Abstract
Rates of change in tree communities following major disturbances are determined by a complex set of interactions between local site factors, landscape history and structure, regional species pools and species life histories. Our analysis focuses on vegetation change following abandonment of agricultural fields or pastures, as this is the most extensive form of major disturbance in Neotropical forests. We consider five tree community attributes: stem density, basal area, species density, species richness and species composition. We describe two case studies, in northeastern Costa Rica and Chiapas, Mexico, where both chronosequence and annual tree dynamics studies are being applied. These case studies show that the rates of change in tree communities often deviate from chronosequence trends. With respect to tree species composition, sites of different ages differ more than a single site followed over time through the same age range. Dynamic changes in basal area within stands, on the other hand, generally followed chronosequence trends. Basal area accumulation was more linked with tree growth rates than with net changes in tree density due to recruitment and mortality. Stem turnover rates were poor predictors of species turnover rates, particularly at longer time-intervals. Effects of the surrounding landscape on tree community dynamics within individual plots are poorly understood, but are likely to be important determinants of species accumulation rates and relative abundance patterns.
Keywords: forest succession, vegetation dynamics, chronosequence, species turnover, Netropics
The more closely vegetational dynamics are observed, the less clear-cut becomes the distinction between climax and successional communities. Vegetation does not really consist of climaxes and successions leading towards them. In a long-range perspective, the vegetation of the Earth's surface is in incessant flux; what we observe in the field are not simply successions and climaxes, but only different kinds and degrees of vegetational stability and instability, different kinds and rates of population change.
R. H. Whittaker (1972, p. 347)
Disturbances are pervasive features of forest ecosystems, to the extent that there is no clear distinction between successional and mature-phase vegetation (Aubréville 1938; Whittaker 1972; Clark 1996). Structure and dynamics of forest vegetation reflect a complex interplay of disturbance events and regeneration processes taking place through time and space (Chazdon 2003). Vegetation structure and composition are often interpreted in terms of how forests have responded to recent and historic disturbance regimes (Whitmore & Burslem 1998). Several attributes of tropical forest structure and composition can recover following large-scale disturbances, natural or anthropogenic, such as hurricanes, fires, logging, swidden agriculture or cattle raising activities (see reviews by Brown & Lugo 1990; Guariguata & Ostertag 2001; Chazdon 2003; Finegan & Nasi 2004; Chazdon in press; Holl in press). Rates of change, however, vary considerably, depending upon what forest attributes are being measured and upon the intensity, duration and frequency of the disturbance. Ultimately, rates of forest change following major disturbances are determined by a complex set of interactions between local site factors, landscape history and structure, regional species pools (including non-native species) and species life histories (Pickett et al. 1987). In this review, we define major disturbances as those that result in the complete or nearly complete removal of vegetation, leading to the establishment of even-aged regrowth. These disturbances may occur at a range of spatial scales, from large gaps in a forest matrix, to small swidden agriculture fields, to large-scale deforestation. In most of the successional studies we discuss here, vegetation removal occurs at spatial scales ranging from 0.5 to 20 ha.
1. Complexities of vegetation change following stand-removing disturbances
Following major disturbances, rates of change of forest structure and composition are strongly affected by the nature and extent of residual vegetation, in the form of resprouts, remnant trees or shrubs, seedling/saplings and/or the soil seed bank (Uhl et al. 1981; Guevara et al. 1986; Galindo-Gonzalez et al. 2000; Slocum & Horvitz 2000; Benitez-Malvido et al. 2001; Elmqvist et al. 2001; Chazdon 2003). Resprouting is the predominant form of regeneration following damaging windstorms, such as hurricanes or cyclones (Yih et al. 1991; Bellingham et al. 1994). The effects of large-scale wind disturbances on forest structure and species composition are therefore generally transitory (Dittus 1985; Walker 1991; Bellingham & Tanner 1995; Burslem et al. 2000; Vandermeer et al. 2000; Pascarella et al. 2004), with rapid recovery of forest structure and composition. Globally, tropical forests subject to frequent hurricanes/cyclones have significantly shorter canopies compared with forests in similar environments that are not prone to such frequent wind-induced disturbances (Whitmore 1990; de Gouvenain & Silander 2003). When residual vegetation and propagule sources are lacking, destroyed by fires, or when soils are highly disturbed or compacted by heavy grazing, bulldozing or high-impact logging, rates of forest regrowth and biomass accumulation decline; under extreme conditions, successional processes may be arrested or diverted by exotic species (Hjerpe et al. 2001; Chinea 2002; Fine 2002; Zarin et al. 2005).
To add further complexity, site factors (such as soil fertility and texture) interact with landscape factors, such as forest cover spatial organization and extent, distance to forested areas and regional species pool, to determine rates of species colonization, accumulation of species and accumulation of biomass above- and belowground (Johnson et al. 2000; Moran et al. 2000; Zarin et al. 2001; Chazdon 2003). A complete understanding of the factors that influence forest vegetation change following major disturbances in tropical forests must incorporate analyses of site attributes as well as landscape configuration and regional species composition. Most studies to date, however, have focused on effects of site attributes (such as land-use history and soils), leaving many unanswered questions regarding the effects of landscape composition and regional land-use dynamics on local patterns and processes of forest succession (Helmer 2000; Chinea & Helmer 2003). These effects are not exclusively unidirectional, as secondary forest development can influence species composition and genetic structure of populations in nearby mature forests as well as in surrounding areas undergoing succession (U. Sezen 2006, unpublished work). As stated by Pickett (1976, p. 117), ‘Successional gradients and the evolutionary and functional responses of populations on them are part of a dynamic, regional process rather than a single site pattern.’
2. Assessing rates of change following major disturbances
Successional processes are not always directional or predictable, and multiple pathways can lead to a range of mature forest types rather than a single stable endpoint (Gleason 1926). Here, we describe the succession process in terms of rates of change in tree community attributes rather than as a recovery process, thereby avoiding the assumption that succession is orderly or deterministic and will eventually reach the original forest structure and species composition present before the disturbance.
Two general approaches have been used to assess rates of vegetation change during succession. Most information on tropical forest succession is derived from chronosequence studies, where temporal changes are inferred from a single-time investigation of a set of forest stands of different ages since disturbance. Rates of change are therefore estimated indirectly, based on assumptions that the same successional process takes place within each stand. The second approach directly documents the rates of change through monitoring vegetation dynamics over time in particular forest stands. Here, we describe results of studies using each approach and examine their strengths and limitations in terms of assessing rates of vegetation change following large-scale disturbance in tropical forest areas. Most of our examples are drawn from studies of wet and seasonally dry Neotropical forests. We then examine whether chronosequence trends can predict tree community changes as observed within individual secondary forest stands over time. We consider two case studies, from northeastern Costa Rica and Chiapas, Mexico, where both approaches are being applied in long-term studies of successional permanent plots. Since chronosequence studies are based on single-time information from a range of sites, these data emphasize cumulative or net effects of ecological processes. In contrast, vegetation dynamics examine incremental change over time and can therefore reveal more about the ecological processes that produce cumulative changes.
Our review focuses on vegetation change following abandonment of agricultural fields or pastures, as this is the most extensive form of major disturbance in tropical forests today, as well as historically. Secondary, degraded and logged forests now cover more area than mature forests within tropical regions (FAO 2004). We examine rates of change for five tree community attributes: stem density; basal area; species density; species richness; and species composition. In two case studies, we examine turnover rates of stems and species for different size classes and time-scales. Stem density and basal area are influenced by rates of tree growth, recruitment and mortality, whereas species density, richness and composition reflect processes of species turnover and community assembly. Collectively, these processes determine changes in species relative abundance, size distributions and dominance over time.
When assessing vegetation change in successional tropical forests, the standard for comparison is usually neighbouring areas of mature or old-growth forests. For many regions, however, these forests have become reduced to small fragments or are degraded to various degrees, and a suitable ‘pristine’ area may not be available for contemporaneous study (Chazdon in press). Moreover, edaphic variation may lead to local differences in forest structure and composition in mature forests (Fanshawe 1952; Duivenvoorden 1995; Clark et al. 1999; Harms et al. 2001; Potts et al. 2002; Valencia et al. 2004) and this is also the case in secondary forests (Herrera & Finegan 1997; Méndez-Bahena 1999; Finegan & Delgado 2000). Many secondary forests are found in small patches isolated from continuous forest cover. As a result, it remains a major challenge to obtain robust data on vegetation change in secondary forests that matches the spatial extent of data collected for mature forest tracts, which are generally larger areas (at least for those that are investigated in ecological studies) and may therefore encompass greater edaphic heterogeneity.
3. Rates of change based on chronosequence studies
Originally framed as a tool for investigating primary succession, chronosequence studies have become a key component of research on secondary forest succession. A space-for-time substitution allows researchers to examine a much larger time frame than would be otherwise feasible and also corrects for potential interannual climatic variation that could confound a time-series (Foster & Tilman 2000). However, chronosequence data only permit inferences of successional change and do not facilitate direct analysis of the underlying processes mediating the change (growth, mortality and recruitment). In addition, the basic assumption of chronosequence studies—that the sites represent points along a continuum, rather than snapshots of independent trajectories—often remains untested.
Chronosequence sites must be carefully chosen to avoid bias in site selection. The best chronosequence studies are those that base their site selection on precise, independently verifiable estimates of site age. For example, Ruiz et al. (2005) selected a chronosequence of tropical dry forest sites on Providencia Island, Colombia, based on a sequence of available aerial photographs and remote sensing data. Ideally, site selection should remain unbiased by prior notions of successional rates, topography or ease of access, although these factors often restrict available study areas. Moreover, successional sites should encompass the same range of heterogeneity in soils and topography as mature forest sites that are being compared.
Chronosequence studies have contributed valuable data used to infer rates of vegetation change in regenerating tropical forests. Our review will largely focus on woody vegetation in moist/wet lowland forests, but we also include studies in tropical dry forests. Most studies have examined relatively young forests (less than 50 years old). Studies of more advanced secondary forests (50–200 years old) often focus on single forest sites in the absence of a chronosequence context (Lang & Knight 1983; Milton et al. 1994; Brearley et al. 2004).
