollen) shows that they are unlikely to have had any large-scale or lasting impact upon the vegetation. Furthermore, unlike the alluvial plains of the Beni basin (discussed below), there is no evidence of any kind of anthropogenic earthwork in any of the forest or savannah ecosystems. It is therefore likely that the Holocene charcoal record reflects a predominantly natural, rather than anthropogenic, fire history and that the vegetation changes were also a consequence of natural environmental change.
(c) Implications of palaeo data
(i) Patterns of biodiversity and current vegetation dynamics
The recent expansion of rainforest across NKMNP within the last two to three millennia, revealed by the two pollen records, raises the possibility that their relatively low local (alpha) diversity, relative to other Amazonian forests further north, may not necessarily be simply due to moisture stress in this ecotonal position, but perhaps be because they are still undergoing succession and species recruitment and have not yet filled all their available niches. Furthermore, although there is evidence for both forest encroachment into savannah and vice versa over recent decades (e.g. from satellite imagery; Killeen 1998), the pollen and stable carbon isotope records indicate that the forest expansion into savannah is the predominant process, and that it most likely does not merely represent minor ecotonal oscillations, but instead constitutes a long-term trend of climate-driven rainforest expansion that began at least 3000 years ago. At the forest–savannah ecotone near Brasília (15°57′ S, 47°56′ W), over 1000 km away, Ratter (1992) has observed giant, relic cerrado trees growing 4–5 km within rainforest as evidence of recent forest encroachment into savannah. The Holocene palaeovegetation data from NKMNP, as well as from other sites in ecotonal areas across southern and eastern Amazonia (Absy et al. 1991; Turcq et al. 1998; De Freitas et al. 2001; Mourguiart & Ledru 2003) suggest that this too could be part of a Late Holocene climate-driven trend.
Conversely, the soil stable carbon isotope data are consistent with the hypothesis that the cerrado savannahs on the Huanchaca plateau owe their high biodiversity to their long geological history, which possibly extends back uninterrupted beyond the Quaternary. Upland savannahs probably persist on the plateau, not because precipitation is insufficient to support forest, but more likely because soils are insufficiently deep or fertile to support forest.
The pollen data suggest that the scattered seasonally flooded savannah patches below the plateau are most likely ancient relicts of a much greater, possibly continuous distribution that covered most low-lying areas prior to their replacement by seasonally flooded rainforest.
Ecological studies of permanent, one hectare, vegetation study plots from several types of forest in NKMNP have revealed consistent increases in plant turnover (i.e. recruitment and mortality), above-ground biomass, basal area, stem density and liana development over the past decade (Phillips et al. 2002, 2004; Baker et al. 2004). It might be tempting to hypothesize that these ecological trends can be explained by the recent spread of rainforest species into NKMNP over recent millennia and that they signify long-term rainforest succession, especially as recruitment leads mortality (Phillips et al. 2004). However, this cannot be the explanation because these recent changes are a consistent feature, not only of NKMNP, but also of dozens of old-growth forest sites, with different Quaternary histories (Mayle et al. 2004), across the entire Amazon basin. None of the alternative explanations suffice either, such as secondary succession of forest following indigenous population collapse after the Spanish Conquest (Wright 2005), or responses to climatic perturbations such as the El Niño Southern Oscillation, because they too cannot account for similar ecological responses across the entire Amazon basin. Although the underlying causal factor and mechanism is still controversial and unresolved (Wright 2005, 2006; Lewis et al. 2006), Phillips et al. (2004) make a forceful argument that the only conceivable driver that could synchronously affect the entire Neotropics in a similar way is the anthropogenic rise in atmospheric CO2 concentrations, causing increased canopy photosynthetic rates, possibly augmented by increasing sunlight.
(d) Future climate change
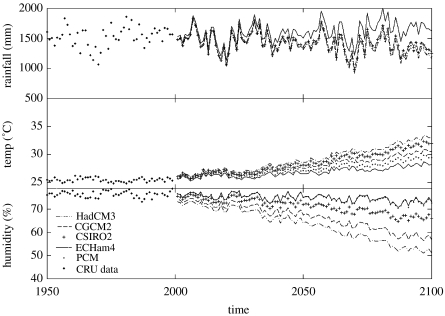
We extracted the predictions of five leading global circulation models (GCMs) for NKMNP during the twenty-first century and compared them with the twentieth century average data, calculated from the Climatic Research Unit global meteorology dataset (New et al. 1999). The range of model outputs indicates a general warming and drying trend (figure 6). The most extreme scenario is predicted by the UK Met Office Hadley Centre ‘HadCm3’ model, which predicts a warming of 7.5°C and a decrease in average relative humidity from 75 to 51%. The most conservative scenario is given by the coupled global climate model (CGCM2), which predicts a rise in temperature of 2.5°C and a decrease in relative humidity of only 3%. The other model results (CSIRO, PCM and ECHam4) are distributed between these two extremes. The effects of climatic change on rainfall are mediated by substantial inter-annual and seasonal variation, but all show a declining trend, with the exception of the CGCM2 model. However, none of these models simulate biosphere–atmosphere feedbacks, which are thought to be important in the Amazon region.
Figure 6.
Climate predictions of five leading global climate models (GCMs) for NKMNP during the twenty-first century, compared with the twentieth century average data calculated from the Climatic Research Unit (CRU) global meteorology data set (New et al. 1999).
These feedbacks are both biophysical and biogeochemical, and their effects include reductions in evapotranspiration and reductions in carbon storage in vegetation and soil. The net effect on a drying and warming climatic trend would be acceleration, but significant uncertainty exists in quantifying such feedbacks, especially over the longer term (Davidson & Janssens 2006; Meir et al. 2006).
Short-term declines in rainfall not only affect evapotranspiration and productivity but also respiration rates in soil, thus modifying the net emissions of CO2 to the atmosphere. Over the longer term, pollen and stable carbon isotope data show that the drier climate of the Mid-Holocene favoured savannah and semi-deciduous forest over humid evergreen rainforest in NKMNP. Any future change in climate towards drier conditions would be expected to cause similar vegetation changes, through increased mortality and subsequent species replacement. These changes in soil and vegetation imply large net emissions of CO2 (through reductions in carbon storage), as well as reductions in evapotranspiration and increases in albedo, although their effects are quantitatively uncertain (Meir & Grace 2005). However, new datasets are emerging that may help improve the modelling of long-term responses (decadal to century scale) of rainforest trees to drought; for example, mortality rates have been found to at least double after both experimental and El Niño-associated drought (e.g. Nakagawa et al. 2000; Nepstad et al. 2004; Meir & Grace 2005), and potential rates of vegetation change have been calculated on this basis (e.g. Condit et al. 1998). However, it is important to note that while the Mid-Holocene vegetation may be considered a reasonable analogue for the future, it is an imperfect analogue because CO2 levels in the twenty-first century are predicted to be at least double those of the Mid-Holocene (ca 270 p.p.m., Indermühle et al. 1999). The effects of a future drier climate may be partially compensated for by the concomitant increase in atmospheric CO2 concentration, which would have the physiological effect of allowing a greater rate of photosynthesis per unit of water loss, enabling trees to survive a longer dry season. Although this effect is incorporated into many biosphere models, its long-term permanence and, indeed, the uniformity of response across species has been questioned (Idso 1999; Körner et al. 2005).
