Abstract
In the Kruger National Park (KNP), South Africa, ecosystem managers use a series of monitoring endpoints, known as thresholds of potential concern (TPCs), to define the upper and the lower levels of accepted variation in ecosystems. For woody vegetation, the current TPC suggests that woody cover should not drop by more than 80% of its ‘highest ever’ value. In this paper, we explore the utility of palaeoecological data in informing TPCs. We use calibrated fossil pollen data to explore variability in vegetation at two sites over the past 5000 years, to provide a long-term record of changes in woody vegetation cover and a context for interpreting more recent vegetation change. The fossil pollen data are calibrated using studies of modern pollen and vegetation from KNP; arboreal pollen percentage was simulated using pollen–landscape modelling software for savannah landscapes of varying woody vegetation cover, and the relationship between vegetation and pollen data was quantified using nonlinear regression. This quadratic equation was then applied to fossil pollen data in order to estimate woody vegetation cover from arboreal pollen percentages. Our results suggest that the TPCs have not been exceeded during the period represented in the pollen record, because estimated woody vegetation cover has remained above 20% of its highest ever value. By comparing the fossil pollen data with TPCs, our study demonstrates how palaeoecological data can be presented in a form that is directly relevant to management objectives.
Keywords: pollen analysis, ecosystem management, modelling, Mosaic, Polflow, Kruger National Park
1. Introduction
Nature has a range of ways to be, but there is a limit to those ways and therefore, human changes must be within those limits.
(Pickett et al. 1992, p. 82)
There is increasing recognition in the ecological literature of the variability and complexity of ecological systems (Pahl-Wostl 1995) and, as a result, new approaches to conservation ecology are developing that are based on a ‘flux of nature’ rather than a ‘balance of nature’ paradigm (Pickett et al. 1992; Fiedler et al. 1997). In order to translate these theoretical advances into management options, however, long-term, scale-specific data are required in order to decide on an acceptable level of variability. It is not sufficient to accept that ecosystems are variable; conservation managers need to know how variable, the processes that drive variability, and the spatial and temporal scales over which variation occurs. They also need to be able to identify critical ecological thresholds at which major changes in ecosystem transitions might occur (Carrión et al. 2001). Thus, there is an emerging role for palaeoecology in defining the temporal variability of ecosystems (Landres et al. 1999) and in informing management interventions that either promote or suppress ecological heterogeneity (Rogers 2003).
This is especially true in the case of savannahs, which are highly dynamic ecosystems; complex interactions between edaphic conditions, herbivory, competition, climate, fires and human management influence ecosystem pattern and process at a range of spatial and temporal scales (Gillson 2004; Vetter 2005). The resulting spatial and temporal heterogeneity raises important questions for ecosystem managers, who need to distinguish habitat degradation, the approach of an ecological threshold, or unprecedented change from the normal variability of the ecosystem. In the Kruger National Park (KNP), South Africa, ecosystem managers use monitoring endpoints, known as thresholds of potential concern (TPCs), to decide when management intervention is needed (Biggs & Rogers 2003). TPCs are a set of operational goals that define the upper and the lower levels along a continuum of change in selected environmental indicators for which the Kruger ecosystem is managed. For woody vegetation cover, the TPC would be exceeded when woody cover for any landscape drops by more than 80% of the highest ever value. For the KNP as a whole, a further TPC suggests that ‘the mean drop parkwide should not exceed 30%’ (Biggs & Rogers 2003, p. 65). As a part of an adaptive management approach, TPCs are being continually adjusted in response to the emergence of new ecological information or changing management goals. Currently, new TPCs are being developed for woody cover with an emphasis on spatial variation at different scales (H. C. Biggs 2006, personal communication). Knowledge of how woody vegetation has changed in the past will help inform present management goals.
As the TPCs for woody cover illustrate, there is a temporal dimension to the TPC since it involves comparison of previous woody cover with woody cover changes observed now. Mills et al. (2003, p. 494) commented that ‘Better knowledge of the variation of plant communities over a longer time will lead to a better understanding of current patterns and processes and thus the realistic setting of TPCs.’
One problem, however, is how to define the highest ever woody cover. There are excellent vegetation records in the KNP, e.g. descriptive surveys and checklists (van Rooyen 1978, unpublished M.Sc. thesis; Gertenbach 1983; Ventner & Gertenbach 1986; W. P. D. Gertenbach 1987, unpublished D.Sc. thesis; F. J. Ventner 1990, unpublished Ph.D. thesis; Zambatis 2002); aerial and fixed-point photographs 1940–present; surveys within studies of herbivore (e.g. Coetzee et al. 1979; Brits et al. 2002), fire (e.g. Trollope et al. 1998; Enslin et al. 2000) and climate (e.g. Scholes et al. 2001; Bond et al. 2003) interactions with vegetation; longer-term studies of vegetation (e.g. Eckhardt et al. 2000; H. C. Eckhardt & H. C. Biggs 2001, unpublished report); and satellite images. It was not until 2002, however, that Veld Condition Assessments (VCAs) included the woody component of vegetation (KNP Veld Condition Assessment 2002, unpublished survey data). Thus, aside from satellite data covering the past ca 30 years and some studies of aerial photographs and vegetation in highly localized areas, to date there have been few sources of information on the long-term variability in woody vegetation cover (Eckhardt et al. 2000). This is especially pertinent, given the current concern over the impact of elephants on large trees in the KNP; specifically whether current levels of elephant abundance can be sustained without loss of vulnerable tree species (Engelbrecht 1979; Brits et al. 2002).
In this paper, we focus on the TPC for woody cover and use a calibrated fossil pollen record from two sites in the northwest of the KNP to explore whether recent changes in tree density are within the normal range of variability for these areas. We calibrate the fossil pollen record by comparing observed arboreal fossil pollen percentages with simulated arboreal pollen loadings from pre-defined landscape scenarios using computer modelling software. The four main stages used to achieve this were as follows:
We analysed fossil pollen from dated sediment cores from two sites in the northwest KNP in order to study changes in the abundance of arboreal fossil pollen over time.