(a) Basal area/aboveground biomass
Trends in basal area are closely linked to trends in aboveground biomass (ABM; Clark & Clark 2000). Both are generally calculated from stem diameters, but the equations used for ABM are often site specific (e.g. Brown 1997; Nelson et al. 1999) and sometimes incorporate data on wood density and tree height. Owing to these factors, basal area is a more conservative metric for comparisons of regrowth across sites where site-specific allometric equations have not been developed. For both ABM and basal area, secondary tropical wet forests exhibit rapid growth in the first years of establishment. Biomass accumulation tends to be tree dominated (greater than 94% in Central Amazonia; Feldpausch et al. 2005), with lianas comprising only a small fraction (DeWalt & Chave 2004; Gehring et al. 2004). In a chronosequence of sites in Bolivia, Peña-Claros (2003) found that secondary forests reached 70% of mature forest basal area in the first 25 years of regrowth. Pascarella et al. (2000) found similar values in the first 25–30 years of regrowth in Puerto Rico.
Saldarriaga et al. (1988) found that ABM increased linearly with stand age up to 40 years in the upper Rio Negro, then levelled off due, they suggested, to mortality of long-lived pioneer species. The pantropical average rate of biomass accumulation in the first 20 years of forest succession has been estimated to be 6.2 mg ha−1 yr−1, although among-site variation is very high (Silver et al. 2000). Reported values are as high as 11 mg ha−1 yr−1 in central Amazonia (Feldpausch et al. 2004) and up to 15 mg ha−1 yr−1 in high altitude forests (Fehse et al. 2002). In contrast to the linear increases in ABM found in other chronosequence studies, Jepsen (2006) reported sigmoidal increase in biomass accumulation in swidden fallows from 2 to 15 years old in Sarawak Malaysia, with initially rapid biomass growth up to 10 years followed by no net biomass accumulation. Based on fitted functions, biomass accumulation reached a maximum rate of 12.7 mg ha−1 yr−1, 4 years after abandonment (Jepsen 2006). In Chiapas, Mexico, secondary forests developed in abandoned pastures and with fallow ages of 12–13 years attained 20% of the carbon storage estimated for nearby undisturbed mature rainforest (94 mg C ha−1) sharing the same geomorphological properties (Balvanera et al. 2005).
The growth of secondary forests can be strongly affected by soil fertility (Moran et al. 2000), the duration and intensity of prior land use (Uhl et al. 1988; Steininger 2000; Finegan & Nasi 2004; Gehring et al. 2005) and fire frequency (Zarin et al. 2005). Swidden fallows and abandoned pastures may follow very different successional trajectories (Uhl et al. 1988; Steininger 2000). Unfortunately, comparisons among studies are hindered by methodological differences in plot size, minimum stem diameter and the need for site-specific allometric equations to calculate biomass (Clark & Clark 2000).
(b) Tree density
Successional changes in stem density may be driven by intrinsic species life-history differences (e.g. in recruitment, growth and mortality rates), density-dependent population processes and additional stand disturbances. Species life-history attributes can lead to episodic recruitment and the development of strong cohort structure in young secondary forests. For example, in the Neotropics, initial colonization is often dominated by relatively short-lived, fast-growing, pioneer genera such as Cecropia, Vismia and Ochroma (Finegan 1996; Vester 1997; Mesquita et al. 2001; Chazdon in press). When these species die off after 25–30 years, or sometimes much earlier, stem density may decline rapidly (see Breugel et al. 2006 and §4).
Tropical secondary forests are generally dense and highly productive, and density-dependent mortality is to be expected, as is the case in secondary forests in other climatic regions (Peet & Christensen 1987; Finegan 1996). Mortality and recruitment processes in mature tropical forests are often density- or frequency dependent (Wills et al. 1997; Harms et al. 2000; Niklas et al. 2003; Uriarte et al. 2005; Wills et al. 2006). The importance of density-dependent effects on stem density and species composition in tropical secondary forests remains unclear, however, due to the paucity of published information from permanent sample plot studies. Breugel et al. (2006) found that stand-level growth, mortality and recruitment were negatively related to stand basal area, but not to stem density. A concentration of growth in the larger trees and relatively high mortality among the smaller trees indicated asymmetric competition, and the resulting pattern of tree thinning strongly influences vegetation dynamics in early succession. Successional changes in density of both trees and regenerating seedlings and saplings may indicate the potential for density-dependent effects on mortality, growth and recruitment (Uriarte et al. 2004). The relative importance of conspecific versus heterospecific effects of density on tree regeneration also remains to be determined in tropical secondary forests (Uriarte et al. 2004). Since shade-tolerant tree species are not yet reproductively mature in young stands, seeds must be dispersed from nearby or distant mature forests or forest fragments. The lack of locally produced seed shadows decreases the potential for conspecific density-dependent effects on seedling recruitment and growth (Janzen 1970; Connell 1971; Uriarte et al. 2005) in young secondary forests. Therefore, if density-dependent processes are operating in young secondary forests, these effects are more likely to be heterospecific for all but the dominant pioneer species. Intraspecific density-dependence mechanisms may still operate in very early successional stages, however, when a single species dominates colonization in abandoned fields.
Unlike basal area and ABM, stem density does not appear to follow a predictable pattern with forest age. Density is potentially influenced by a wide variety of factors, operating at a range of spatial and temporal scales that vary in their effects on different size classes. Stem density in secondary forests is generally higher than in old-growth forests, even when comparing relatively large size classes (e.g. greater than or equal to 10 cm DBH). In Costa Rica, density of stems greater than or equal to 10 cm DBH ranged from 547 to 687 stems ha−1 in 15–20 years old secondary forests compared with 506–527 stems ha−1 in old growth (Guariguata et al. 1997). Stem density may peak at an intermediate age range, although these patterns will vary with diameter size limits. Feldpausch et al. (2005) detected a peak in stem density at 6–8 years post-abandonment in central Amazonian secondary forests. In Puerto Rico, stem density greater than 2.5 cm DBH increased up to ca 25 years post-abandonment (Aide et al. 1995) and then stabilized.
The overall pattern of stem density along chronosequences may yield few discernible trends, but analysing the density of species, in particular functional groups or within specific size classes, will likely be a more fruitful line of inquiry. These comparisons will be facilitated using the same minimum stem diameter in sample plots at similar successional stages.
(c) Species density and species richness
As the forest develops, new species begin to colonize and recruit, leading to a gradual accumulation of species over time. We distinguish between species density (number of species per sample area) and species richness (number of species per standardized number of individuals sampled; Gotelli & Colwell 2001). Species density is highly sensitive to stem density (Denslow 1995; Chazdon et al. 1998), which varies during succession in an unpredictable manner as discussed above. An alternate approach is to use an index such as Shannon's H or Fisher's alpha to assess species diversity; these measures can also be influenced by sample size, however. Species richness can also be estimated from sample data using a variety of approaches (Colwell & Coddington 1994; Chazdon et al. 1998). Numerous studies have documented increasing diversity with forest age, although direct comparisons are difficult due to lack of standardization of diversity measures (see reviews by Chazdon in press, Holl in press). Aide et al. (1995) found a positive relationship between stand age and species density in a 60-year chronosequence in Puerto Rico, and Saldarriaga et al. (1988) found similar Shannon diversity in 40 years old secondary forest and old growth in very small plots. In the earliest study of secondary forest diversity, Eggeling (1947) found a peak in species density of stems greater than or equal to 10 cm at intermediate ages, followed by a decline later in succession. Sheil (2001) applied rarefaction methods to these data to correct for differences in stem density and confirmed the mid-successional peak. Andel (2001) found higher diversity (Fisher's alpha) in old secondary forests in northwest Guyana than in neighbouring old-growth forests.
Like basal area and ABM, species accumulation in secondary forests can be strongly influenced by soil fertility and land-use history (Finegan & Delgado 2000; Pascarella et al. 2000). Rates of species accumulation are often slower on abandoned pasture compared with abandoned crop cultivation in regenerating forests of Puerto Rico (Aide et al. 1995).
(d) Species composition
Whereas species density may quickly reach the level of old growth, species composition remains distinct for much longer or may never fully recover (Corlett 1992; Clark 1996; Finegan 1996). Pioneer species dominate the community during the first years of regeneration, leading to a forest composition very distinct from that of nearby old growth (Chazdon 2003; Peña-Claros 2003; Chazdon in press). The legacy of early species colonization may persist for decades or even centuries, as some pioneer species can be very long lived (Budowski 1970; Gemerden et al. 2003; Chazdon in press).
One of the central questions regarding forest succession is whether floristic change follows a predictable pathway. Egler (1954) proposed two contrasting models of forest succession, which Gomez-Pompa & Vazquez-Yanes (1981) adapted for tropical forests. In the ‘relay floristics’ model, forest development proceeds via successive waves of colonization. The species composition of each wave is predictable, as is the sequence of replacement. In the ‘initial floristic composition’ model, most of the colonists arrive at the onset of forest regeneration, but reach peak abundances at different points in succession. Neither of these models adequately describes actual trends in species composition in successional tropical forests, however. Few chronosequence studies support the relay floristics model, although many species of early successional pioneers fail to recruit after canopy closure (Finegan 1996; Chazdon in press; Holl in press; Breugel et al. in preparation). Studies in eastern Amazonia, Bolivia, and Mexico found that species from all functional groups (including shade-tolerant species) established very early in succession and continued to establish after canopy closure (Uhl et al. 1988; Peña-Claros 2003; Breugel et al. in preparation), supporting the initial floristic composition model. In Mesoamerica, studies of tree life histories have revealed a number of distinct functional groups of tree species, which become abundant during different phases of succession (Budowski 1965, 1970; Finegan 1984, 1996; Dalling et al. 2000; Capers et al. 2005, Chazdon in press). The resulting waves of recruitment and mortality may lead to wide fluctuations in stem density and may also account for the observed mid-successional peak in species richness (Sheil 1999). Further study will reveal whether these waves reflect species differences (seed dispersal modes, seedling establishment requirements, growth rates, final size and longevity) and/or landscape effects (dispersal limitation and species pool available for colonization).