Fully coupling biosphere processes to a GCM has only been achieved by a few modelling groups (Cox et al. 2000; Friedlingstein et al. 2003, 2006) and while current models show a positive feedback with climatic change, uncertainty in the relevant ecological and physiological mechanisms translates into modelled differences in climatic and vegetation change scenarios (Meir et al. 2006). In addition, the compounding effect of anthropogenic influences is particularly important for the time frame relevant to conservation. Deforestation is expected to reduce evapotranspiration, further reducing rainfall regionally (Costa & Foley 2000), with the additional potential for climatic effects further afield (Avissar & Werth 2005). Coupled with other land-use changes and increased fire frequency, a significant positive feedback with climatic warming and drying is possible (Nepstad et al. 2004). Thus, fully coupling land–atmosphere feedbacks into a climatic change scenario may well show that current GCM model outputs are under-estimates of climatic change in the Amazon region. As a result, an intensified drying and warming trend is likely over both short and longer time frames, leading to conversion of humid evergreen rainforests in NKMNP to an ecosystem with a lower leaf area index and with seasonal vegetation growth (i.e. semi-deciduous dry forest and savannah).
(e) Implications for conservation
The key message from the palaeo data and GCM outputs is that the long-term trend of rainforest expansion in NKMNP over the last several millennia is likely to be reversed over the course of the twenty-first century as total annual evapotranspiration exceeds precipitation. Although recruitment currently leads mortality in rainforest communities (Baker et al. 2004), we predict that for most rainforest species reduced available moisture will eventually cause an increase in net mortality and reductions in biomass, stem density and basal area gain. However, the recent increased abundance of lianas (Phillips et al. 2002) may continue further, as increased tree mortality leads to more tree-fall gaps (Schnitzer & Bongers 2002). Humid evergreen rainforest would be susceptible to not only increased drought, but also the accompanied increase in fire frequency.
If the Mid-Holocene forests serve as a reasonable analogue, one would predict that future climate warming and drought would lead to progressive replacement of rainforest species by the more drought-adapted and fire-adapted tree species of the Chiquitano dry forest that lies immediately south of NKMNP. However, seed source availability is a crucial and under-recognized constraint on predicted vegetation change. In the case of NKMNP, unfortunately, much of the Chiquitano dry forest is unprotected and currently experiences among the highest rates of deforestation in the world (Steininger et al. 2001a,b). To allow for this rainforest–dry forest ecotonal shift to occur, there is an urgent need to protect the tracts of semi-deciduous dry forest that lie immediately south of NKMNP, establishing a ‘latitudinal landscape corridor’. This would not be an easy task as most of the land is privately owned and a development corridor will inevitably emerge along the road between Santa Cruz, Bolivia, and Cuiabá, Brazil (Killeen et al. 2003).
If the GCM climate simulations for the twenty-first century are correct, then it is possible that many rainforest species will become locally extinct in NKMNP within the next 100 years, especially as many such species must already be close to their climatic thresholds in this ecotonal region. Rainforest species on the Huanchaca plateau would be expected to be under greatest threat due to their geographic isolation within forest patches surrounded by savannah. Even in lowlands, below the plateau, movements of bird and animal rainforest species northward to more central parts of Amazonia would be highly limited due to the extensive deforestation that has already occurred in neighbouring Rondônia (Skole & Tucker 1993; see figure 1), although the humid forest habitats along the Río Iténez could potentially serve as a biodiversity corridor allowing northward migration (Killeen et al. 2003).
The Holocene palaeodata show that savannah ecosystems would also be expected to expand at the expense of rainforest as climate becomes more arid in the future. From a conservation standpoint, this would be a positive outcome, as it is the savannahs of NKMNP, rather than the rainforests, that contain the most endangered species (Da Silva & Bates 2002), and the Neotropical savannah biome is under far greater threat (from cattle-ranching, agriculture and human population expansion) than the Amazon rainforests (e.g. Silva et al. 2006). The Huanchaca plateau probably contains the largest protected tract of undisturbed cerrado (upland) savannahs (approx. 42 000 ha) anywhere in the Neotropics (Killeen 1998; Da Silva & Bates 2002), which together with the seasonally flooded savannahs below are home to some of the most threatened savannah megafauna on the continent, such as the pampas deer (Ozotoceros bezoarticus), the marsh deer (Blastocerus dichotomus), the maned wolf (Chrysocyon brachyurus), the greater rhea (Rhea americana) and the giant anteater (Myrmecophaga tridacyla). In the absence of hunting, future climate change could actually favour expansion of these endangered species.
(f) Fire
Aside from climate change, the key influence upon the ecosystems of NKMNP in the future is likely to be anthropogenic fires that get out of control and enter the park from beyond its borders; for example from slash-and-burn agriculture and/or burning of ranch-land in the neighbouring seasonally flooded savannahs and deforested areas along the Brazilian border (Killeen 1991). Although the frequency of forest fires in NKMNP is unknown, large-scale forest fires were observed in 1987, 1993 and 1995 (Killeen et al. 2003). The frequency of such fires would undoubtedly increase under a warmer, drier climate, speeding up the conversion of forest to savannah, especially at forest edges. The challenge for park managers is to prevent anthropogenic fires, while at the same time allowing natural, lightning-induced fires to take hold, although differentiating between the two in practice will often not be possible. A key challenge is to determine the effect of differing frequencies and intensities of fire upon the forest–savannah mosaic, not only with regard to the dynamic between these two biomes, but also their respective structure and species composition. Unfortunately, most palaeo-charcoal records have neither the temporal nor spatial resolution to address this issue.