Using pollen–landscape modelling software (Mosaic and Polflow), we simulated savannah landscapes of varying woody cover and estimated the percentage of arboreal pollen associated with each landscape, based on modern pollen and vegetation data from KNP.
Outputs from these simulations were used in a regression of woody vegetation cover on arboreal pollen percentage (from step ii), generating an equation relating percentage of arboreal fossil pollen to percentage of woody vegetation cover.
We applied this equation to the fossil pollen data in order to estimate changes in woody vegetation cover over time.
Using this approach, we presented fossil pollen data as estimates of woody vegetation cover, a form directly relevant to the TPCs used in ecosystem management.
2. Study area and sample sites
The KNP covers an area of approximately 20 000 km2 and lies in northeastern South Africa, bordering with Mozambique to the east and Zimbabwe to the north (figure 1). Its boundaries are demarcated by the Lebombo Hills in the east, the Luvuvhu and Limpopo Rivers in the north and the Crocodile River in the south. In addition to the Crocodile River, three other permanent rivers (the Olifants, Letaba and the Sabie) flow from west to east across the KNP and there are a multitude of seasonal rivers. To the west of the KNP boundary, there are communal areas and private and provincial game reserves.
Figure 1.
Location of the Kruger National Park.
The climate of Kruger is temperate in the south, and tropical and subtropical in the north. The average annual rainfall is 730 mm per year in the southwest and 400 mm per year in the far north, with high inter-annual variability. The geology of the KNP is divided along a central north–south axis, with basalt plains at about 200 m.a.s.l. to the east and undulating granite to the west, rising to 800 m granitic hills in the southwest. There are also sandstone hills in the northeast.
The vegetation is sub-arid to semi-arid wooded savannah, with a heterogeneous structuring varying from open grassy plains with low shrubs to dense woodlands and riverine forests. There are over 400 tree species, of which the genera Acacia, Combretum, Sclerocarya and Colophospermum are common. The major vegetation types are influenced by the underlying geology, a pattern which is modified by soils, fire, grazing and topography (Eckhardt et al. 2000). The vegetation and land classifications most widely used for management and research are based on Gertenbach's landscapes (Gertenbach 1983). Gertenbach recognized 35 landscapes within the KNP, a landscape being defined as an area with a specific geomorphology, climate, soil and vegetation pattern together with the associated fauna.
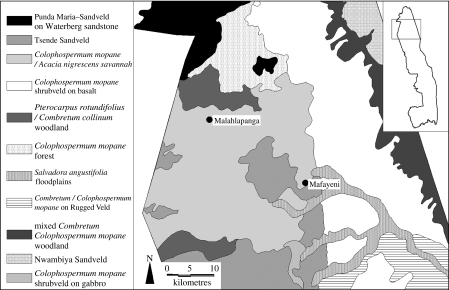
The sediment coring sample sites are located in landscape 12: Colophospermum mopane/Acacia nigrescens savannah, and border the Tsende Sandveld landscape (Gertenbach 1983) (figure 2). These landscapes are in the poorly grassed granite areas of the northwest (north of the Olifants River), where weeping bushwillow (Combretum collinum) and mopane (Colophospermum mopane) are dominant. The sample sites, Mafayeni (23°00′47.4″ S, 31°14′15.9″ E) and Malahlapanga (22°53′20.0″ S, 31°02′25.8″ E), consist of spring-fed sediment basins less than 50 m in diameter, associated with peat beds.
Figure 2.
Location of Mafayeni and Malahlapanga sample sites.
3. Material and methods
(a) Fossil pollen analysis and accelerator mass spectrometry radiocarbon dating
Sediment cores were collected from Mafayeni and from Malahlapanga in 50 cm sections using a Russian corer (Wright et al. 1965). Sediment cores were wrapped in clingfilm and aluminium foil and were transported to the UK, where they were stored at 4°C.
Sediment cores were sub-sampled using parallel razor blades inserted 5 mm apart into the sediment core at 2 cm intervals. Sediment volume (1 cm3) was determined by volumetric displacement in distilled water. Fossil pollen was extracted from the sub-samples using standard methods, which successively remove sulphur compounds, carbonates, humic acids, macrofossils, silicates and cellulose (Bennett & Willis 2001). The samples were stained with aqueous safranin, dehydrated using tertiary butyl alcohol (TBA) and mixed with silicon oil. In order to ensure statistical significance, for each sub-sample, between 400 and 800 terrestrial grains (Maher 1972) were identified using a Nikon Eclipse 400 microscope at ×400 magnification. Pollen grains were identified using keys and plates (van Campo 1960; Bonnefille 1971; Bonnefille & Riollet 1980; Hamilton 1982; Sowunmi 1995) as well as reference material collected from the National Museums of Kenya, Nairobi and the Daubeny Herbarium, University of Oxford.
The pollen sum included both terrestrial and aquatic taxa, because in the pollen–landscape modelling program, aquatic habitats were included as part of the mixed grassland matrix. In order to assess changes in woody vegetation over time, pollen data were subdivided into arboreal and non-arboreal pollen. Arboreal pollen included trees and shrubs, i.e. it represents woody vegetation taxa. Non-arboreal taxa were grouped together and included Poaceae, Cyperaceae, other herbaceous taxa and aquatics. The pollen groupings thus represented woody patches in a mixed grassland matrix, which also included a wet area (the coring site).
Confidence limits for the percentage of arboreal taxa were calculated in PsimPoll (Bennett 1998) using a modification of Maher's lognormal distributions with additional use of propagation of errors methods. The maximum number of simulations (1000) was used in order to improve the accuracy of estimates of confidence intervals.