The initial floristic composition of successional forests may influence species composition for long periods of time, though the effects of early species composition are often confounded with those of prior land use. In the Brazilian Amazon, researchers have identified two different floristic pathways on abandoned lands. Cecropia-dominated forests generally arise on less severely impacted lands, while Vismia-dominated forests develop on more heavily used or more frequently burned areas (Steininger 2000, Mesquita et al. 2001). Cecropia species are short lived, driving a rapid turnover in species composition at 25–30 years post-abandonment and potentially leading to a more rapid recovery of old-growth forest species. Vismia species, in contrast, are longer lived and resprout frequently and may arrest the floristic turnover (Vester 1997; Steininger 2000; Mesquita et al. 2001; Lucas et al. 2002). Detailed long-term studies are needed to evaluate the influence of land-use history and initial floristic composition on successional pathways and species turnover.
An important question in forest regeneration is whether secondary forests converge with the vegetation of nearby old-growth forests or whether the community composition remains distinct. Terborgh et al. (1996) found directional succession occurring on floodplain forests in Peru, returning to the composition of old-growth forests. A key factor in convergence here, however, would seem to be that the regional context of this study was that of forest cover with little or no human disturbance. Are successional trajectories more variable in human-influenced or -dominated landscapes? Sheil (1999) re-evaluated Eggeling's (1947) plots in Budongo Forest, Uganda, and also detected directional changes in floristic composition conforming to Eggeling's (1947) predicted successional trajectory.
In other situations, the outcome of succession is less clear with measurable differences in floristic composition persisting for up to centuries after abandonment (Corlett 1992; Clark 1996; Finegan 1996; Gemerden et al. 2003). In some cases, succession can be arrested due to intensive land use. Fern thickets of Dicranopteris pectinata (Willd.) Underw. colonized large areas in the Dominican Republic after abandonment of agriculture in the early 1970s and inhibited forest regrowth since then (Slocum et al. 2004). Observations from the Montes Azules Reserve in southern Mexico suggest that fern thickets can arrest succession for very prolonged periods: pure and thick carpets (0.5–4 ha in extent) of D. pectinata are found immersed in old-growth rainforest in areas that were inhabited by ancient Mayan people. Ceramic pieces found beneath such carpets (ca 1300 years old) suggest that intensive crop cultivation in poor soil led to this long-lasting, arrested successional phenomenon (M. Martínez-Ramos 2006, personal observations). At present, no tree regeneration is occurring within the fern carpets even though they are completely surrounded by mature forest (Suazo Ortuño 1998).
Chazdon (in press) points out another uncertainty inherent in inferring successional trajectories: the composition of ‘mature’ forest varies edaphically and harbours the legacy of ancient disturbances. Establishing appropriate reference points for mature forest composition may pose problems for reconstruction of late successional pathways.
(e) Rates of change in tropical dry forests
Tropical dry forests compose 42% of tropical forests worldwide, but are far less studied than tropical wet or moist forests (Mooney et al. 1995; Sanchez-Azofeifa et al. 2005). Dry forests are floristically and structurally simpler than wet forests, with fewer canopy strata, lower leaf area index and more frequent vegetative reproduction (Murphy & Lugo 1986). Highly seasonal rainfall distribution strongly limits conditions for seed germination and seedling establishment (Ceccon et al. 2006; Vieira & Scariot 2006). These differences are likely to affect rates of change in vegetation structure and composition following major disturbances (Ewel 1977; Vieira & Scariot 2006).
The prevalence of anemochorous species in dry forests (Sabogal 1992; Bullock 1995; Gentry 1995; Vieira & Scariot 2006) can have contrasting effects on rates of successional change. In seasonally dry tropical forests of Bolivia, 63% of the canopy tree species are wind dispersed (Justiniano & Fredericksen 2000). Small, wind-dispersed seeds arrive at higher densities than animal-dispersed ones (Holl 1999) and are more resistant to desiccation (Vieira & Scariot 2006). Seed dispersal in dry forests may therefore be less negatively affected by forest fragmentation than in wet forests (Gillespie 1999), although dispersal limitation is still a major limitation for dry forest regeneration (Janzen 1988). These factors may promote rapid structural regeneration in tropical dry forests (Ceccon et al. 2002; Ruiz et al. 2005; Vieira & Scariot 2006). If early regeneration of tropical dry forests is dominated by wind-dispersed species, however, the frugivore community of the area may be impoverished and rates of species accumulation may be negatively affected (Janzen 1988). Seedling survival is generally very low in tropical dry forests (Gerhardt & Hytteborn 1992; Swaine 1992; Ceccon et al. 2006), and the seed bank has a reduced role in seedling regeneration (Rico-Gray & García-Franco 1992; Ray & Brown 1994) compared with wetter forests. Rates of seed predation may be as high as in wet forests, although more studies are needed (Hammond 1995).
Fire likely plays a more important role in tropical dry forest succession (Murphy & Lugo 1986; Vieira & Scariot 2006). Owing to strong seasonality in moisture and nutrient availability, light conditions and gap dynamics are less important for tropical dry forests compared with wet forests (Gerhardt 1996). However, seedling growth and survival in a tropical dry forest of Yucatan, Mexico, was affected by light availability (Ceccon et al. 2003, 2004), and at Chamela, Mexico, seedlings of a wide array of tropical dry forest tree species, differing in seed mass and successional status, showed strong differential growth responses to experimental levels of light (Rincón & Huante 1993; Huante & Rincón 1998) and soil nutrients (Huante et al. 1995a,b). Such seedling functional responses suggest that tropical dry forest species may be sensitive to environmental heterogeneity promoted by disturbance and forest regeneration.
Resprouting assumes a greater importance in secondary dry forest regeneration than in wet forests (Murphy & Lugo 1986; Kennard et al. 2002; McLaren & McDonald 2003; Vieira et al. 2006). By allowing survival through a disturbance (Swaine 1992; Miller & Kauffman 1998; Marod et al. 2002), resprouting uncouples initial species composition from seed availability (Ceccon et al. 2006; Vieira & Scariot 2006). Consequently, resprouting lessens the impact of important barriers commonly related to seed regeneration—lack of seed sources and animal dispersers, short seed longevity, impoverished soil seed banks, lack of suitable microhabitats for seed germination, seed predation, seedling herbivory and competition with aggressive vegetation (Guariguata et al. 1995; Wunderle 1997; Wijdeven & Kuzee 2000; Zimmerman et al. 2000).
The small number of chronosequence studies conducted in tropical dry forest suggest that dry forests have fewer seral stages than wet forests (Ewel 1980; Kennard 2002; Lebrija-Trejos 2006, unpublished work). As in wet forests, basal area and ABM increase rapidly with stand age (Brown & Lugo 1990; Ray & Brown 1994; Kennard 2002; Ruiz et al. 2005; Toledo & Salick 2006). Based on nine secondary tropical dry forests, ABM increased significantly with stand age in young stands (0–20 years post-abandonment), but not in a wider age range (up to 50 years; Silver et al. 2000). Life zone (wet, moist and dry) did not significantly affect ABM during the first 20 years of succession, although rates of ABM accumulation were higher in wet versus moist forests (Silver et al. 2000). Generally, forest biomass in tropical forests increases with precipitation levels (Brown & Lugo 1982; Murphy & Lugo 1986), although more studies in secondary tropical dry forests are needed. In dry forests of Southern Yucatan, Mexico, ABM increased linearly with stand age in swidden up to 25 years old (Read & Lawrence 2003). Read & Lawrence (2003) projected that at the continuing rate of 2.3–3.4 mg ha−1 yr−1, ABM will reach levels observed in mature forests of the region within 55–95 years, similar to rates estimated by Hughes et al. (1999) for the humid region of Los Tuxtlas. The contribution of small stems (1–4.9 cm DBH) to ABM decreased during succession, whereas large stems (greater than 10 cm DBH) assumed an increasing importance. Increases in tree height during succession were important determinants of ABM changes (Read & Lawrence 2003).
Changes in stem density in tropical dry forests show inconsistent trends with stand age across studies. As in wet forests, much of the variation in stem density with age is due to fluctuations in the density of small stems (Kennard 2002; Toledo & Salick 2006). At the tropical dry forest of Chamela, a chronosequence study showed that stem density (DBH>1 cm) increased rapidly with fallow age, so that secondary forest plots (1000 m2 in size) of 8–12 years have similar stem density than old-growth forest plots. Species richness in these secondary forest plots, however, was still 15% lower than that of the old-growth forest (P. Balvanera, G. Ibarra-Manríquez, A. Pérez-Jiménez & M. Martinez-Ramos 2006, unpublished data). Although species density showed a peak in 11–16 years old tropical dry forests of Providencia Island, Colombia, species richness of stems (number of species/187 stems greater than 2.5 cm DBH) increased linearly with stand age (Ruiz et al. 2005). As with wet forests, species composition in secondary dry forests lags behind species density and structural measures (basal area and biomass) in terms of relative rates of change during succession (Ceccon et al. 2002; Toledo & Salick 2006).
The effect of land use on secondary forest regeneration is poorly understood in dry forests. One recent study of 45–50-year-old subtropical dry forests in Puerto Rico showed strong effects of land use on rates of recovery of basal area, tree height and biomass (Colón & Lugo 2006). Species density and basal area recovered faster in areas used for charcoal production compared with abandoned home sites or farms. Severe disturbances that removed vegetation and disturbed soil and root systems promoted establishment of invasive, exotic species, with long-term effects on successional pathways (Gonzalez Iturbe et al. 2002; Colón & Lugo 2006). Further comparative studies are needed to draw conclusive comparisons between rates of change during succession in wet versus dry tropical forests. To our knowledge, no published studies have examined vegetation dynamics over time within secondary dry forest stands, although a few studies are now underway in Mexico (F. Bongers and M. Martínez-Ramos 2006, personal communication).
4. Case studies of vegetation dynamics in relation to chronosequence trends
Long-term vegetation dynamics studies are a necessary counterpart to chronosequence studies. While chronosequence studies may reveal patterns, studies of vegetation dynamics provide insight into the processes that drive vegetation change: recruitment, mortality, growth rates and species turnover (Bakker et al. 1996; Foster & Tilman 2000). One fruitful combination of approaches has been chronosequence resampling (Foster & Tilman 2000), in which individual sites within a chronosequence are followed over time.