3. Seasonally flooded savannahs of the Bolivian Beni Basin
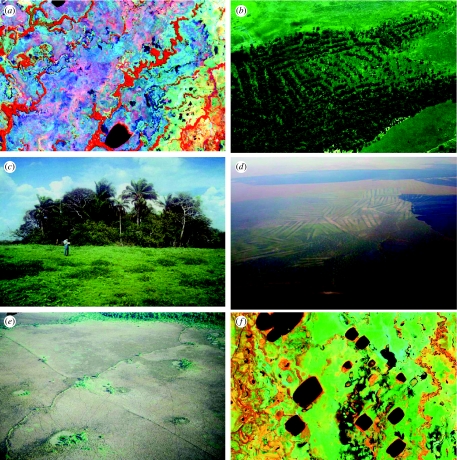
The Llanos de Moxos (figure 1) is a 160 000 km2 expanse of seasonally inundated savannahs, interspersed with a complex mosaic of forest ‘islands’ and riverine gallery forests (figure 7a), occupying the extremely flat Beni–Mamoré–Iténez basin in SW Amazonia (10°40′–16°25′ S), situated between the Precambrian Shield to the east and the Andes to the west and south. Numerous white-water rivers and hundreds of shallow, flat-bottomed lakes cover the landscape. The spatial distribution of vegetation is closely related to micro-topography. Flat, low-lying areas, which are seasonally inundated for several months a year during the rainy season, by flooding from rivers and ponding of rainwater, are covered by completely open, tree-less savannah (locally referred to as pampa). Conversely, forest islands (islas) are restricted to raised areas (mounds) which are sufficiently high to escape flooding.
Figure 7.
Earthmounds, vegetation and lakes of the Llanos de Moxos. (a) Remote-sensing image showing the forest–savannah mosaic of the Llanos de Moxos, palaeofluvial features and gallery forests (red) trending SW–NE, with intervening open savannahs (light blue). (b) Forest island formation upon abandoned pre-Hispanic raised fields. (c) Forest island upon relict natural levee surface. (d) Aerial photo showing rectangular, parallel raised fields in the Llanos de Moxos. (e) Oblique aerial photograph of a linear zigzag feature (running bottom left to top right) in the savannahs of Baures region, Llanos de Moxos, interpreted by Erickson (2000) as a fish weir. Circular features surrounded by palms (20 m diameter) are interpreted by Erickson (2000) as artificial ponds. N.B. The diagonal feature (running top left to bottom right) is a contemporary path. (Photo courtesy of Clark Erickson, University of Pennsylvania Museum of Archaeology and Anthropology). (f) Remote-sensing image showing rectangular lakes of the Llanos de Moxos oriented SW–NE.
The extent to which this forest–savannah mosaic is natural or a human artefact has been debated since the 1960s. Beck (1983) argues that these savannahs are the legacy of centuries, or even millennia, of deforestation by pre-Conquest indigenous peoples, and subsequently large-scale cattle ranching over the last several centuries. However, we argue that, at a regional scale at least, the seasonally flooded savannahs are natural, resulting from the unique suite of edaphic, hydrological and climatic conditions of the basin. Heavy, shallow soils with impermeable clay pans are inundated with stagnant waters at high temperature for several months of the year, resulting in anaerobic conditions and physiological heat stress, followed by a severe dry season. Very few woody species are able to endure this combination of abiotic factors.
However, at a fine spatial scale there is extensive archaeological evidence showing that pre-Conquest indigenous peoples made significant modifications to this savannah environment, principally by constructing a vast array of different types of earth mounds (Denevan 1966; Erickson 1995, 2000). As these artificial landforms were above the flood-level, they provided suitable habitats for colonization by trees once they were abandoned.
(a) Forest islands: natural or anthropogenic?
To what extent is the mosaic of forest islands scattered across the Llanos de Moxos a natural phenomenon, or an artefact of landscape engineering by humans as argued by Denevan (1966) and Erickson (1995)? Accounts from early Spanish chroniclers such as Cabello Balboa (ca 1600–1604; in Jiménez de la Espada 1965) and Recio de León (1623; in Maurtua 1906) reveal that the Moxos savannahs, which are today only sparsely populated, supported dense native populations during the sixteenth and early seventeenth centuries. Denevan (1966) was the first to provide detailed archaeological evidence that Palaeo-Indians made large-scale changes to the topography of much of the region. He showed that numerous forest islands (figure 7b,c), generally ranging from 10 to 50 m diameter and at least 2 m above the flooded savannah surface, were comprised largely of rubble made up of broken pottery and were constructed to allow human habitation above the floodwaters. Once abandoned, these mounds were colonized by trees forming forest islands. Denevan (1966) concluded that the primary determinant of the basic vegetation patterns in the Llanos de Moxos savannahs is the degree of flooding, which is determined by local relief, but he demonstrated that much of this relief was created by earthmoving activities of the pre-Hispanic peoples of the region.
However, while some forest islands do indeed appear to cover artificial earth mounds, closer examination reveals a variety of forest island types that differ in species composition, morphometry, soil type and physical setting; these clearly must have had different modes of origin. Many of the smallest forest islands, 10 m or less in diameter, have been created by termites rather than humans. Such islands are regularly spaced apart, sometimes less than 50 m, and form on earth mounds created by eroding termite mounds. Such termite savannahs are not only characteristic of seasonally flooded areas of NKMNP (Killeen 1998), discussed earlier, but are also abundant in many parts of the Llanos de Moxos (Denevan 1966; Beck 1983; Hanagarth 1993). Aerial photography and, more recently, LANDSAT imagery, have demonstrated that many forest islands are eroded relics of natural levees or terraces of abandoned river channels, and therefore constitute fragments of former gallery forest (Braun 1961; Hanagarth & Sarmiento 1990). Corroboratory evidence comes from their underlying sandy soils, which contrasts with the clays of the surrounding savannah (Langstroth 1996).
It is therefore clear that a single model cannot be used to explain the origin of these forest islands. While some are demonstrably artificial, whereby trees have colonized abandoned Palaeo-Indian earthworks, others have formed by entirely natural processes. Furthermore, the degree to which some supposedly artificial mounds are entirely anthropogenic in origin, or modified natural mounds, is also often unclear.
(b) Anthropogenic modification of the seasonally flooded savannahs
In addition to creating mounds, which later become forest islands, there is abundant evidence for other kinds of anthropogenic earthworks constructed in pre-Hispanic times, as recently highlighted by Mann (2000) and reviewed by Calandra & Salceda (2004). These earthworks have been described in detail by Denevan (1966), Erickson (1995, 2000) and Langstroth (1996) and are summarized below. Elongate, rectangular raised fields are common throughout the Moxos, especially west of the River Mamoré, often arranged in parallel series (figure 7d). Circular mounds (lomas) are also common, especially within gallery forests, and have been variously interpreted as garbage piles, house mounds, ceremonial mounds or burial mounds. Linear ridges that traverse the savannahs, and often connect mounds and forest islands, have been interpreted as causeways, which may have performed multiple functions such as transportation, hydraulic control and boundary markers. Canals have also been built to divert water from streams and rivers to areas where water is scarce in the dry season and to connect the natural south–north fluvial system with a series of east–west navigational channels. Some of the most impressive earthworks are the complex networks of linear zigzag structures (1–2 m wide and 20–50 cm high; figure 7e), covering 525 km2 of the Baures region in the east of the Beni basin, interpreted by Erickson (2000) as fish weirs, which supposedly trapped fish in the seasonally flooded savannahs as the flood-waters receded at the end of the rainy season. These features are clearly anthropogenic, not only owing to their angular zigzag shapes, but also because they were constructed from an assortment of rock, soil, reeds, branches and logs, as well as basketry.