Accelerator mass spectrometry (AMS) radiocarbon dating of sieved, acid-washed sediment was used to establish the age of the base of the cores, and at one further point higher in each sediment series. A sub-sample of bulk sediment was dispersed in water and examined under binocular magnification to identify and remove any modern rootlets (none were found). The sample was then treated in three stages (acid–alkali–acid) to remove humic and fulvic acids, and secondary carbonates. The sample was treated with 4% HCl, 0.5 M NaOH and 4% HCl for 2 h per treatment. Between treatments, the sample was washed several times with deionized water. The pre-treated sample was combusted in a closed quartz tube with CuO at 900°C and the resultant CO2 was converted to graphite by reduction with H2. 14C concentration was measured in a 1.5 SDH-Pelletron model compact carbon accelerator mass spectrometer by comparing simultaneously the 14C, 13C and 12C beams of each sample with those of oxalic acid, standard CO2 and coal background material. Conventional 14C ages were calculated with a δ13C correction for isotopic fractionation. The 1-sigma errors include both counting statistics and uncertainty connected with the subtraction of background readings.
(b) Modelling of savannah landscapes and simulation of their associated pollen signals
The aim of the pollen–landscape modelling was to derive a relationship between the percentage of woody vegetation cover in savannah landscapes and the percentage of arboreal pollen in the pollen rain associated with this landscape. (This equation is derived in §3c and applied in §3d to estimate woody vegetation cover from fossil pollen data.) In order to simulate pollen signals for modelled savannah landscapes:
Savannah landscapes of varying woody cover were simulated in Mosaic (Middleton & Bunting 2004). In each landscape simulation, 200 m diameter, circular patches of woody vegetation were located randomly within a 10×10 km mixed grassland matrix (pixel size 10 m, 1000 rows×1000 columns). Nineteen different landscape scenarios were simulated, in which woody cover increased at 5% intervals from 5 to 95% (see table 1 and figure 3 for details of simulated landscapes).
Based on modern pollen and vegetation data from KNP (Duffin & Bunting in press), Polflow (Bunting & Middleton 2005) was used to estimate the percentage of arboreal pollen in each simulated savannah landscape, using the Prentice–Sugita pollen dispersal and deposition model incorporated in Polflow (Prentice 1985; Sugita 1993, 1994). For each savannah landscape (5–95% woody vegetation cover), 10 estimates of arboreal pollen percentage were simulated, each one being the mean of eight simulated sample points randomly located within each landscape (the sample point locations were randomly generated and thus could fall either within a woody patch or on the grassland matrix).
Table 1.
Nineteen different savannah landscapes simulated in Mosaic and the associated estimated arboreal pollen percentages, simulated in Polflow.
| woody vegetation cover (%) | grassland matrix cover (%) | number of sample points in each landscape | number of replicates | mean simulated arboreal pollen percentage |
|---|---|---|---|---|
| 5 | 95 | 8 | 10 | 1.51 |
| 10 | 90 | 8 | 10 | 3.79 |
| 15 | 85 | 8 | 10 | 7.87 |
| 20 | 80 | 8 | 10 | 7.81 |
| 25 | 75 | 8 | 10 | 13.91 |
| 30 | 70 | 8 | 10 | 14.77 |
| 35 | 65 | 8 | 10 | 17.46 |
| 40 | 60 | 8 | 10 | 20.67 |
| 45 | 55 | 8 | 10 | 20.70 |
| 50 | 50 | 8 | 10 | 22.65 |
| 55 | 45 | 8 | 10 | 34.33 |
| 60 | 40 | 8 | 10 | 35.73 |
| 65 | 35 | 8 | 10 | 39.04 |
| 70 | 30 | 8 | 10 | 47.95 |
| 75 | 25 | 8 | 10 | 56.48 |
| 80 | 20 | 8 | 10 | 61.24 |
| 85 | 15 | 8 | 10 | 65.54 |
| 90 | 10 | 8 | 10 | 76.05 |
| 95 | 5 | 8 | 10 | 88.68 |
Figure 3.
Examples of landscape scenarios created with Mosaic, with woody vegetation patches (black circles) at (a) 20, (b) 40, (c) 60 and (d) 80% cover in a grassland matrix (grey background).
The 19 different landscape scenarios allowed quantification of the effect of woody cover percentage on arboreal pollen percentage, while the eight sample points within each landscape simulated the effect of the distance of pollen sample site from woody vegetation patch on the percentage of arboreal pollen. In this way, the sample scheme used captured the effects of landscape heterogeneity on simulated arboreal pollen percentage. In total, Polflow was used to simulate 1520 arboreal pollen percentages for 19 landscapes scenarios. Mean estimated arboreal pollen percentages for each landscape scenario are shown in table 1.
(c) Pollen–vegetation relationship
In order to derive a relationship between the percentage of woody vegetation cover in savannah landscapes and the percentage of arboreal pollen in the pollen rain from the simulations described in §3b, the estimated arboreal pollen percentages (from Polflow) were plotted against the percentage of woody vegetation cover in the 10×10 km landscape scenarios (simulated in Mosaic).
Regressions between these two variables provided the quantitative relationship between the woody vegetation cover and the percentage of arboreal pollen (see §§3b and 4b). The graphs of woody vegetation cover and simulated pollen loading were analysed using curve estimation in SPSS. The significance of linear, logarithmic, inverse, quadratic, compound, power, growth and exponential regressions was assessed using analysis of variance. Comparison of adjusted R2 was used to assess which curve best explained the variance of the simulated woody vegetation data. The coefficients and the constants for the best-fit curve were used to generate an equation relating woody vegetation cover to arboreal-simulated pollen percentage. The 95% confidence limits for the equation were calculated based on 1.96×standard error of the coefficients and constant. These analyses were carried out using SPSS v. 11 (SPSS, Inc., Chicago, IL).
In these analyses, percentage of woody vegetation cover was treated as the dependent variable because the equation generated from the regression is used in §3d to calibrate arboreal fossil pollen percentages, and this technique (inverse regression) minimizes the root mean squared error of prediction when the equation is used to infer changes in arboreal fossil pollen percentage over time (Krutchkoff 1967; Osborne 1991).