Currently, there is a paucity of time-series data on secondary tropical forest succession. The longest running set of observations come from Sheil's (1998, 1999, 2001) re-examination of Eggeling's (1947) plots in Budongo forest, Uganda. Another long-term vegetation dynamics study documents forest recovery following anthropogenic and cyclone disturbance in the Solomon Islands (Whitmore 1974; Burslem et al. 2000).
Here, we describe successional vegetation dynamics based on case studies in northeastern Costa Rica and Chiapas, Mexico. These studies encompass different land-use histories, different stem size classes and different temporal scales. As we illustrate below, some of the community-level patterns observed in these dynamics studies are remarkably consistent with chronosequence predictions. However, these vegetation dynamics studies also reveal idiosyncratic patterns driven by initial species composition, site factors, land-use history and landscape composition. These studies also provide insight into the mechanisms and drivers of successional change following the abandonment of agricultural lands.
(a) Case study: Sarapiquí region, Costa Rica
In wet lowlands of northeastern Costa Rica, Chazdon and collaborators have monitored vegetation dynamics annually for 8 years (1997–2004) in four 1 ha plots of secondary forest on abandoned pastures in the Caribbean lowlands (Capers et al. 2005; Chazdon et al. 2005), and Finegan and collaborators have monitored forest dynamics in four plots over 16 years (1987–2003); three plots are 1 ha and one plot (initially 1-year old) is 0.3 ha. These four plots studied by Finegan were not used for pasture, but were cleared and prepared for planting and then abandoned or were used for one cycle of cultivation. Although these sites had lighter land use than the sites studied by Chazdon, results from both the sets of plots are combined in some of the analyses presented here.
Lowland forests of the Sarapiquí region of northeastern Costa Rica are classified as tropical wet forest (sensu Holdridge et al. 1975), receiving ca 3900 mm of precipitation annually (Sanford et al. 1994). The driest months, February–April, still receive more than 100 mm of rain in most years (but see Chazdon et al. 2005). The average monthly temperature is 25.8°C, with little annual variation. The region is a patchwork of cattle pastures, agricultural areas (bananas, heart of palm and pineapple), residential areas, forest fragments and second-growth forests. Soil fertility varies throughout this region due to erosion of old and more recent lava flows, nutrient-enriched alluvium associated with flood zones and phosphorus-enriched zones associated with geothermal waters at La Selva Biological Station (Pringle 1991; Sollins et al. 1994). These factors, as well as variation in land-use history (logging versus pasture versus crops), affect species composition and regeneration in both secondary and mature forests of the region (Herrera & Finegan 1997; Clark et al. 1999; Finegan & Delgado 2000).
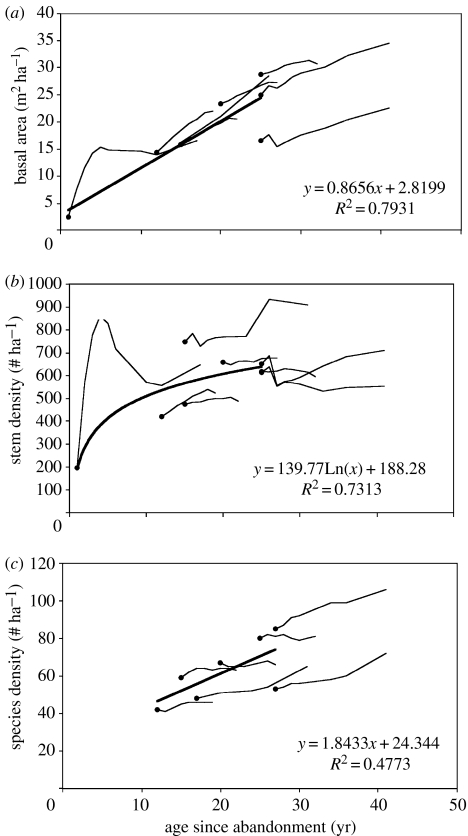
In the time-series analyses shown here, we combine data for seven 1 ha plots and one 0.3 ha plot for basal area and stem density. Analysing the plots as a chronosequence across the initial age range from 1 to 25 years, the tree basal area in the first 25 years of regrowth increased linearly with time since abandonment (R2=0.79; figure 1a). Resampling these plots over time revealed few deviations from the initial chronosequence projection (figure 1a). These results, along with findings from many chronosequence studies, support the view that the basal area is a predictable emergent feature of regenerating forest communities on sites with low to intermediate intensities of agricultural use. At least for sites less than 40 years following abandonment, dominated by long-lived pioneers like Goethalsia meiantha, Laetia procera, Simarouba amara and Vochysia ferruginea, the basal area increases linearly with time. Basal area in secondary forests over 25 years is similar to or higher than values from mature forests of this area (Guariguata et al. 1997). The only site where basal area decreased over time was the youngest site (figure 1a); in this case, decreases in basal area from 6 to 9 years were due to the decline of the initially dominant pioneer species, Ochroma pyramidale.
Figure 1.
Chronosequence data for trees greater than or equal to 10 cm DBH in eight secondary forest monitoring plots in northeastern Costa Rica (black dots) and successional trajectories within the same set of plots. (a) Basal area (m2 ha−1); (b) stem density (number of stems ha−1); and (c) species density (number of species ha−1). Data on species density were available for only seven plots (each 1 ha).
Tree density also showed a clear chronosequence trend, but the increase with stand age was nonlinear (figure 1b). Within sites, however, tree density showed highly variable dynamics, and did not conform well to chronosequence trends. The youngest site showed a rapid increase for the first 4 years, followed by a rapid decline in density, due to the dynamics of the dominant species, O. pyramidale. Density changes in the older sites varied widely over time; some plots showed no changes in tree density, whereas others showed decreases or increases (figure 1b).
The remarkably consistent patterns in basal area despite the inconsistent temporal patterns of density suggest that at the stand level, basal area increments are driven by overall tree growth increments rather than by changes in stem density, a pattern also observed in even-aged temperate forests (Peet & Christensen 1987). Indeed, diameter growth rates in young forests are significantly higher than rates in older stands, even when comparing the same species and size classes (R. L. Chazdon 2006, unpublished work). In the Lindero Sur site (initially 12 years old), the per capita basal area increment for trees (greater than or equal to 10 cm DBH) was more than six times higher than in the Cuatro Rios site (initially 25 years old). Accumulation of basal area and biomass is driven by growth rates of large trees and can proceed rapidly even if stem density is declining (as occurs in even-aged stands during self-thinning; White & Harper 1970). Tree mortality in these secondary forests is concentrated in the smaller stem size classes (less than 10 cm; Chazdon et al. 2005) and has relatively little impact on basal area.
Species density rises during forest regeneration in this chronosequence, although the relationship is not as consistent as the pattern observed for basal area (R2=0.47; figure 1c). Although there is a significant increase in species density with age since abandonment, the former-pasture sites showed little or no change in species density over time. In part, this stabilization of species density reflects species turnover within plots and replacement of early colonizing tree species with later recruiting species, with little or no net change in the number of species. These results show, however, that changes in species density over time cannot explain initial differences among plots in the chronosequence. Thus, chronosequence patterns in species density that appear to be based on plot age may actually reflect intrinsic differences in levels of tree diversity among plots that are predominantly unrelated to age.
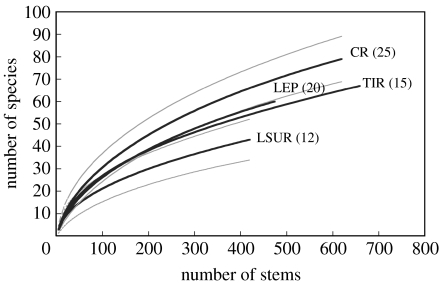
Similar conclusions apply to comparisons of tree species richness greater than 10 cm DBH for four former-pasture sites studied by Chazdon et al. (2005), based on rarefaction analyses. In both 1997 and 2004, species richness was significantly higher in the oldest site (25 years, Cuatro Rios; 66 species/420 stems) compared with the youngest site (12 years, Lindero Sur; 43 species/420 stems; p<0.05), but the two sites of intermediate age did not differ from the others (figure 2). Interpreting these trends as a chronosequence suggests that tree species richness increases slowly during succession compared with other forest structural characteristics. Within each plot, species richness (number of trees/420 stems) for trees greater than 10 cm DBH was also compared between 1997 and 2004. Tree species richness did not change significantly within any of these plots over the 7-year study period, in parallel with observations on species density. Thus, for species richness, plot-level changes did not conform to the overall chronosequence trend.
Figure 2.
Sample-based rarefaction curves for trees greater than 10 cm DBH in 1997 (initial census) for four second-growth forest 1 ha plots in northeastern Costa Rica on former pasture. Site ages are given in parentheses next to each solid line. Grey lines indicate 95% confidence intervals for species richness based on sample-based rarefaction (Mao Tau; Colwell et al. 2004).
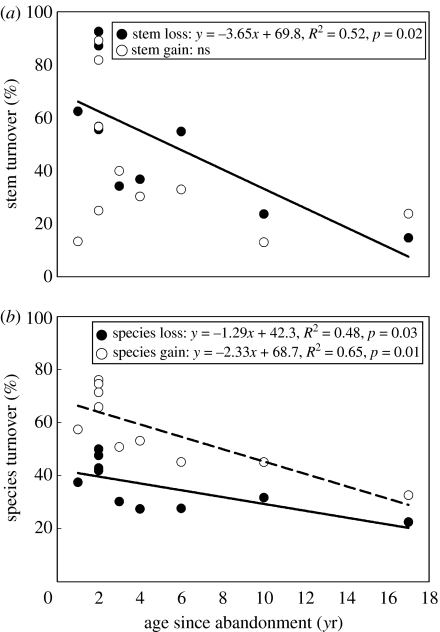
Species richness remained stable or increased despite substantial turnover rates for stems and species (figure 3a). Younger forests (less than 15 years since abandonment) are certainly more dynamic in overall stem density and population sizes than older sites (Chazdon et al. 2005), but this dynamism at the level of stems and species does not necessarily translate into significant changes in species richness. For the former-pasture sites, rates of stem gain were highest (31.6%) in the youngest site and decreased with forest age, whereas rates of stem loss remained relatively constant (13.5–16.5%) across sites (figure 3a). Rates of species turnover were lower than rates of stem turnover in all the four former-pasture plots (figure 3a). In the two younger plots (Lindero Sur and Tirimbina), rates of species gain exceeded rates of species loss, whereas the reverse was found for the two older plots (Lindero El Peje and Cuatro Rios; figure 3a).