All these forms of landscape engineering indicate that the Moxos savannahs supported intensive fishing and farming industries, supplying, and maintained by, a much larger human population than exists in this part of Bolivia today. The chronology for these various earthworks is limited, but dates from basal units indicate that these societies modified the landscape extensively over at least the past three millennia and collapsed around the time of the Spanish Conquest (Erickson et al. 1991; Langstroth 1996).
(c) Quaternary history of the Llanos de Moxos
Beyond the last three millennia of extensive human impact, the Quaternary vegetation history of the Llanos de Moxos is unknown. Although the area is covered with hundreds of lakes (figure 7f), only a couple of attempts have been made to core any of them, and those have been unsuccessful due to the extremely stiff clay sediments that are impenetrable beyond 20 or 30 cm depth with a standard manual piston-type corer, as discovered by one of the authors (F. Mayle) and other palaeoecologists (H. Wright 1995, personal communication; M. Bush 2003, personal communication). Due to the lack of data, ideas about the mode of origin and ages of these lakes are entirely speculative. Social scientists have argued that many are artificial due to their commonly square or rectangular shapes, flat and shallow bathymetry (less than 2 m deep), and raised margins (see Romero & Pastó 2004). However, while this may be true for some of the small ponds, it is hard to envisage that these cultures had the resources to excavate large lakes, several kilometres in diameter, from the hard savannah clays, especially as the volume of sediment to be removed would have been much greater than that of the known earthworks.
If the majority of lakes are natural in origin, then how can their distinctly rectangular shapes and uniform orientation along a SW–NE axis be explained? Plafker (1964, 1974) has hypothesized that they were caused by fracturing of the underlying Precambrian basement rocks, which affected the alignment of superficial jointing in the overlying unconsolidated Tertiary and Quaternary sediments, causing them to subside and form shallow lake basins. However, Clapperton (1993), Dumont & Fournier (1994) and Langstroth (1996) have queried this mechanism as it is unclear how basement fracturing can be propagated to the surface through more than 800 m of soft unconsolidated sediments. These authors suggest that these oriented lakes instead constitute palaeo-deflation basins that formed under more arid climatic conditions of the past. Dumont & Fournier interpret the existence of indurated clay caps beneath some of the lakes as additional evidence of a formerly drier climate. Furthermore, the orientation of the long axis of the lakes is almost exactly perpendicular to the axis of the Holocene dunes of the Piraí basin in Santa Cruz, consistent with an aeolian mechanism (Livingstone 1954; Carson & Hussey 1962; Dumont & Fournier 1994).
In the absence of empirical palaeovegetation data from the Beni, insights into the age of the forest–savannah mosaic may be gleaned by considering species' endemicity. Although not recognized as a centre of endemicity, the Llanos de Moxos is home to a few notable endemic vertebrates such as the Beni anaconda (Eunectes beniensis), the blue-throated macaw (Ara glaucogularis), and the Beni and Olalla's titi monkeys (Callicebus modestus and Callicebus olallae, respectively). The Beni anaconda is confined to the savannah wetlands, while the macaw and titi monkeys are restricted to forest islands. The latter are most significant because they are not found in larger contiguous forests or extensive gallery forests. The presence of endemic vertebrate species suggests that the forest–savannah mosaic that characterizes the Beni has at least a long Quaternary history, although the spatial configuration of these two ecosystems may well have changed over time.
(d) Implications of artificial earthworks
(i) Amazonian anthropology
The issue of whether or not the acidic, nutrient-poor soils of the Amazon basin were ever able to yield sufficient food to support large, complex, hierarchical societies has been a matter of vigorous debate among anthropologists for decades (Denevan 1966, 2001; Meggers 1996; Heckenberger 2005). However, there is increasing evidence that Amazonia did indeed support dense populations in regions which are sparsely populated today: for example, black terra preta soils throughout the central Amazon (Lehmann et al. 2003; Glaser 2007) and recently discovered complex earthworks in southern Amazon rainforests of Mato Grosso, Brazil (Heckenberger 2005; Heckenberger et al. 2003, 2007). The archaeological evidence from the Llanos de Moxos can therefore not be considered as an anomaly but adds to a growing body of evidence that Amazonia as a whole supported dense, complex societies that had major impacts upon their ecosystems that persist to this day.
(ii) Conservation
Using their ecoregion approach to assessing conservation status, Dinerstein et al. (1995) categorized the Beni savannahs of Bolivia as ‘bioregionally outstanding’ owing to their diverse grassland flora which supports a rich fauna of large mammals and birds. They were classified as ‘endangered’ and with a ‘high priority at regional scale’ due to the serious threats of wildlife exploitation and grassland degradation due to overgrazing from extensive cattle-ranching and the high frequency of burning. These threats are predicted to become more severe over coming decades and, most recently, rice farming has begun to take its toll in the Beni.
This conservation assessment was based solely upon ‘conservation status’ and ‘biological distinctiveness’. The extent to which the ‘biological distinctiveness’ of the Beni is anthropogenically derived was therefore not considered. This brings into question the suitability of standard biodiversity-based conservation approaches for regions such as the Beni that have a strong legacy of human intervention in the landscape. Essentially ‘virgin’ forest–savannah environments, such as those within NKMNP, are clearly ideal for traditional conservation approaches, whereby the ecosystems are designated as a national park from which human activities are excluded. By contrast, any plans to conserve the forest–savannah mosaic of the Llanos de Moxos in a similar way, by excluding all human land use, may end up inadvertently changing the vegetation mosaic and species composition in unforeseen ways. Several important tree species may well have been managed by native peoples over centuries or millennia and are harvested to this day for a variety of uses. For example, the tree Sorocea, which grows on earth mounds, is commonly used by the Sirionó Indians to make beer (Mann 2000), while the palm Mauritia flexuosa, which grows in the surrounding seasonally flooded savannahs, produces up to 5000 edible fruits per year from a single tree and 10–60 t of fruit from a single hectare (Erickson 2000). Although such tree species may have become established on earth mounds by natural means, such as bird dispersal (Langstroth 1996), rather than planting, native people could have encouraged expansion of such species over the other non-utilized species. The relative abundance of such taxa may change once people are excluded.