(d) Calibrating arboreal fossil pollen percentages
The equation generated in §3c was applied to the fossil pollen data (see §3a) in order to convert arboreal fossil pollen percentages into quantitative estimates of woody vegetation cover over time. For each fossil pollen sub-sample, woody vegetation cover (y) was estimated from the arboreal fossil pollen percentage (x) and the coefficients and the constants in the above equation. Estimated woody vegetation cover was plotted against depth to show changes in woody vegetation cover over time.
For each estimate of woody vegetation cover, confidence limits were plotted, firstly for the quadratic equation alone and secondly for the fossil pollen data and quadratic equation combined. To combine the errors and calculate the combined confidence limits, for each fossil pollen data point, the lower confidence limits for the regression coefficients and constants were applied in a quadratic equation along with the lower confidence limit for the pollen data, to give an overall lower confidence limit for estimated woody vegetation cover. Similarly, the upper confidence limits for the regression coefficients and constants were used alongside the upper confidence limit for the pollen data to calculate an overall upper confidence limit for woody vegetation cover (table 4).
Table 4.
Maximum arboreal pollen percentages and estimated maximum woody vegetation cover (with 95% confidence limits) for Mafayeni and Malahlapanga.
| site | maximum arboreal pollen (%) | maximum estimated woody cover (%) | confidence limits for woody vegetation cover | |||
|---|---|---|---|---|---|---|
| quadratic (coefficients and constants) | quadratic and pollen combined | |||||
| lower | upper | lower | upper | |||
| Mafayeni | 33.02 | 55.16 | 46.04 | 64.28 | 42.04 | 70.60 |
| Malahlapanga | 6.91 | 19.49 | 15.97 | 23.01 | 13.44 | 27.01 |
In order to confirm that the estimates of woody vegetation are realistic, woody vegetation cover was estimated from modern pollen samples using the equation described above, and these estimates were compared with vegetation survey data at Mafayeni and Malahlapanga (KNP Veld Condition Assessment 2002, unpublished survey data). Estimated and measured woody vegetation cover was then compared statistically in SPSS using a t-test.
4. Results
(a) Fossil pollen analysis and chronology
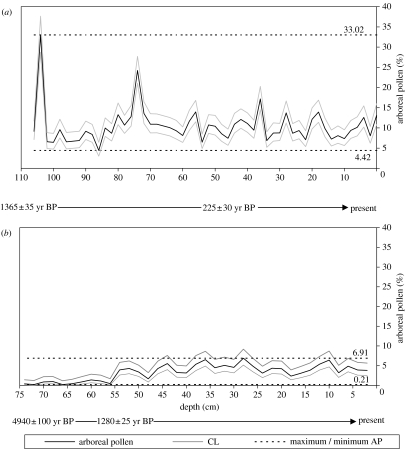
Figure 4 shows the percentage of arboreal fossil pollen at Mafayeni and Malahlapanga. Minimum and maximum arboreal fossil pollen percentages are indicated on the diagram, as well as 95% confidence limits.
Figure 4.
Variation in arboreal pollen percentages at (a) Mafayeni and (b) Malahlapanga, with maximum and minimum arboreal fossil pollen and 95% confidence limits (CL) indicated.
In the sediment sequence from Mafayeni, over 400 terrestrial pollen grains were counted per sub-sample (over 33 000 pollen grains in total). The percentage of arboreal fossil pollen varied between 4.4 and 33.0% over the past ca 1400 years, with a mean of 10.5%. There is a peak of 33.0% in arboreal fossil pollen at 104 cm depth near the start of the sequence, and a further peak at 74 cm depth, when arboreal fossil pollen reaches a maximum of 24.2%. Between these two peaks (102–76 cm depth), the percentage of arboreal fossil pollen varies between 4.4 and 13.3% (mean of 8.7%). From 72 cm upwards, the percentage of arboreal fossil pollen varies between 6.4 and 17.2% (mean of 10.5%).
AMS radiocarbon dating gave a radiocarbon age of 1365±35 calendar years before the present (yr BP) at the base of the core from Mafayeni (110 cm depth). Calibration of this radiocarbon date suggests that the sediment sequence began accumulating between AD 640 and 680 (68.2% probability). A further radiocarbon date at 46 cm depth gave a radiocarbon age of 225±30 yr BP and could not be calibrated due to the complication of fossil fuel burning during the industrial revolution.
Compared with the data from Mafayeni, the percentage of arboreal fossil pollen is lower and less variable in the sediment sequence from Malahlapanga. Over 400 terrestrial grains were counted per sub-sample (over 20 000 pollen grains in total for this site). Over the past ca 5000 years, the percentage of arboreal fossil pollen has varied between 0.2 and 6.9%, with a mean arboreal fossil pollen percentage of 3.3% for the whole sequence. The percentage of arboreal fossil pollen is lower in the oldest section of the sequence; from 74 to 56 cm, mean arboreal fossil pollen percentage is just 0.7% compared with 4.3% in the rest of the sequence. AMS radiocarbon dating gave a radiocarbon age of 4940±100 yr BP for the base of the Malahlapanga sequence (72 cm), suggesting that this sediment sequence began accumulating between 3940 and 3810 BC (68.2% probability). A further radiocarbon date at 55 cm depth, where the transition in arboreal fossil pollen abundance occurs, gave a radiocarbon age of 1280±25 yr BP. Calibration of the date suggests that the transition in pollen composition occurs between AD 685 and 740.
(b) Relationship between arboreal fossil pollen percentage and percentage of woody cover
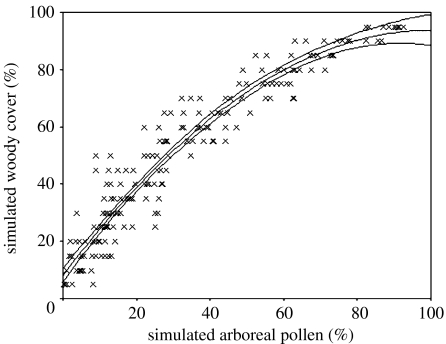
Percentage of woody vegetation cover, simulated in Mosaic, was plotted against the simulated arboreal pollen percentages from Polflow in a scattergraph. The relationship between percentage of woody vegetation cover and simulated arboreal pollen is curvilinear (figure 5).