Figure 3.
Percentage of stems greater than or equal to 10 cm DBH lost through mortality and gained through recruitment and percentage of species lost and gained in secondary forest plots in northeastern Costa Rica from (a) 1997 to 2004 in four plots on abandoned pasture; LSUR, Lindero Sur; TIR, Tirimbina; LEP, Lindero El Peje; CR, Cuatro Rios and (b) 1989 to 2003 in three plots abandoned after light land use. Initial plot age since abandonment is shown in parentheses.
A slightly different pattern of stem and species turnover was observed in the three 1 ha light-use sites studied by Finegan from 1989 to 2003, a 14-year period (figure 3b). Over this extended time period, rates of stem gain were higher than rates of stem loss in two plots (initially 15 and 25 years old). Rates of species gain exceeded species loss in all the three plots; in plot 1 (initially 25 years old), species gains were substantial despite no net change in stem density. In plot 2 (initially 25 years old), rates of stem gain exceeded rates of species gain, because most species of new recruits were already present in the plot. Higher rates of stem loss than species loss in all the three plots indicate cohort decline in species that were initially abundant. Half of the total species lost from the three sites belonged to pioneer genera (Cecropia, Croton, Trema and Vismia) and the family Melastomataceae. Plot differences in stem and species turnover rates may also reflect variation in soil fertility and proximity to seed sources. We lack a clear understanding of how these factors interact with stand demography to bring about changes in species richness over time in tropical secondary forests. We do know, however, that these changes are slow and may take centuries (Finegan 1996; Chazdon 2003).
For trees greater than or equal to 10 cm DBH, the four former-pasture plots differed significantly in species composition in 2004 (p<0.05; table 1). Similarity values (Chao Jaccard Abundance Estimator; Chao et al. 2005) ranged from 0.44 to 0.80. The 15-year-old plot (Tirimbina), which has the longest history of disturbance and isolation, was the most divergent. The same pairwise similarities were also computed within each site between 1997 and 2004 to determine whether species composition changed significantly over time (table 2). None of the four sites showed a significant change in species composition over time, however, suggesting that age differences are not the major factor contributing to differences in species composition. Each plot appears to follow an idiosyncratic pathway of species accumulation, likely driven by edaphic factors, land-use history and landscape matrix.
Table 1.
Pairwise similarity for trees greater than 10 cm DBH in four second-growth forest plots in Costa Rica in 2004, based on the Chao Jaccard Abundance Estimator ±95% confidence limits (Chao et al. 2005). (Plot age since abandonment is given in parentheses. Values along the diagonal are similarities within each site between 1997 and 2004. Tree species composition differed significantly across plots of different ages, but not over time within plots.)
| Lindero Sur (12) | Tirimbina (15) | Lindero El Peje (20) | Cuatro Rios (25) | |
|---|---|---|---|---|
| Lindero Sur | 1.0±0.0 | 0.48±0.14 | 0.80±0.12 | 0.61±0.12 |
| Tirimbina | 1.0±0.0 | 0.48±0.16 | 0.44±0.15 | |
| Lindero El Peje | 1.0±0.0 | 0.75±0.09 | ||
| Cuatro Rios | 1.0±0.0 |
Table 2.
Pairwise similarity for trees greater than 1 cm DBH in 10 second-growth forest plots in Chiapas, Mexico, based on the Chao Jaccard Abundance Estimator ±95% confidence limits (Chao et al. 2005) (Values along the diagonal are similarities within each site between the first and the last census (3-year interval). Number in parentheses gives plot age since abandonment. Tree species composition showed significant differences over time in only three of the plots (indicated in bold)).
| R(1) | F(2) | H(2) | P(2) | R(2) | G(3) | F(4) | S(8) | H(10) | H(17) | |
|---|---|---|---|---|---|---|---|---|---|---|
| R1 | 0.98±0.03 | 0.93±0.16 | 0.95±0.25 | 0.92±0.10 | 0.81±0.13 | 0.87±0.16 | 0.87±0.14 | 0.69±0.22 | 0.38±0.28 | 0.29±0.27 |
| F2 | 0.75±0.17 | 0.92±0.22 | 0.49±0.29 | 0.69±0.17 | 0.34±0.22 | 0.62±0.22 | 0.74±0.17 | 0.83±0.23 | 0.04±0.08 | |
| H2 | 0.83±0.17 | 0.99±0.15 | 0.96±0.20 | 0.41±0.26 | 0.98±0.16 | 0.83±0.17 | 0.85±0.22 | 0.02±0.08 | ||
| P2 | 0.85±0.13 | 0.94±0.07 | 1.0±0.17 | 0.92±0.10 | 0.40±0.21 | 0.90±0.22 | 0.26±0.19 | |||
| R2 | 0.78±0.10 | 0.86±0.14 | 0.85±0.11 | 0.67±0.14 | 0.55±0.22 | 0.21±0.18 | ||||
| G3 | 1.0±0.04 | 0.51±0.24 | 0.41±0.23 | 0.75±0.28 | 0.33±0.28 | |||||
| F4 | 1.0±0.03 | 0.7±0.21 | 0.87±0.17 | 0.84±0.17 | ||||||
| S8 | 1.0±0.05 | 0.87±0.12 | 0.22±0.19 | |||||||
| H10 | 1.0±0.03 | 0.28±0.24 | ||||||||
| H17 | 1.0±0.04 |
(b) Case study: Chiapas, Mexico
The second case study took place in the Marquéz de Comillas region, Chiapas, Mexico, where Breugel, Martínez-Ramos, Bongers and collaborators have monitored secondary forest succession in ten 500 m2 secondary forest plots since 2000. The climate of this region is cooler, drier and more seasonal than that of the Sarapiquí region. The average annual rainfall is ca 3000 mm, with less than 100 mm per month falling in the dry season (February–April). The mean annual temperature is ca 24°C. The original vegetation consists mainly of lowland tropical rainforests and semi-deciduous forests (Ibarra-Manríquez & Martínez-Ramos 2002). Today, the region is a mosaic of small-scale agriculture, pastures, mostly young (less than 10 years) secondary forests and remnants of old-growth forests. In the area, three morphological units are found, of which the Low Hills topographic unit with sandy and limestone-based soils with low pH (less than 5.5; Siebe et al. 1996) is most common. Geomorphology and former land use, more specifically abandoned pastures versus abandoned cornfields, have been shown to affect successional patterns in this region (Méndez-Bahena 1999). Sites were selected in Low Hills on former cornfields (milpas), with fallows ranging from 1.5 to 17 years post-abandonment and mostly with only one cycle of cultivation. In these plots, growth, mortality and recruitment of trees greater than or equal to 1 cm DBH are being monitored annually; here we present data for the first 3 years examined. The vegetation in these sites is expected to exhibit faster dynamics than the larger size classes and older sites described in the case study of northeastern Costa Rica.
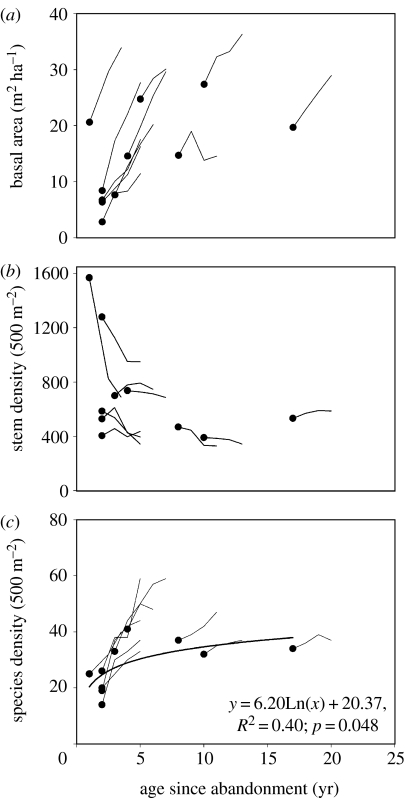
Both among-plot comparisons and within-plot dynamics showed high variability for all the three parameters (basal area, stem density and species density; figure 4). In contrast to the Sarapiquí study, fallow age was not a significant predictor of initial basal area when the plots were analysed as a chronosequence (figure 4a). Initial basal area varied widely among plots with one of the highest values for the 1-year-old plot (due to extremely high density and fast growth of early recruits). Basal area increased in all but one plot by an average of 126% during the first 3 years of the study. The only exception to this trend was in one of the intermediate-aged plots (8 years at the beginning of the study), where, as observed in the youngest plot in the Sarapiquí study, massive mortality of the pioneer species, Ochroma pyramidale, drove an overall decline in basal area (Breugel et al. 2006). In every other case, there was an increase in basal area ranging from 33 to 304% in the 3-year period, resulting in several plots with basal area over 25 m2. These are surprisingly high values, higher than the average basal area in neighbouring old-growth forest plots on comparable sites (M. Martínez-Ramos et al. 2006, unpublished work). We have to realize however that this does not directly translate into a relatively high biomass, mainly owing to lower plant stature and low average specific wood density of the dominant species in our plots (Balvanera et al. 2005; M. van Breugel et al. 2006, unpublished work).
Figure 4.
Chronosequence data for trees greater than or equal to 1 cm DBH in 10 secondary forest monitoring plots in Chiapas, Mexico (black dots) and successional trajectories within the same set of plots. (a) Basal area (m2 ha−1); (b) stem density (number of stems 500 m−2) and (c) species density (number of species 500 m−2).
Net changes in basal area sometimes obscured faster dynamics in the constituent processes: growth, mortality and recruitment. Breugel et al. (2006) analysed rates of change of these parameters as a function of age, and all three declined significantly with stand age. However, diameter increment growth rates exceeded mortality and recruitment in all stands although the margin was widest in the youngest sites. In these sites, large losses in basal area from mortality were offset by larger gains from growth and recruitment.