Furthermore, several tribes (e.g. Moxo, Movima, Canichana, Baure and Itonama) whose ancestors helped create the anthropogenic landscape of earthworks, fish weirs, causeways, etc. still occupy the Beni basin today and receive some form of protection via ‘indigenous lands’. Any conservation policy that aimed to supposedly conserve the landscape of the Llanos de Moxos by excluding the very people whose ancestors helped create it would surely be misguided and counter-productive. A more appropriate conservation strategy may be one that incorporates traditional indigenous land-use practices and local economies, given the evidence that the Llanos de Moxos has supported dense human societies over several millennia. Ultimately, biologists need to engage with anthropologists and archaeologists to adopt a conservation strategy that takes account of not only its biological value, but also its rich archaeological heritage. The level and type of human land use that this biota has sustained over past millennia needs to be understood in order to determine what type and intensity of land use is sustainable for this environment in the future.
It is unclear how a future drier climate might affect the Llanos de Moxos. On one hand, reduced seasonal inundation might make hydrological conditions suitable for tree populations to expand beyond the raised mounds to the surrounding low-lying plains. However, the species composition of these forests could change significantly, with more drought-tolerant tree taxa from the Gran Chaco deciduous woodlands to the south spreading north into the Beni and out-competing semi-deciduous and rainforest species. On the other hand, irrespective of the direction of future climate change, the riverine gallery forests could serve as important migration corridors for species interchange between the different types of tropical forest bordering the basin (e.g. between rainforest to the north and Chaco dry woodland to the south). Regardless of the anthropogenic legacy within these gallery forests, they should clearly be protected from future deforestation to maintain them as intact corridors.
4. Conclusions
The forest–savannah environments of NKMNP and the Llanos de Moxos in lowland Bolivia differ markedly from one another in a variety of ways: their abiotic controls; biota; geomorphological settings; current land use; and environmental histories (particularly with respect to human impact). Consideration of their respective palaeoecological and archaeological histories demonstrates markedly differing legacies of past human disturbance versus climatically driven vegetation change, which in turn call for differing CCS that reflect their contrasting histories.
NKMNP contains largely ‘virgin’ ecosystems. The palaeoecological records show no evidence that they have experienced any significant human impact over the past 50 000 years. Fossil pollen and stable carbon isotope data demonstrate that present-day rainforest encroachment into savannah is significant, but is a part of long-term trend (over the last three millennia) of southward expansion of rainforest through the park, replacing dry forest and savannah communities. This ecotonal shift appears to have been an entirely natural process caused, either directly or indirectly, by increasing precipitation.
In contrast, there is abundant archaeological evidence that the forest–savannah mosaic of the Llanos de Moxos in the Beni basin has been altered extensively by Palaeo-Indians over centuries and millennia prior to the Spanish Conquest, largely by building a variety of different kinds of earth mound that persist today. Although some forest islands are clearly natural features, such as gallery forest fragments, others are demonstrably artificial, whereby Palaeo-Indians have built earthworks, which, once abandoned, have become colonized by trees. Therefore, at least some of the forest expansion in the Llanos de Moxos during the Late Holocene was not climatically driven, but instead was facilitated by humans creating edaphically suitable conditions for trees to grow.
The contrasting legacies of past human impact between these two forest–savannah transition zones have important implications for conservation planners. On one hand, the traditional approach of creating a national park, from which human land use is excluded, appears to be appropriate for the ecosystems of NKMNP which have no discernible legacy of past human disturbance. On the other hand, this approach would be inappropriate for the Llanos de Moxos, where much of the landscape owes its very character to past human activity. The dilemma for conservationists is determining the type and intensity of land use that is appropriate for this anthropogenically modified landscape.
How will the vegetation in these two ecotonal regions be affected by the drier and warmer climatic conditions predicted for the twenty-first century? The Late Holocene rainforest expansion in NKMNP is likely to be reversed, as more drought-tolerant species are favoured, resulting in semi-deciduous dry forest and savannah communities, akin to those that dominated NKMNP during the Early/Mid Holocene. In the Llanos de Moxos, future increased drought could result in drought-tolerant tree taxa from the Gran Chaco deciduous woodlands to the south spreading northward and out-competing semi-deciduous and rainforest species in future.
Landscape corridors are needed to facilitate these latitudinal shifts in species ranges. The riverine gallery forests that traverse the Beni basin, generally from south to north, should be targeted as natural corridors allowing species interchange between the Chaco dry woodlands to the south and the humid Amazonian rainforests to the north. In order to facilitate northward spread of semi-deciduous dry forest species into NKMNP, it is imperative that the protected area network is expanded to form a contiguous latitudinal landscape corridor that connects the rainforests of NKMNP with the poorly protected Chiquitano dry forest to the south.
We hope that knowledge of the long-term dynamic history of forest–savannah environments, considered in the context of ecological observations and modelled future climate change scenarios, can help conservationists develop the most appropriate conservation strategies for these biodiverse and often-neglected habitats.
Acknowledgments
We are grateful to Kathy Willis and Lindsey Gillson for inviting us to contribute this paper, and William Denevan and the two reviewers, Keith Bennett and Kathy Willis, whose comments and suggestions improved the paper.
Footnotes
One contribution of 14 to a Theme Issue ‘Biodiversity hotspots through time: using the past to manage the future’.
References
- Absy M.L, et al. Mise en évidence de quatre phases d'ouverture de la forêt dense dans le sud-est de l'Amazonie au cours des 60,000 dernières années. Première comparaison avec d'autres régions tropicales. Comptes Rendus de l'Academie des Sciences de Paris. 1991;312:673–678. [Google Scholar]
- Avissar R, Werth D. Global hydroclimatological teleconnections resulting from tropical deforestation. J. Hydrometeorol. 2005;6:134–145. doi:10.1175/JHM406.1 [Google Scholar]
- Baker P.A, Seltzer G.O, Fritz S.C, Dunbar R.B, Grove M.J, Tapia P.M, Cross S.L, Rowe H.D, Broda J.P. The History of South American tropical precipitation for the past 25,000 years. Science. 2001;291:640–643. doi: 10.1126/science.291.5504.640. doi:10.1126/science.291.5504.640 [DOI] [PubMed] [Google Scholar]
- Baker T.R, et al. Increasing biomass in Amazonian forest plots. Phil. Trans. R. Soc. B. 2004;359:353–356. doi: 10.1098/rstb.2003.1422. doi:10.1098/rstb.2003.1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates J.M, Stotz D.F, Schulenberg T.S. Avifauna of Parque Nacional Noel Kempff Mercado. In: Killeen T.J, Schulenberg T.S, editors. A biological assessment of Parque Nacional Noel Kempff Mercado, Bolivia. RAP working papers 10. Conservation International; Washington, DC: 1998. pp. 112–119. [Google Scholar]
- Beck S.G. Vegetationsökologische Grundlagen der Viehwirtschaft in den Überschwemmungs-Savannen des Río Yacuma (Departamento Beni, Bolivien) Dissertationes Botanicae. 1983;80:1–186. [Google Scholar]
- Braun, O. 1961 Cultivo de pastos en el Beni. La Paz, Servicio Agrícola Interamericano.