Figure 5.
Scattergraph of simulated woody vegetation cover against simulated arboreal pollen percentages. The curve is described by a quadratic regression, with 95% confidence limits indicated.
The F values of linear, logarithmic, inverse, quadratic, compound, power, growth and exponential regressions were highly statistically significant (p<0.0001; table 2).
Table 2.
Significance and R2 for regressions of simulated woody vegetation cover on simulated arboreal pollen percentage.
| curve | significance of F-value | adjusted R2 |
|---|---|---|
| linear | <0.000 | 0.8882 |
| logarithmic | <0.000 | 0.7722 |
| inverse | <0.000 | 0.2216 |
| quadratic | <0.000 | 0.9259 |
| compound | <0.000 | 0.6635 |
| power | <0.000 | 0.8742 |
| growth | <0.000 | 0.6635 |
| exponential | <0.000 | 0.6635 |
The adjusted R2 values (table 2) indicate that the highest proportion of the variation (92.6%) in the simulated data is explained by a quadratic regression,
| (4.1) |
The coefficients (a and b) and constant (c) for the quadratic regression, with 95% confidence limits, are shown in table 3.
Table 3.
Coefficients and constants (with confidence limits) for the quadratic equation describing the regression of woody cover percentage on arboreal pollen percentage.
| coefficient | s.e. | 1.96 s.e. | upper confidence limit | lower confidence limit | |
|---|---|---|---|---|---|
| a (coefficient) | −0.00848 | 0.000862 | 0.00169 | −0.00679 | −0.01017 |
| b (coefficient) | 1.704758 | 0.074924 | 0.146851 | 1.851609 | 1.557907 |
| c (constant) | 8.116342 | 1.237946 | 2.426374 | 10.54272 | 5.689968 |
Thus, from table 3, the quadratic equation describing the regression of woody cover percentage on arboreal pollen percentage is
| (4.2) |
where y is the percentage of woody vegetation cover and x is the percentage of arboreal pollen.
(c) Calibrated fossil pollen data
In order to calibrate fossil pollen data in terms of estimated variations in woody vegetation cover, the quadratic equation generated in §4b was applied to the percentages of arboreal pollen shown in figure 4. Woody vegetation cover (y) was estimated from the arboreal fossil pollen percentage (x) and the coefficients (a and b) and the constant (c) in equation (2). Two sets of confidence limits for the estimates of woody vegetation cover were calculated, first for the coefficients and constant in the quadratic equation (tables 3 and 4) and second for the fossil pollen data and quadratic equation combined.
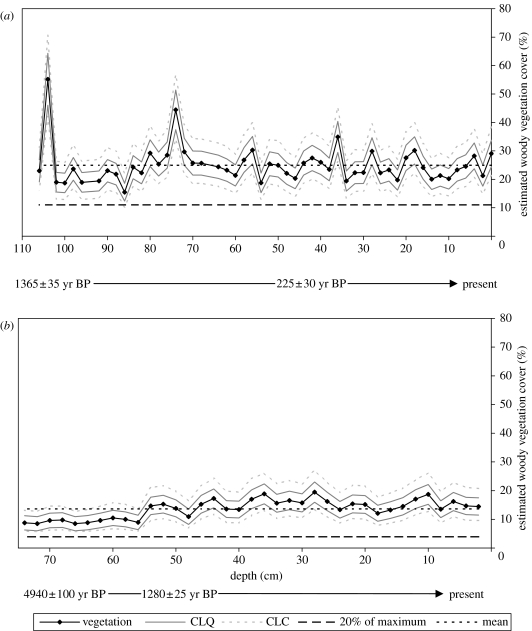
For the fossil pollen sequence from Mafayeni, the estimated woody vegetation cover varied between 15 and 55% over the past ca 1400 years, with a mean of 25%. The 95% confidence limits for the estimate of maximum woody cover for Mafayeni are 42–71%.
The sequence from Malahlapanga had a minimum and a maximum estimated woody vegetation cover of 8 and 19%, with a mean of 14%. The 95% confidence limits for the estimate of maximum woody vegetation cover for Malahlapanga are 13–27%.
The TPC specifies that woody vegetation cover should not fall below 80% of its highest ever value. Therefore, in order to discuss estimates of woody vegetation cover in terms relevant to the TPCs, 20% of the highest ever woody vegetation cover (to represent the 80% drop in woody vegetation cover specified in the TPC) were calculated as 11% for Mafayeni and 4% for Malahlapanga.
Estimated woody vegetation cover plotted against depth is shown in figure 6. Confidence limits for the estimates of woody cover are also indicated. The overall confidence limits for estimated woody vegetation cover are asymmetric owing to the method used to calculate confidence limits of the pollen data. Mean woody vegetation cover and 20% of the highest ever woody vegetation cover (11% for Mafayeni and 4% for Malahlapanga) are also indicated in figure 6.
Figure 6.
Estimations of woody vegetation cover: (a) over the past ca 1400 years at Mafayeni and (b) over the past ca 4900 years at Malahlapanga. The 95% confidence limits are shown for quadratic regression alone (CLQ) and for the quadratic regression and pollen data combined (CLC). The mean estimated woody vegetation cover and 20% of the maximum estimated woody vegetation cover are also shown.
Estimates of present-day woody vegetation cover, derived from modern pollen surface samples, were comparable to VCA vegetation survey data for the areas surrounding each site. At Mafayeni, our estimates of modern woody vegetation cover were between 21.3 and 38.4% (lower and upper 95% confidence limits). Measurements of woody cover at the six VCA sites falling within a 10 km radius of Mafayeni were between 3.7 and 36.2%. A comparison of means test showed no statistical difference between woody vegetation cover from VCA and modern pollen estimates (p=0.658 if unequal variances assumed at 95% confidence). In other words, there was no significant difference between the mean woody vegetation cover estimated from pollen data and the measured woody vegetation cover from vegetation survey data. Similarly, at Malahlapanga, woody vegetation cover estimated from surface pollen data was estimated to be between 9.6 and 20.8%. This was comparable to measurements at seven VCA survey sites within a 10 km radius of Malahlapanga, where woody cover was measured to be between 4.9 and 22.9%. Again, there was no statistical difference between the means (p=0.915). We are therefore confident that our estimates of woody vegetation cover reflect real woody vegetation cover.