Initial stem density was not related to stand age, and stem density within plots followed unpredictable trajectories (figure 4b). Although density generally decreased within plots over the 3 years, rates of change varied widely among plots. The most dramatic decline in density was shown in the two plots with densities over 1000 trees per plot, suggesting strong density-dependent effects on mortality. Rates of change in the other plots were not related to initial density. An important factor contributing to variation in initial density and the subsequent changes might be variation in species composition, at least in young secondary forests. Variation in stem density of a few very dominant species such as Trema micrantha, Trichospermum mexicanum and Cecropia peltata reflects to a large extent initial differences in stem density in most plots. Variation in rates of change in stem density is therefore largely explained by the interspecific differences in demographic rates of a few dominant species (Breugel et al. 2006, Breugel et al. in preparation).
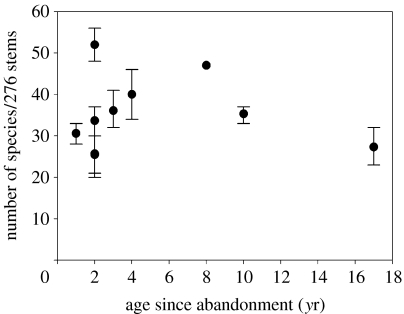
Species density showed a nonlinear chronosequence trend across the initial age range (R2=0.40; figure 4c). However, this trend is rather dependent on which census is used, as there was no significant relationship between age and density for the last census. In most individual plots, species density increased dramatically, in most cases much faster than suggested by the chronosequence trend. Especially in the youngest plots (up to 3 years old), changes were very rapid and several plots had a higher species density than in the older plots at the end of the 3-year interval. These trends also applied to species richness. When stands were compared after rarefaction to account for effects of stem density, species richness did not differ significantly with age for any of the censuses (figure 5). Within plots, however, species richness (number of trees/276 stems) increased over the 3-year period on average by 81.6% (±18.4 s.e.) and this increase was significant in all but the oldest site.
Figure 5.
Species richness in secondary forest plots in Chiapas, Mexico in year 3 of the study. Species richness was rarefied to the lowest stem density among plots and was not related to plot age for any of the 3 years. Bars give the 95% confidence intervals.
The fact that species richness increases significantly within these stands, but not within the Costa Rican abandoned pasture sites, may reflect the smaller diameter cut-off as well as the younger age of the sites. The probability that a recruit belongs to a new species may be higher in early succession simply because species density is lower, i.e. with succession an increasing proportion of the local species pool would already have arrived at the site. In the first years of succession, fast growing pioneer species still constitute a considerable fraction of new recruits, apparently because high mortality in the early phases of secondary forests opens up new recruitment possibilities (Breugel et al. 2006; in preparation). The lower diameter limit implies that newly established individuals will be included in the tree community more rapidly.
Rates of stem turnover (greater than 1 cm DBH) were very high, with values of stem gains from 13 to 90% and stem losses between 15 and 93% (figure 6). Stem loss was negatively related to age (R2=0.52), but there was no relationship between stem gain and age (figure 6a). Species turnover was very high as well, with species gains up to 75% and species losses up to 50%. Both species loss and gain (R2=0.48 and 0.65, respectively; figure 6b) were negatively related to age. In contrast to the results presented for an 8-year period for larger stems in the Sarapiquí plots (figure 3a), dynamics at the level of stems did translate in species dynamics, as species loss was significantly related to stem loss and species gain to stem gain (R2=0.58 and 0.65, respectively).
Figure 6.
Turnover of stems and species in secondary forest plots in Chiapas, Mexico. (a) Percentage of stems greater than or equal to 1 cm DBH lost through mortality and gained through recruitment. Stem gain was not significantly related to age, but stem loss decreased significantly with plot age as shown by regression equation. (b) Percentage of species lost and gained; both species loss and gain decreased significantly as a function of plot age.
The rate of successional dynamics, expressed in species and stem turnover rates, thus decreases with plot age in this series of plots. Although dynamics in the older Mexican plots do not seem to be very different from the Sarapiquí light-use plots (figure 3b), a robust comparison is difficult owing to the differences in diameter limits, plot size and census period.
Similarity in species composition (Chao Jaccard Abundance Estimator; Chao et al. 2005) between pairs of plots ranged from almost total dissimilarity (0.02) to complete similarity (1.00; table 2). Across plots, only seven species dominated the sites, of which T. mexicanum, T. micrantha and C. peltata were by far the most abundant. Since only a few species were dominant in these plots, variation in their abundance strongly influenced overall similarity between plots. For example, the plots F2 and H2, both dominated by T. mexicanum, were highly similar. H17 on the other hand was dominated by Vochysia guatemalensis, a species that was not found in the first two plots and consequently similarity was very low. We also compared pairwise similarities between initial species composition and species composition after 3 years in each plot (table 2). Only in the three 2-year-old plots did species composition change significantly over the 3-year period. As was observed in the Costa Rican case study, among-plot differences in species composition are not reflected in compositional changes over time within plots; therefore, they seem to be the result of other factors, such as the interaction between site factors (soil, land-use history) and factors related to species colonization (e.g. distance to seed sources, regional species pool), rather than being strongly determined by time since agricultural abandonment.
5. Synthesis
A major finding in our vegetation dynamics studies in successional tropical forests is that trends which appear to reflect age differences across sites (based on statistical analyses of chronosequence data) may actually have relatively little to do with age since abandonment. Studies of vegetation dynamics are a crucial counterpart to chronosequence studies and provide an essential ‘reality check’ in our understanding of rates of vegetation change and the factors that influence them at local, landscape and regional scales. The potential for misinterpretation of successional trends may be minimized to some extent if chronosequence studies include replicate plots of similar age.
At least during the first 30 years of secondary regrowth, our case studies show that chronosequence data do not correctly reflect true species dynamics. Clearly, factors other than age since abandonment have influence on species density, species richness and rates of recruitment of new species. For example, none of our studies take landscape context explicitly into account as a source of variation in stand dynamic processes. Availability and proximity of seed sources can vary largely across plots.
Young stands show substantial variation in initial stem density, basal area and species composition, reflecting local patterns of species colonization and effects of remnant vegetation. In the Chiapas sites, species richness was not related to age since abandonment, but showed increases over 3 years within sites. In contrast, species richness increased with age since abandonment across the Costa Rican sites, but remained unchanged over 7 years within four former-pasture sites. Our limited data suggest that initial compositional differences often lead to divergence in species composition, at least over time-scales of 5–15 years. Successional forests of different ages within a particular landscape generally differ more in tree species composition than a single site followed over time through the same age range (tables 1 and 2). This observation reveals the need for more detailed and longer term dynamics studies.
In contrast to species composition, basal area changes are relatively easy to predict from the chronosequence studies. Stand-level basal area and biomass accumulation in secondary forests are more affected by diameter and height growth rates of standing trees than by net changes in density due to tree recruitment and mortality. Stem density, species density and species turnover are far less predictable from a static snapshot of sites of different ages and are more rapid in younger sites and in smaller size classes. Across plots of different ages, stem turnover rates were poor predictors of species turnover rates, especially when evaluated over longer time-intervals (figures 2 and 4). Large changes in population size for a small number of species can strongly influence rates of stem turnover at the plot level, but these dynamics will have little or no impact on species turnover rates. Thus, the factors that drive species-level demographic changes appear to be relatively independent of those affecting species loss, gain and accumulation.
The case studies in Costa Rica (sites older than 10 years; all stems greater than 10 cm DBH) and Chiapas (most sites less than 10 years old; all stems greater than 1 cm DBH) highlight important effects of plot age and stem size class on rates of vegetation change. Tree communities in the Costa Rican former-pasture plots appear to have stabilized over 7 years in terms of species density, species richness and species composition, whereas the younger and smaller communities in Chiapas are showing significant changes in these attributes over only 3 years. The focus on stems greater than 10 cm DBH ignores much of the dynamic behaviour of small stems, including changes in abundance, species richness and growth forms (Capers et al. 2005). Particularly in older successional stands that have a closed canopy, new species of trees arriving as seedlings will take many years (perhaps up to several decades) to become evident in tree size classes (above 5 cm DBH). Long-term vegetation dynamics studies that encompass both small and large size classes will capture these colonization and recruitment events more effectively and will also be more directly comparable across regions.
Our work also highlights gaps in our current understanding of rates of change during forest succession in tropical regions. Although tree dynamics of young and intermediate aged stands are now being characterized, no studies have examined vegetation dynamics in old secondary forests (more than 45 years since abandonment) within a chronosequence context. Moreover, the effects of the surrounding landscape on dynamic processes within individual plots are poorly understood. In particular, the regional species pool and the extent of seed dispersal from neighbouring forest fragments are likely to be strong determinants of species accumulation rates and relative abundance patterns within successional forests. Although we know that mature forests have higher species density and species richness of trees compared with second-growth forests in northeastern Costa Rica and Chiapas, Mexico (Guariguata et al. 1997; Ibarra-Manríquez & Martínez-Ramos 2002), we have little basis for predicting rates of change in tree community composition during succession, particularly during later phases (Chazdon in press). Tropical forest succession is an idiosyncratic process, driven by many factors; the more we understand about how it operates within sites, the more accurately we will be able to generalize about how this complex process operates at large scales.
Acknowledgments
Research on vegetation dynamics in tropical secondary forests in northeastern Costa Rica was supported by grants to Robin Chazdon and collaborators from the Andrew Mellon Foundation, the US National Science Foundation, the University of Connecticut Research Foundation and the Organization for Tropical Studies. Studies of secondary succession in Chiapas and Chamela were supported by SEMARNAT-CONACYT grant 2002-C01-0597 to Miguel Martínez-Ramos. Research on secondary forest succession in Chiapas was supported by NWO-WOTRO grant W85-326 to Frans Bongers and Michiel van Breugel. We thank the many field assistants and students who participated in the fieldwork in Costa Rica and Chiapas. Finegan's work in Costa Rica was made possible by funding from the Leverhulme Trust (London), the UK Government's Overseas Development Administration (now Department for International Development) and the Swiss Development Cooperation. He thanks the many people who have supported fieldwork and data management, particularly Lucrecia Guillén, Hugo Brenes, Vicente Herra and Edwin Pereira. We thank the National Centre for Ecological Analysis and Synthesis in Santa Barbara, California (funded jointly by the NSF, the State of California and the University of California, Santa Barbara) for funding the working group on Biodiversity and Conservation Value in Agricultural Landscapes of Mesoamerica that brought most of us together to synthesize our data on secondary forest vegetation dynamics.