- Burbridge R.E, Mayle F.E, Killeen T.J. Fifty-thousand-year vegetation and climate history of Noel Kempff Mercado National Park, Bolivian Amazon. Quatern. Res. 2004;61:215–230. doi:10.1016/j.yqres.2003.12.004 [Google Scholar]
- Bush M.B. Amazonian speciation: a necessarily complex model. J. Biogeogr. 1994;21:5–17. doi:10.2307/2845600 [Google Scholar]
- Bush M.B. Distributional change and conservation on the Andean flank: a palaeoecological perspective. Global Ecol. Biogeogr. 2002;11:463–473. doi:10.1046/j.1466-822X.2002.00305.x [Google Scholar]
- Bush M.B, Silman M.R. Observations on Late Pleistocene cooling and precipitation in the lowland Neotropics. J. Quatern. Sci. 2004;19:677–684. doi:10.1002/jqs.883 [Google Scholar]
- Calandra H.A, Salceda S.A. Amazonia boliviana: arqueología de los Llanos de Mojos. Acta Amaz. 2004;32:155–163. [Google Scholar]
- Carson C.E, Hussey K.M. The oriented lakes of arctic Alaska. J. Geol. 1962;70:417–439. [Google Scholar]
- Clapperton C. Elsevier; Amsterdam, The Netherlands: 1993. Quaternary geology and geomorphology of South America. [Google Scholar]
- Condit R. Ecological implications of changes in drought patterns: shifts in forest composition in Panama. Clim. Change. 1998;39:413–427. doi:10.1023/A:1005395806800 [Google Scholar]
- Costa M.H, Foley J.A. Combined effects of deforestation and doubled atmospheric CO2 concentrations on the climate of Amazonia. J. Climatol. 2000;13:18–34. doi:10.1175/1520-0442(2000)013<0018:CEODAD>2.0.CO;2 [Google Scholar]
- Cox P.M, Betts R.A, Jones C.D, Spall S.A, Totterdell I.J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature. 2000;408:184–187. doi: 10.1038/35041539. doi:10.1038/35041539 [DOI] [PubMed] [Google Scholar]
- Cross S.L, Baker P.A, Seltzer G.O, Fritz S.C, Dunbar R.B. A new estimate of the Holocene lowstand level of Lake Titicaca, central Andes, and implications for tropical palaeohydrology. The Holocene. 2000;10:21–32. doi:10.1191/095968300671452546 [Google Scholar]
- D'Agostino K, Seltzer G, Baker P, Fritz S, Dunbar R. Late-Quaternary lowstands of Lake Titicaca: evidence from high-resolution seismic data. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002;179:97–111. [Google Scholar]
- Da Silva J.M.C, Bates J.M. Biogeographic patterns and conservation in the South American Cerrado: a tropical savannah hotspot. BioScience. 2002;52:225–233. doi:10.1641/0006-3568(2002)052[0225:BPACIT]2.0.CO;2 [Google Scholar]
- Davidson E.A, Janssens I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440:165–173. doi: 10.1038/nature04514. doi:10.1038/nature04514 [DOI] [PubMed] [Google Scholar]
- De Freitas H.A, Pessenda L.C.R, Aravena R, Gouveia S.E.M, De Souza Ribeiro A, Boulet R. Late Quaternary vegetation dynamics in the southern Amazon basin inferred from carbon isotopes in soil organic matter. Quatern. Res. 2001;55:39–46. doi:10.1006/qres.2000.2192 [Google Scholar]
- Denevan W.M. The aboriginal cultural geography of the Llanos de Mojos of Bolivia. Ibero-Americana. 1966;48:1–60. [Google Scholar]
- Denevan W.M. Oxford University Press; New York, NY: 2001. Cultivated landscapes of native Amazonia and the Andes. [Google Scholar]
- Dinerstein E, Olson D.M, Graham D.J, Webster A.L, Primm S.A, Bookbinder M.P, Ledec G. The World Bank; Washington, DC: 1995. A conservation assessment of the terrestrial ecoregions of Latin America and the Caribbean. [Google Scholar]
- Dumont J.F, Fournier M. Geodynamic environment of Quaternary morphostructures of the Subandean foreland basins of Peru and Bolivia: characteristics and study methods. Quatern. Int. 1994;21:129–142. [Google Scholar]
- Emmons L.H. Mammal fauna of Parque Nacional Noel Kempff Mercado. In: Killeen T.J, Schulenberg T.S, editors. A biological assessment of Parque Nacional Noel Kempff Mercado, Bolivia. RAP working papers 10. Conservation International; Washington, DC: 1998. pp. 129–135. [Google Scholar]
- Erickson C.L. Archaeological methods for the study of ancient landscapes of the Llanos de Mojos in the Bolivian Amazon. In: Stahl P, editor. Archaeology in the American tropics: current analytical methods and applications. Cambridge University Press; Cambridge, UK: 1995. [Google Scholar]
- Erickson C.L. An artificial landscape-scale fishery in the Bolivian Amazon. Nature. 2000;408:190–193. doi: 10.1038/35041555. doi:10.1038/35041555 [DOI] [PubMed] [Google Scholar]
- Erickson, C. L., Esteves, C. J., Winkler, V. W. & Michel, L. M. 1991 Estudio preliminar de los sistemas agricolas precolombinos en el departamento del Beni, Bolivia. Informe de los trabajos de campo efectuados durante el mes de Julio de 1990. La Paz/Philadelphia: Instituto Boliviano de Cultura, Instituto Nacional de Arqueologia, The University Museum and Department of Anthropology, University of Pennsylvania.
- Friedlingstein P, Dufresne J.-L, Cox P.M, Rayner P. How positive is the feedback between climate change and the carbon cycle? Tellus. 2003;55B:692–700. [Google Scholar]
- Friedlingstein, P. et al 2006 Climate–carbon cycle feedback analysis: results from the C4MIP model intercomparison. J. Climate19, 3337–3353.
- Furley P.A, Proctor J, Ratter J.A, editors. The nature and dynamics of forest–savannah boundaries. Chapman and Hall; London, UK: 1992. [Google Scholar]
- Glaser B. Prehistorically modified soils of central Amazonia: a model for sustainable agriculture in the twenty-first century. Phil. Trans. R. Soc. B. 2007;362:187–196. doi: 10.1098/rstb.2006.1978. doi:10.1098/rstb.2006.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling, W. D. 2004 Characterisation of Amazonian forest and savannah ecosystems by their modern pollen spectra. Ph.D. thesis, Department of Geography, University of Leicester, UK.
- Gosling W.D, Mayle F.E, Tate N.J, Killeen T.J. Modern pollen-rain characteristics of tall terra firme moist evergreen forest, southern Amazonia. Quatern. Res. 2005;64:284–297. doi:10.1016/j.yqres.2005.08.008 [Google Scholar]
- Hanagarth, W. 1993 Acerca de la geoecología de las sabanas del Beni La Paz, Instituto de Ecología.