5. Discussion
(a) Fossil pollen as an indicator of vegetation change over time
The fossil pollen data show considerable variation in the abundance of arboreal pollen over time. The pollen sequence from Mafayeni, dated from 1400 yr BP, varied throughout the sequence, whereas the pollen sequence from Malahlapanga, dated from 4900 yr BP, was less variable but showed a clear transition in tree abundance part-way through the sequence. The difference in variability, sediment age and the much lower proportion of arboreal pollen at Malahlapanga is indicative of the local heterogeneity of the savannah landscapes in KNP.
Bioturbation is inevitable in most sediment basins, and this is especially so in ecosystems where large mammals are present. The fact that clear peaks and trends were apparent in the pollen data, however, as well as the chronology developed from radiocarbon dates, indicates the preservation of a temporal sequence. The effect of bioturbation would be to smooth the pollen data, and thus the fossil pollen sequences presented here are likely to provide a conservative estimate of vegetation variability over time.
(b) Relationship between simulated pollen and landscape data
The relationship between arboreal pollen percentage and woody vegetation cover was explored using pollen–landscape modelling software (Mosaic and Polflow). The relationship between vegetation and pollen is not linear for percentages, due to the relative over- and under-representation of taxa in the pollen sum, known as the Fagerlind effect (Fagerlind 1952). This nonlinearity is apparent in the graph of simulated woody cover against simulated arboreal pollen percentage, and the observed curvilinear relationship between woody vegetation cover and arboreal pollen percentage was best described by a quadratic regression.
In patchy landscapes like savannahs, considerable variation in arboreal pollen percentage is expected, due to the effects of distance of the pollen sample point from the woody vegetation patch. This variation is reflected in the confidence limits for the coefficients and constants of the quadratic regression equation.
(c) Calibration of fossil pollen data
Many savannah trees are poor pollen producers and are under-represented in the pollen data. As a result, the arboreal pollen percentages are lower than the percentage of woody vegetation cover in the landscape. The quadratic expression developed above and described in §5b converts arboreal percentages into estimates of woody cover percentages. Owing to the under-representation of savannah tree pollen, it follows that estimated woody vegetation cover is higher than arboreal pollen percentage. Applying the quadratic equation to arboreal pollen percentage from fossil pollen data resulted in estimates (with confidence limits) of changes in woody vegetation cover over time.
Over the past 1400 years, the mean and maximum woody vegetation cover around the Mafayeni site was estimated to be 25 and 55%, respectively. The 95% confidence limits for the estimated maximum woody cover are 42 and 71%. At Malahlapanga, the mean and maximum estimated woody vegetation cover was 13 and 19%, respectively, over the past 4900 years, with 95% confidence limits for the estimated maximum woody cover of 13–27% (figure 6).
There are several sources of uncertainty in the calibration of the fossil pollen data, which explain the relatively wide confidence limits for the estimates of woody vegetation cover. First, there is uncertainty inherent in the pollen data, as reflected in the confidence limits shown in figure 4. As with any sub-sample, the pollen data provide estimates of a population mean. Despite low pollen productivity of savannah trees, the relatively narrow confidence limits achieved for the pollen data were a result of counting a very large number of pollen grains. Providing confidence limits for the pollen data allows this source of uncertainty to be quantified in the estimates of woody vegetation cover.
Second, there are limitations in the methodology used for estimating pollen productivity. The pollen productivity estimates used in this paper are taken from pollen surface samples at sites that are not randomly located in the landscape and this may introduce bias. Further, assumptions intrinsic in the fall speed and pollen productivity estimates will affect the estimates of woody vegetation cover. Third, we have developed a single estimate of pollen productivity and fall speed for all arboreal taxa, and it may be that separate estimates of these parameters are required for all 35 vegetation landscapes in KNP.
Further work will increase the precision of woody vegetation cover estimates. Pollen productivity estimates can be improved using more detailed vegetation data at a larger number of sites. Stratifying these data according to landscape may allow the development of pollen productivity estimates that are specific to the various different vegetation landscapes in the KNP (Gertenbach 1983). Nevertheless, mean woody vegetation cover estimated from modern pollen surface samples was not statistically different from measured woody vegetation cover from vegetation surveys, suggesting that realistic estimates of woody vegetation cover can be derived from arboreal pollen percentages.
(d) Thresholds of potential concern and management implications
For woody vegetation cover, the TPC would be exceeded when woody cover for any landscape drops by more than 80% of the highest ever value. For the KNP as a whole, a further TPC suggests that ‘the mean drop parkwide should not exceed 30%’ (Biggs & Rogers 2003, p. 65).
Calibration of fossil pollen datasets from Mafayeni (figure 6) shows that 95% confidence limits for estimated woody vegetation cover have been well above 11% (20% of the highest ever value) for almost all of the past 1400 years. The lowest estimated woody vegetation cover for the whole series was 15%, with a lower confidence limit of 10%. This is the only point at which estimated woody cover is below 11%. At Malahlapanga, there is less variation in estimated tree cover, and the estimated woody vegetation cover was above 20% of the highest ever value (4%) for the whole of the past 4900 years. Thus, our pollen data show no evidence of the TPC being exceeded.
The difference in the highest ever and mean woody vegetation cover between these two sites highlights the importance of site-specific TPCs for the different landscapes in KNP. Therefore, we are not able to comment on parkwide trends in woody vegetation cover as our palaeo-datasets are specific to two areas in the northwest of the KNP. Thus, our results cannot be extrapolated to the parkwide TPC for woody vegetation.