Footnotes
One contribution of 14 to a Theme Issue ‘Biodiversity hotspots through time: using the past to manage the future’.
References
- Aide T.M, Zimmermann J.K, Herrera L, Rosario M, Serrano M. Forest recovery in abandoned tropical pastures in Puerto Rico. Forest Ecol. Manage. 1995;77:77–86. doi:10.1016/0378-1127(95)03576-V [Google Scholar]
- Andel T. van. Floristic composition and diversity of mixed primary and secondary forests in northwest Guyana. Biodivers. Conserv. 2001;10:1645–1682. doi:10.1023/A:1012069717077 [Google Scholar]
- Aubréville A. La forêt coloniale: les forêts de l'Afrique occidentale française. Ann. Acad. Sci. Coloniale. 1938;9:1–245. [Google Scholar]
- Bakker J.P, Olff H, Willems J.H, Zobel M. Why do we need permanent plots in the study of long-term vegetation dynamics? J. Veg. Sci. 1996;7:147–156. doi:10.2307/3236314 [Google Scholar]
- Balvanera P, Kremen C.K, Martínez-Ramos M. Applying community structure analysis to ecosystem function: examples from pollination and carbon storage. Ecol. Appl. 2005;15:360–375. [Google Scholar]
- Bellingham P.J, Tanner E.V.J. Damage and responsiveness of Jamaican montane tree species after disturbance by a hurricane. Ecology. 1995;76:2562–2580. doi:10.2307/2265828 [Google Scholar]
- Bellingham P.J, Tanner E.V.J, Healey J.R. Sprouting of trees in Jamaican montane forests, after a hurricane. J. Ecol. 1994;82:747–758. doi:10.2307/2261440 [Google Scholar]
- Benitez-Malvido J, Martínez-Ramos M, Ceccon E. Seed rain vs seed bank, and the effect of vegetation cover on the recruitment of tree seedlings in tropical successional vegetation. Dissertatione Botanicae. 2001;346:285–203. [Google Scholar]
- Brearley F.Q, Prajadinata S, Kidd P.S, Proctor J, Suriantata Structure and floristics of an old secondary rain forest in Central Kalimantan, Indonesia, and a comparison with adjacent primary forest. Forest Ecol. Manage. 2004;195:385–397. doi:10.1016/j.foreco.2004.02.048 [Google Scholar]
- Breugel, M. van, Martínez-Ramos, M., & Bongers, F. 2006 Community dynamics during early secondary succession in Mexican tropical rain forests. J. Trop. Ecol 22, 663–674.
- Breugel, M., van Bongers, F., & Martínez-Ramos, M. In preparation. Community-level species dynamics during early secondary forest succession: a test of the Initial Floristic Composition hypothesis.
- Brown, S. 1997 Estimating biomass and biomass change of tropical forests: a primer. Forestry Paper 134, FAO, Rome.
- Brown S, Lugo A.E. The storage and production of organic matter in tropical forests and their role in the global carbon cycle. Biotropica. 1982;14:161–187. doi:10.2307/2388024 [Google Scholar]
- Brown S, Lugo A.E. Tropical secondary forests. J. Trop. Ecol. 1990;6:1–32. [Google Scholar]
- Budowski G. Distribution of tropical American rain forest species in the light of successional processes. Turrialba. 1965;15:40–42. [Google Scholar]
- Budowski G. The distinction between old secondary and climax species in tropical Central American lowland forests. Trop. Ecol. 1970;11:44–48. [Google Scholar]
- Bullock S.H. Plant reproduction in Neotropical dry forests. In: Bullock S.H, Mooney H.A, Medina E, editors. Seasonally dry tropical forests. Cambridge University Press; Cambridge, UK: 1995. pp. 277–303. [Google Scholar]
- Burslem D, Whitmore T.C, Brown G.C. Short-term effects of cyclone impact and long-term recovery of tropical rain forest on Kolombangara, Solomon Islands. J. Ecol. 2000;88:1063–1078. doi:10.1046/j.1365-2745.2000.00517.x [Google Scholar]
- Capers R.S, Chazdon R.L, Redondo Brenes A, Vilchez Alvarado B. Successional dynamics of woody seedling communities in tropical secondary forests. J. Ecol. 2005;93:1071–1084. doi:10.1111/j.1365-2745.2005.01050.x [Google Scholar]
- Ceccon E, Olmsted I, Vázquez-Yanes C, Campo-Alves J. Vegetation and soil properties in two tropical dry forests of differing regeneration status in Yucatan. Agrociencia. 2002;36:621–631. [Google Scholar]
- Ceccon E, Huante P, Campo-Alves J. Effects of nitrogen and phosphorus fertilization on the survival and recruitment of seedlings of dominant tree species in two abandoned tropical dry forests in Yucatán, Mexico. Forest Ecol. Manage. 2003;182:387–402. doi:10.1016/S0378-1127(03)00085-9 [Google Scholar]
- Ceccon E, Sanchéz S, Campo-Alves J. Tree seedling dynamics in two abandoned tropical dry forests of different successional status in Yucatán, Mexico: a field experiment with N and P fertilization. Plant Ecol. 2004;170:12–26. [Google Scholar]
- Ceccon E, Huante P, Rincón E. Abiotic factors influencing tropical dry forests regeneration. Brazil. Arch. Biol. Technol. 2006;49:305–312. [Google Scholar]
- Chao A, Chazdon R.L, Colwell R.K, Shen T.-J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005;8:148–159. doi:10.1111/j.1461-0248.2004.00707.x [Google Scholar]
- Chazdon R.L. Tropical forest recovery: legacies of human impact and natural disturbances. Persp. Plant Ecol. Evol. Syst. 2003;6:51–71. doi:10.1078/1433-8319-00042 [Google Scholar]
- Chazdon, R. L. In press. Successional vegetation dynamics of tropical forests. In Tropical plant community ecology (ed. W. P. Carson & S. A. Schnitzer). Oxford, UK: Blackwell Publishing.
- Chazdon R.L, Colwell R.K, Denslow J.S, Guariguata M.R. Statistical methods for estimating species richness of woody regeneration in primary and secondary rain forests of NE Costa Rica. In: Dallmeier F, Comiskey J, editors. Forest biodiversity research, monitoring and modeling: conceptual background and old world case studies. Parthenon Publishing; Paris, France: 1998. pp. 285–309. [Google Scholar]
- Chazdon R.L, Redondo Brenes A, Vilchez Alvarado B. Effects of climate and stand age on annual tree dynamics in tropical second-growth rain forests. Ecology. 2005;86:1808–1815. [Google Scholar]
- Chinea J.D. Tropical forest succession on abandoned farms in the Humacao Municipality of eastern Puerto Rico. Forest Ecol. Manage. 2002;167:195–207. doi:10.1016/S0378-1127(01)00693-4 [Google Scholar]
- Chinea J.D, Helmer E.H. Diversity and composition of tropical secondary forests recovering from large-scale clearing: results from the 1990 inventory in Puerto Rico. Forest Ecol. Manage. 2003;180:227–240. doi:10.1016/S0378-1127(02)00565-0 [Google Scholar]
- Clark D. Abolishing virginity. J. Trop. Ecol. 1996;12:735–739. [Google Scholar]
- Clark D.B, Clark D.A. Landscape-scale variation in forest structure and biomass in a tropical rain forest. Forest Ecol. Manage. 2000;137:185–198. doi:10.1016/S0378-1127(99)00327-8 [Google Scholar]
- Clark D.B, Palmer M.W, Clark D.A. Edaphic factors and the landscape-scale distributions of tropical rain forest trees. Ecology. 1999;80:2662–2675. doi:10.2307/177248 [Google Scholar]
- Colón S.M, Lugo A.E. Recovery of a subtropical dry forest after abandonment of different land uses. Biotropica. 2006;38:354–364. doi:10.1111/j.1744-7429.2006.00159.x [Google Scholar]
- Colwell R.K, Coddington J.A. Estimating terrestrial biodiversity through extrapolation. Phil. Trans. R. Soc. B. 1994;345:101–118. doi: 10.1098/rstb.1994.0091. [DOI] [PubMed] [Google Scholar]
- Colwell R.K, Mao C.X, Chang J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology. 2004;85:2717–2727. [Google Scholar]
- Connell J.H. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: Den Boer P.J, Gradwell G, editors. Dynamics of numbers in populations. Proc. Advanced Study Institute. Center for Agricultural Publication and Documentation; Wageningen, The Netherlands: 1971. pp. 298–312. [Google Scholar]
- Corlett R.T. The ecological transformation of Singapore, 1819–1990. J. Biogeogr. 1992;19:411–420. doi:10.2307/2845569 [Google Scholar]
- Dalling J.S, Winter K, Hubbell S.P, Hamrick J.L, Nason J.D, Murawski D.A. The unusual life history of Alseis blackiana: a shade-persistent pioneer tree? Ecology. 2000;82:933–945. doi:10.2307/2679893 [Google Scholar]
- de Gouvenain R.C, Silander J.A., Jr Do tropical storm regimes influence the structure of tropical lowland rain forests? Biotropica. 2003;35:166–180. [Google Scholar]
- Denslow J.S. Disturbance and diversity in tropical rain forests: the density effect. Ecol. Appl. 1995;5:962–968. [Google Scholar]
- DeWalt S.J, Chave J. Structure and biomass of four lowland Neotropical forests. Biotropica. 2004;36:7–19. [Google Scholar]
- Dittus W.P.J. The influence of cyclones on the dry evergreen forest of Sri Lanka. Biotropica. 1985;17:1–14. doi:10.2307/2388371 [Google Scholar]
- Duivenvoorden J.F. Tree species composition and rain forest–environment relationships in the middle Caquetá area, Colombia, NW Amazonia. Vegetatio. 1995;120:91–113. doi:10.1007/BF00034341 [Google Scholar]
- Eggeling W.J. Observations on the ecology of the Budongo Rain Forest, Uganda. J. Ecol. 1947;34:20–87. doi:10.2307/2256760 [Google Scholar]
- Egler F.E. Vegetation science concepts: I. Initial floristic composition—a factor in old-field vegetation development. Vegetatio. 1954;4:412–417. doi:10.1007/BF00275587 [Google Scholar]
- Elmqvist, T., Wall, M., Berggren, A. L., Blix, L., Fritioff, A. & Rinman, U. 2001 Tropical forest reorganization after cyclone and fire disturbance in Samoa: remnant trees as biological legacies. Conserv. Ecol.5: art. no.-10.