- Hanagarth W, Sarmiento J. Reporte preliminar sobre la geoecología de la sabana de Espíritu y sus alrededores (Llanos de Moxos, departamento del Beni, Bolivia) Ecología en Bolivia. 1990;16:47–75. [Google Scholar]
- Hannah L, Midgley G.F, Lovejoy T, Bond W.J, Bush M, Lovett J.C, Scott D, Woodward F.I. Conservation of biodiversity in a changing climate. Conserv. Biol. 2002;16:264–268. doi: 10.1046/j.1523-1739.2002.00465.x. doi:10.1046/j.1523-1739.2002.00465.x [DOI] [PubMed] [Google Scholar]
- Heckenberger M.J. Routledge; New York, NY: 2005. The ecology of power. [Google Scholar]
- Heckenberger M.J, Kuikuro A, Kuikuro U.T, Russell J.C, Schmidt M, Fausto C, Franchetto B. Amazonia 1492: pristine forest or cultural parkland? Science. 2003;301:1710–1714. doi: 10.1126/science.1086112. doi:10.1126/science.1086112 [DOI] [PubMed] [Google Scholar]
- Heckenberger M.J, Russell J.C, Toney J.R, Schmidt M.J. The legacy of cultural landscapes in the Brazilian Amazon: implications for biodiversity. Phil. Trans. R. Soc. B. 2007;362:197–208. doi: 10.1098/rstb.2006.1979. doi:10.1098/rstb.2006.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton J.T, Ding Y, Griggs D.J, Noguer M, van der Linden P.J, Dai X, Maskell K, Johnson C.A, editors. Climate Change 2001: the scientific basis. Contribution of working group 1 to the Third Assessment Report of the IPCC. Cambridge University Press; Cambridge, UK: 2001. [Google Scholar]
- Idso S.B. The long-term response of trees to atmospheric CO2 enrichment. Global Change Biol. 1999;5:493–495. doi:10.1046/j.1365-2486.1999.00240.x [Google Scholar]
- Indermühle A, et al. Holocene carbon-cycle dynamics based on CO2 trapped in ice at Taylor Dome, Antarctica. Nature. 1999;398:121–126. doi:10.1038/18158 [Google Scholar]
- Jiménez de la Espada, M. 1965 Relaciones geográficas de Indias: Perú Madrid: Atlas.
- Killeen T.J. The effect of grazing on native Gramineae in Concepción, Santa Cruz, Bolivia. Trop. Grasslands. 1991;25:12–19. [Google Scholar]
- Killeen T.J. Vegetation and flora of Noel Kempff Mercado National Park. In: Killeen T.J, Schulenberg T.S, editors. A biological assessment of Parque Nacional Noel Kempff Mercado, Bolivia. RAP working papers 10. Conservation International; Washington, DC: 1998. pp. 61–85. [Google Scholar]
- Killeen T.J, Schulenberg T.S, editors. A biological assessment of Parque Nacional Noel Kempff Mercado, Bolivia. RAP working papers 10. Conservation International; Washington, DC: 1998. [Google Scholar]
- Killeen T.J, Siles T.M, Grimwood T, Tieszen L.L, Steininger M.K, Tucker C.J, Panfil S. Habitat heterogeneity on a forest–savannah ecotone in Noel Kempff Mercado National Park (Santa Cruz, Bolivia): implications for the long-term conservation of biodiversity in a changing climate. In: Bradshaw G.A, Marquet P.A, editors. How landscapes change: human disturbance and ecosystem fragmentation in the Americas. Ecological Studies. vol. 162. Springer; Berlin, Germany: 2003. pp. 285–312. [Google Scholar]
- Körner C, Asshoff R, Bignucolo O, Hättenschwiler S, Keel S.G, Peláez-Riedl S, Pepin S, Siegwolf R.T.W, Zotz G. Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science. 2005;309:1360–1362. doi: 10.1126/science.1113977. doi:10.1126/science.1113977 [DOI] [PubMed] [Google Scholar]
- Langstroth, R. P. 1996 Forest islands in an Amazonian savannah of northeastern Bolivia. Ph.D. thesis, University of Washington-Madison. 434 lvs.
- Lehmann J, Kern D.C, Glaser B, Woods W.I, editors. Amazonian dark earths: origin, properties, management. Kluwer; Dordrecht, The Netherlands: 2003. [Google Scholar]
- Lewis S.L, Phillips O.L, Baker T.R. Impacts of global atmospheric change on tropical forests. Trends Ecol. Evol. 2006;21:173–174. doi: 10.1016/j.tree.2006.02.001. doi:10.1016/j.tree.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Litherland M, Power G. The geological and geomorphic evolution of Serrania Huanchaca (Eastern Bolivia): the Lost World. J. S. Am. Earth Sci. 1989;2:1–17. doi:10.1016/0895-9811(89)90023-0 [Google Scholar]
- Livingstone D.A. On the orientation of lake basins. Am. J. Sci. 1954;252:547–554. [Google Scholar]
- Malcolm J.R, Liu C, Neilson R.P, Hansen L, Hannah L. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 2006;20:538–548. doi: 10.1111/j.1523-1739.2006.00364.x. doi:10.1111/j.1523-1739.2006.00364.x [DOI] [PubMed] [Google Scholar]
- Mann C.C. Earthmovers of the Amazon. Science. 2000;287:786–789. doi: 10.1126/science.321.5893.1148. doi:10.1126/science.287.5454.786 [DOI] [PubMed] [Google Scholar]
- Maurtua, V. 1906 Juicio de límites entre Perú y Bolivia Madrid, Imprenta de los Hijos de M.G. Hernández.
- Mayle F.E, Burbridge R, Killeen T.J. Millennial-scale dynamics of southern Amazonian rain forests. Science. 2000;290:2291–2294. doi: 10.1126/science.290.5500.2291. doi:10.1126/science.290.5500.2291 [DOI] [PubMed] [Google Scholar]
- Mayle F.E, Beerling D.J, Gosling W.D, Bush M.B. Responses of Amazonian ecosystems to climatic and atmospheric CO2 changes since the Last Glacial Maximum. Phil. Trans. R. Soc. B. 2004;359:499–514. doi: 10.1098/rstb.2003.1434. doi:10.1098/rstb.2003.1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggers B.J. Smithsonian Institution; Washington, DC: 1996. Amazonia: man and culture in a counterfeit paradise; p. 214. [Google Scholar]
- Meir P, Grace J. The response to drought by tropical rain forest ecosystems. In: Malhi Y, Phillips O, editors. Tropical forests and global climate change. Oxford University Press; Oxford, UK: 2005. pp. 75–84. [Google Scholar]
- Meir, P., Cox, P. M. & Grace, J. 2006 The influence of terrestrial ecosystems on climate. Trends Ecol. Evol 21, 254–260. [DOI] [PubMed]
- Metcalfe, P. R. 2005 Late Quaternary history of the rainforest-savannah boundary of southwest Amazonia. Ph.D. thesis, University of Wales, Swansea, UK.