The TPC compares the present-day vegetation with an extreme point in its history—the ‘highest ever’ value, which is not likely to be representative of the long-term situation. Furthermore, it is difficult to define the highest ever value, because the highest ever value will change depending on how far back in time it is possible to look. Thus, it may be that the highest ever woody cover is problematic as a benchmark for guiding management decisions, because the time-scales need to be defined and appropriate to the rate of change in woody vegetation abundance and tree lifespan.
When assessing the variability of biological systems, variation is usually measured relative to the mean, and it may be that a TPC based on deviation from the mean might provide a more useful guide to monitoring changes in woody vegetation cover. An alternative TPC for woody vegetation cover could be developed, based on a long-term mean of woody vegetation cover. This would be more representative of the usual range of variation in woody cover. However, a TPC of this type would preclude extreme peaks and troughs in woody vegetation cover, and thus potentially reduce the temporal heterogeneity of the ecosystem. A new TPC currently under development is based on the rate of change in woody vegetation cover for different tree height classes (H. C. Eckhardt 2006, personal communication) and changes in woody cover at different spatial scales (H. C. Biggs 2006, personal communication).
Whichever benchmark is adopted, it is important that management interventions do not prevent conservation of processes that generate heterogeneity, as it is these processes which maintain the resilience of the ecosystem (Holling 1973). The challenge is to develop a TPC that helps managers to maintain heterogeneity without exceeding resilience of the ecosystems.
6. Conclusions
In this paper, palaeoecological data from KNP were interpreted in terms of changing woody vegetation cover over time. Fossil pollen data are often interpreted qualitatively, but here we have produced quantitative estimates of woody vegetation cover by calibrating fossil pollen data using a mathematical expression describing the relationship between the arboreal pollen percentage and the percentage of woody vegetation cover in the landscape. This pollen–landscape modelling technique was developed in northern Europe and North America (Sugita 1994; Sugita et al. 1999), but to our knowledge this is the first time it has been applied to data from a lowland African savannah. The methodology used here can be applied to new palaeo-datasets from KNP as they emerge. Further work will increase the precision of woody vegetation cover estimates.
We have derived mathematical expressions that enable fossil pollen data to be interpreted in terms compatible with ecological data on present-day woody vegetation cover. This approach provides a tool for interpreting palaeoecological records in terms that are relevant to management objectives (TPC). TPCs provide a conceptual tool that enables ecosystem managers to apply variability concepts in their management plans, by distinguishing normal ‘background’ variability from unprecedented change or degradation. TPCs will be continuously redefined as a part of the ongoing process of strategic adaptive management in KNP.
Acknowledgments
We are grateful to the Trapnell Fund, the Andrew W. Mellon Foundation and the University of Oxford for funding of this research. We thank the staff and scientists at the Kruger National Park for their excellent support throughout this research. Harry Biggs, Holger Eckhardt and Nick Zambatis deserve our particular thanks. Professor Keith Bennett advised on the statistical analysis of pollen data and the Psimpoll Program. AMS radiocarbon dating was carried out at the Poznań Radiocarbon Laboratory, Poland. This paper is a contribution to the POLLANDCAL network sponsored by NorFA (Nordic Council of Advanced Studies). We are very thankful to all POLLANDCAL members for their useful and inspiring discussions and in particular to Shinya Sugita, Jane Bunting and Dick Middleton. Thanks go to Kathy Willis and members of the Oxford Long-Term Ecology Laboratory for their valuable support and stimulating discussion.
Footnotes
One contribution of 14 to a Theme Issue ‘Biodiversity hotspots through time: using the past to manage the future’.
References
- Bennett, K.D. 1998 Psimpoll 4.25. http://www.kv.geo.uu.se/psimpoll.html
- Bennett K.D, Willis K.J. Pollen. In: Smol J.P, Birks H.J.B, Last W.M, editors. Tracking environmental change using lake sediments. Terrestrial, algal, and siliceous indicators. vol. 3. Kluwer; Dordrecht, The Netherlands: 2001. pp. 5–32. [Google Scholar]
- Biggs H.C, Rogers K.H. An adaptive system to link science, monitoring, and management in practice. In: du Toit J.T, Rogers K.H, Biggs H.C, editors. The Kruger experience: ecology and management of savanna heterogeneity. Island Press; Washington, DC: 2003. pp. 59–80. [Google Scholar]
- Bond W.J, Midgley G.F, Woodward F.I. What controls South African vegetation—climate or fire? S. Afr. J. Bot. 2003;69:1–13. [Google Scholar]
- Bonnefille R. Atlas des pollens d'Éthiopie principales espéces des forêts de montagne. Pollen et Spores. 1971;13:15–72. [Google Scholar]
- Bonnefille R, Riollet G. Centre National de la Re´cherche Scientifique; Paris, France: 1980. Pollens des savannes d'Afrique Orientale. [Google Scholar]
- Brits J, van Rooyen M.W, van Rooyen N. Ecological impact of large herbivores on the woody vegetation at selected watering points on the eastern basaltic soils in the Kruger National Park. Afr. J. Ecol. 2002;40:53–60. doi:10.1046/j.0141-6707.2001.00344.x [Google Scholar]
- Bunting M.J, Middleton R. Modelling pollen dispersal and deposition using humpol software, including simulating windroses and irregular lakes. Rev. Palaeobot. Palynol. 2005;134:185–196. [Google Scholar]
- Carrión J.S, Andrade A, Bennett K.D, Munuera M, Navarro C. Crossing forest thresholds. Inertia and collapse in a Holocene sequence from south-central Spain. The Holocene. 2001;11:635–653. [Google Scholar]
- Coetzee B.J, Engelbrecht A.H, Joubert S.C.J, Retief P.F. Elephant impact on Sclerocarya caffra trees in Acacia nigrescens tropical plains thornveld of the Kruger National Park. Koedoe. 1979;22:39–60. [Google Scholar]
- Duffin, K. I. & Bunting, M. J. In press. Relative pollen productivity and fall speed estimates for southern African savanna taxa. Veg. Hist. Archaeobot
- Eckhardt H.C, van Wilgen B.W, Biggs H.C. Trends in woody vegetation cover in the Kruger National Park, South Africa, between 1940–1998. Afr. J. Ecol. 2000;38:108–115. doi:10.1046/j.1365-2028.2000.00217.x [Google Scholar]
- Engelbrecht A.H. Olifantinvloed op Acacia nigrescens bome in 'n gedeelte van die Punda Maria-Sandveld van die Nasionale Krugerwildtuin (Afrikaans) Koedoe. 1979;22:29–37. [Google Scholar]
- Enslin B.W, Potgieter A.L.F, Biggs H.C, Biggs R. Long term effects of fire frequency and season on the woody vegetation dynamics of the Sclerocarya birrea/Acacia nigrescens savanna of the Kruger National Park. Koedoe. 2000;43:27–37. [Google Scholar]
- Fagerlind F. The real significance of pollen diagrams. Botanisker Notiser. 1952;105:185–224. [Google Scholar]
- Fiedler P.L, White P.S, Leidy R.A. The paradigm shift in ecology and its implications for conservation. In: Pickett S.T.A, Ostfeld R.S, Shachak M, Likens G.E, editors. The ecological basis of conservation: heterogeneity, ecosystems, and biodiversity. Chapman and Hall; New York, NY: 1997. pp. 83–92. [Google Scholar]
- Gertenbach W.P.D. Landscapes of the Kruger National Park. Koedoe. 1983;26:9–121. [Google Scholar]
- Gillson L. Evidence of hierarchical patch dynamics in and east African savanna? Landscape Ecol. 2004;19:883–894. doi:10.1007/s10980-004-0248-5 [Google Scholar]
- Hamilton A. Academic Press; London, UK: 1982. Environmental History of East Africa. [Google Scholar]
- Holling C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973;4:1–23. doi:10.1146/annurev.es.04.110173.000245 [Google Scholar]
- Krutchkoff R.G. Classical and inverse regression methods of calibration. Technometrics. 1967;9:425–439. [Google Scholar]
- Landres P.B, Morgan P, Swanson F.J. Overview of the use of natural variability concepts in managing ecological systems. Ecol. Appl. 1999;9:1179–1188. [Google Scholar]
- Maher L. Nomograms for computing the 0.95 confidence limits of pollen data. Rev. Palaeobot. Palynol. 1972;13:95–214. doi:10.1016/0034-6667(72)90039-5 [Google Scholar]
- Middleton R, Bunting M.J. Mosaic v.1.1: landscape scenario creation software for simulation of pollen dispersal and deposition. Rev. Palaeobot. Palynol. 2004;132:61–66. [Google Scholar]
- Mills M.G.L, Lubchenco J, Robertson W, Biggs H.C, Mabunda D. Reflections on the Kruger experience and reaching forward. In: Toit J.T. du, Rogers K.H, Biggs H.C., editors. The Kruger experience ecology and management of savanna heterogeneity. Island Press; Washington, DC: 2003. pp. 488–501. [Google Scholar]
- Osborne C. Statistical calibration: a review. Int. Statist. Rev. 1991;59:309–336. [Google Scholar]
- Pahl-Wostl C. John Wiley; New York, NY: 1995. The dynamic nature of ecosystems: chaos and order entwined. [Google Scholar]
- Pickett S.T, Parker V.T, Fiedler P.L. The new paradigm in ecology: implications for conservation biology above the species level. In: Fiedler P.L, Jain S.K, editors. Conservation Biology. Chapman and Hall; New York, NY: 1992. pp. 65–88. [Google Scholar]
- Prentice I.C. Pollen representation, source area and basin size: towards a unified theory of pollen analysis. Quatern. Res. 1985;23:76–86. doi:10.1016/0033-5894(85)90073-0 [Google Scholar]
- Rogers K.H. Adopting a heterogeneity paradigm: implications for management of protected savannas. In: du Toit J.T, Rogers K.H, Biggs H.C, editors. The Kruger experience: ecology and management of savanna heterogeneity. Island Press; Washington, DC: 2003. pp. 41–58. [Google Scholar]
- Scholes R.J, et al. The environment and vegetation of the flux measurement site near Skukuza, Kruger National Park. Koedoe. 2001;44:73–83. [Google Scholar]
- Sowunmi A.S. Pollen of Nigerian plants. Grana. 1995;34:121–141. [Google Scholar]
- Sugita S. A model of pollen source area for an entire lake surface. Quatern. Res. 1993;39:239–244. doi:10.1006/qres.1993.1027 [Google Scholar]
- Sugita S. Pollen representation of vegetation in quaternary sediments: theory and method in patchy vegetation. J. Ecol. 1994;82:881–897. doi:10.2307/2261452 [Google Scholar]
- Sugita S, Gaillard M.-J, Broström A. Landscape openness and pollen records: a simulation approach. The Holocene. 1999;9:409–421. doi:10.1191/095968399666429937 [Google Scholar]
- Trollope W.S.W, Trollope L.A, Biggs H.C, Pienaar D, Potgieter A.L.F. Long-term changes in the woody vegetation of the Kruger National Park, with special reference to the effects of elephants and fire. Koedoe. 1998;41:103–112. [Google Scholar]
- van Campo M. Palynologie africaine, IV. Bull. Inst. fr. Afrique noire. 1960;22A:1165–1199. [Google Scholar]
- Ventner F.J, Gertenbach W.P.D. A cursory review of the climate and vegetation of the Kruger National Park. Koedoe. 1986;29:139–148. [Google Scholar]
- Vetter S. Rangelands at equilibrium and non-equilibrium: recent developments in the debate. J. Arid Environ. 2005;62:321–341. doi:10.1016/j.jaridenv.2004.11.015 [Google Scholar]
- Wright H, Livingstone D, Cushing E. Coring devices for lake sediments. In: Kimmel B, Raup D, editors. Handbook of paleontological techniques. Freeman; San Francisco, CA: 1965. pp. 494–520. [Google Scholar]
- Zambatis, N. 2002 Checklist of species of the Kruger National Park Skukuza: Skukuza Herbarium.