- Mourguiart P, Ledru M.-P. Last Glacial Maximum in an Andean cloud forest environment (eastern Cordillera, Bolivia) Geology. 2003;31:195–198. doi:10.1130/0091-7613(2003)031<0195:LGMIAA>2.0.CO;2 [Google Scholar]
- Myers N, Mittermeier R.A, Mittermeier C.G, da Fonseca G.A.B, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. doi:10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Nakagawa M, et al. Impact of severe drought associated with the 1997–1998 El Nino in a tropical forest in Sarawak. J. Trop. Ecol. 2000;16:355–367. doi:10.1017/S0266467400001450 [Google Scholar]
- Nepstad D, Lefebvre P, Lopes da Silva U, Tomasella J, Schlesinger P, Solorzano L, Mutinhow P, Ray D, Guerreira Benito J. Amazon drought and its implications for forest flammability and tree growth: a basin-wide analysis. Global Change Biol. 2004;10:704–717. doi:10.1111/j.1529-8817.2003.00772.x [Google Scholar]
- New M, Hulme M, Jones P. Representing twentieth-century space-time climate variability. Part I: development of a 1961–90 mean monthly terrestrial climatology. J. Clim. 1999;12:829–856. doi:10.1175/1520-0442(1999)012<0829:RTCSTC>2.0.CO;2 [Google Scholar]
- Olson D.M, Dinerstein E. The Global 200: a representation approach to conserving the Earth's most biologically valuable ecoregions. Conserv. Biol. 1998;12:502–515. doi:10.1046/j.1523-1739.1998.012003502.x [Google Scholar]
- Olson D.M, et al. Terrestrial ecoregions of the world: a new map of life on Earth. BioScience. 2001;51:933–938. doi:10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2 [Google Scholar]
- Phillips O.L, et al. Increasing dominance of large lianas in Amazonian forests. Nature. 2002;418:770–774. doi: 10.1038/nature00926. doi:10.1038/nature00926 [DOI] [PubMed] [Google Scholar]
- Phillips O.L, et al. Pattern and process in Amazon tree turnover: 1976–2001. Phil. Trans. R. Soc. B. 2004;359:381–408. doi: 10.1098/rstb.2003.1438. doi:10.1098/rstb.2003.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plafker G. Oriented lakes and lineaments of northeastern Bolivia. Geol. Soc. Am. Bull. 1964;75:503–522. [Google Scholar]
- Plafker, G. 1974 Tectonic implications of oriented lakes and lineaments in northeastern Bolivia. In Proc. 1st Int. Conf. on the New Basement Tectonics, Utah Geological Association, 5, pp. 519–527.
- Ratter J.A. Transitions between cerrado and forest vegetation in Brazil. In: Furley P.A, Proctor J, Ratter J.A, editors. Nature and dynamics of forest–savannah boundaries. Chapman & Hall; London, UK: 1992. pp. 417–429. [Google Scholar]
- Romero I, Pastó E, editors. Moxos: Una limnocultura: Cultura y medioambiente en la Amazonía boliviana. Centre d'Estudis Amazònics; Barcelona, Spain: 2004. 212 pp. [Google Scholar]
- Schnitzer S.A, Bongers F. The ecology of lianas and their role in forests. Trends Ecol. Evol. 2002;17:223–230. doi:10.1016/S0169-5347(02)02491-6 [Google Scholar]
- Silva J.F, Fariñas M.R, Felfili J.M, Klink C.A. Spatial heterogeneity, land use and conservation in the cerrado region of Brazil. J. Biogeogr. 2006;33:536–548. doi:10.1111/j.1365-2699.2005.01422.x [Google Scholar]
- Skole D, Tucker C.J. Tropical deforestation and habitat fragmentation in the Amazon: satellite data from 1978 to 1988. Science. 1993;260:1905–1910. doi: 10.1126/science.260.5116.1905. doi:10.1126/science.260.5116.1905 [DOI] [PubMed] [Google Scholar]
- Smith T.B, Wayne R.K, Girman D.J, Bruford M.W. A role for ecotones in generating rainforest biodiversity. Science. 1997;276:1855–1857. doi:10.1126/science.276.5320.1855 [Google Scholar]
- Smith T.B, Kark S, Schneider C.J, Wayne R.K, Moritz C. Biodiversity hotspots and beyond: the need for preserving environmental gradients. Trends Ecol. Evol. 2001;16:431. doi:10.1016/S0169-5347(01)02201-7 [Google Scholar]
- Steininger M.K, Tucker C.J, Ersts P, Killeen T.J, Villegas Z, Hecht S.B. Clearance and fragmentation of tropical deciduous forest in the Tierras Bajas, Santa Cruz, Bolivia. Conserv. Biol. 2001a;15:856–866. doi:10.1046/j.1523-1739.2001.015004856.x [Google Scholar]
- Steininger M.K, Tucker C.J, Townshend J.R.G, Killeen T.J, Desch A, Bell V, Ersts P. Tropical deforestation in the Bolivian Amazon. Environ. Conserv. 2001b;28:127–134. doi:10.1017/S0376892901000133 [Google Scholar]
- Stute M, Forster M, Frischkorn H, Serejo A, Clark J.F, Schlosser P, Broecker W.S, Bonani G. Cooling of tropical Brazil (5°C) during the Last Glacial Maximum. Science. 1995;269:379–383. doi: 10.1126/science.269.5222.379. doi:10.1126/science.269.5222.379 [DOI] [PubMed] [Google Scholar]
- Turcq B, Sifeddine A, Martin L, Absy M.L, Soubies F, Suguio K, Volkmer-Ribeiro C. Amazonia rainforest fires: a lacustrine record of 7000 years. Ambio. 1998;27:139–142. [Google Scholar]
- Willis K.J, Gillson L, Brncic T.M, Figueroa-Rangel B.L. Providing baselines for biodiversity measurement. Trends Ecol. Evol. 2005;20:107–108. doi: 10.1016/j.tree.2004.12.003. doi:10.1016/j.tree.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Wright S.J. Tropical forests in a changing environment. Trends Ecol. Evol. 2005;20:553–560. doi: 10.1016/j.tree.2005.07.009. doi:10.1016/j.tree.2005.07.009 [DOI] [PubMed] [Google Scholar]
- Wright S.J. Response to Lewis et al.: the uncertain response of tropical forests to global change. Trends Ecol. Evol. 2006;21:174–175. doi:10.1016/j.tree.2006.02.003 [Google Scholar]