- Ewel J.J. Differences between wet and dry tropical successional tropical ecosystems. Geo-Eco-Trop. 1977;1:103–117. [Google Scholar]
- Ewel J. Tropical succession: manifold routes to maturity. Biotropica. 1980;12(Suppl.):2–7. doi:10.2307/2388149 [Google Scholar]
- Fanshawe D.B. The vegetation of British Guiana: a preliminary review. Imperial Forestry Institute, University of Oxford; Oxford, UK: 1952. p. 96. [Google Scholar]
- FAO. 2004 Forest Resources Assessment Programme. Working Paper 83/E. Food and Agriculture Organization of the United Nations, Rome.
- Fehse J, Hofstede R, Aguirre N, Paladines C, Kooijman A, Sevink J. High altitude tropical secondary forests: a competitive carbon sink? Forest Ecol. Manage. 2002;163:9–25. doi:10.1016/S0378-1127(01)00535-7 [Google Scholar]
- Feldpausch T.R, Rondon M.A, Fernandes E.C.M, Riha S.J, Wandelli E. Carbon and nutrient accumulation in secondary forests regenerating on pastures in central Amazonia. Ecol. Appl. 2004;14:S164–S176. [Google Scholar]
- Feldpausch T.R, Riha S.J, Fernandes E.C.M, Wandelli E. Development of forest structure and leaf area in secondary forests regenerating on abandoned pastures in Central Amazonia. Earth Interactions. 2005;9:1–22. doi:10.1175/EI140.1 [Google Scholar]
- Fine P.V.A. The invasibility of tropical forests by exotic plants. J. Trop. Ecol. 2002;18:687–705. doi:10.1017/S0266467402002456 [Google Scholar]
- Finegan B. Forest succession. Nature. 1984;312:109–114. doi:10.1038/312109a0 [Google Scholar]
- Finegan B. Pattern and process in Neotropical secondary rain forests: the first 100 years of succession. Trends Ecol. Evol. 1996;11:119–124. doi: 10.1016/0169-5347(96)81090-1. doi:10.1016/0169-5347(96)81090-1 [DOI] [PubMed] [Google Scholar]
- Finegan B, Delgado D. Structural and floristic heterogeneity in a 30-year-old Costa Rican rain forest restored, on pasture through natural secondary succession. Restoration Ecol. 2000;8:380–393. doi:10.1046/j.1526-100x.2000.80053.x [Google Scholar]
- Finegan B, Nasi R. The biodiversity and conservation potential of shifting cultivation landscapes. In: Schroth G, da Fonseca G.A.B, Harvey C.A, Gascon C, Vasconcelos H.L, Isaac A.N, editors. Agroforestry and biodiversity conservation in tropical landscapes. Island Press; Washington, DC: 2004. pp. 153–197. [Google Scholar]
- Foster B.L, Tilman D. Dynamic and static views of succession: testing the descriptive power of the chronosequence approach. Plant Ecol. 2000;146:1–10. doi:10.1023/A:1009895103017 [Google Scholar]
- Galindo-Gonzalez J, Guevara S, Sosa V.J. Bat- and bird-generated seed rains at isolated trees in pastures in a tropical rainforest. Conserv. Biol. 2000;14:1693–1703. doi: 10.1111/j.1523-1739.2000.99072.x. doi:10.1046/j.1523-1739.2000.99072.x [DOI] [PubMed] [Google Scholar]
- Gehring C, Park S, Denich M. Liana allometric biomass equations for Amazonian primary and secondary forest. Forest Ecol. Manage. 2004;195:69–83. doi:10.1016/j.foreco.2004.02.054 [Google Scholar]
- Gehring C, Denich M, Vlek P.L.G. Resilience of secondary forest regrowth after slash-and-burn agriculture in central Amazonia. J. Trop. Ecol. 2005;21:519–527. doi:10.1017/S0266467405002543 [Google Scholar]
- Gemerden B.S, Olff H, Parren M.P.E, Bongers F. The pristine rain forest? Remnants of historical human impacts on current tree species composition and diversity. J. Biogeogr. 2003;30:1381–1390. doi:10.1046/j.1365-2699.2003.00937.x [Google Scholar]
- Gentry A.H. Diversity and floristic composition of Neotropical dry forests. In: Bullock S.H, Mooney H.A, Medina E, editors. Seasonally dry tropical forests. Cambridge University Press; Cambridge, UK: 1995. pp. 277–303. [Google Scholar]
- Gerhardt K. Effects of root competition and canopy openness on survival and growth of tree seedlings in a tropical seasonal dry forest. Forest Ecol. Manage. 1996;82:33–48. doi:10.1016/0378-1127(95)03700-4 [Google Scholar]
- Gerhardt K, Hytteborn H. Natural dynamics and regeneration methods in tropical dry forests—an introduction. J. Veg. Sci. 1992;3:361–364. doi:10.2307/3235761 [Google Scholar]
- Gillespie T.W. Life history characteristics and rarity of woody plants in tropical dry forest fragments of Central America. J. Trop. Ecol. 1999;15:637–649. doi:10.1017/S0266467499001066 [Google Scholar]
- Gleason H.A. The individualistic concept of the plant association. Bull. Torrey Bot. Club. 1926;53:7–26. doi:10.2307/2479933 [Google Scholar]
- Gomez-Pompa A, Vazquez-Yanes C. Successional studies of a rain forest in Mexico. In: West D.C, Shugart H.H, Botkin D.B, editors. Forest succession: concepts and application. Springer; New York, NY: 1981. pp. 246–266. [Google Scholar]
- Gonzalez Iturbe J.A, Olmsted I, Tun-Dzul F. Tropical dry forest recovery after long term Henequen (sisal, Agave fourcroydes Lem) plantation in northern Yucatan, Mexico. Forest Ecol. Manage. 2002;167:67–82. doi:10.1016/S0378-1127(01)00689-2 [Google Scholar]
- Gotelli N.J, Colwell R.K. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001;4:379–391. doi:10.1046/j.1461-0248.2001.00230.x [Google Scholar]
- Guariguata M, Ostertag R. Neotropical secondary forest succession: changes in structural and functional characteristics. Forest Ecol. Manage. 2001;148:185–206. doi:10.1016/S0378-1127(00)00535-1 [Google Scholar]
- Guariguata M.R, Rheingans R, Montagnini F. Early woody invasion under tree plantations in Costa Rica: implications for forest restoration. Restoration Ecol. 1995;3:252–260. doi:10.1111/j.1526-100X.1995.tb00092.x [Google Scholar]
- Guariguata M.R, Chazdon R.L, Denslow J.S, Dupuy J.M, Anderson L. Structure and floristics of secondary and old-growth forest stands in lowland Costa Rica. Plant Ecol. 1997;132:107–120. doi:10.1023/A:1009726421352 [Google Scholar]
- Guevara S, Purata S.E, van der Maarel E. The role of remnant forest trees in tropical secondary succession. Vegetatio. 1986;66:77–84. [Google Scholar]
- Hammond D.S. Post-dispersal seed and seedling mortality of tropical dry forest trees after shifting agriculture, Chiapas, Mexico. J. Trop. Ecol. 1995;11:295–313. [Google Scholar]
- Harms K.E, Wright S.J, Calderón O, Hernández A, Herre A.E. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature. 2000;404:493–495. doi: 10.1038/35006630. doi:10.1038/35006630 [DOI] [PubMed] [Google Scholar]
- Harms K.E, Condit R, Hubbell S.P, Foster R.B. Habitat associations of trees and shrubs in a 50-ha Neotropical forest plot. J. Ecol. 2001;89:947–959. doi:10.1111/j.1365-2745.2001.00615.x [Google Scholar]
- Helmer E.H. The landscape ecology of tropical secondary forest in montane Costa Rica. Ecosystems. 2000;3:98–114. doi:10.1007/s100210000013 [Google Scholar]
- Herrera B, Finegan B. Substrate conditions, foliar nutrients and the distributions of two canopy tree species in a Costa Rican secondary rain forest. Plant Soil. 1997;191:259–267. doi:10.1023/A:1004209915530 [Google Scholar]
- Hjerpe J, Hedenas H, Elmqvist T. Tropical rain forest recovery from cyclone damage and fire in Samoa. Biotropica. 2001;33:249–259. [Google Scholar]
- Holdridge L.R, Grenke W.G, Haheway W.H, Liang T, Tosi J.J.A. Forest environments in tropical life zones. Pergamon Press; New York, NY: 1975. [Google Scholar]
- Holl K.D. Factors limiting tropical rain forest regeneration in abandoned pasture: seed rain, seed germination, microclimate, and soil. Biotropica. 1999;31:229–242. doi:10.1111/j.1744-7429.1999.tb00135.x [Google Scholar]
- Holl, K. D. In press. Old field succession in the Neotropics. In Old fields: dynamics and restoration of abandoned farmland (ed. V. A. Cramer & R. J. Hobbs).
- Huante P, Rincón E. Responses to light changes in tropical woody seedlings with contrasting growth rates. Oecologia. 1998;113:53–66. doi: 10.1007/s004420050353. doi:10.1007/s004420050353 [DOI] [PubMed] [Google Scholar]
- Huante P, Rincón E, Acosta I. Nutrient availability and growth rate of 34 woody species from a tropical deciduous forest in Mexico. Funct. Ecol. 1995a;9:849–858. doi:10.2307/2389982 [Google Scholar]
- Huante P, Rincón E, Chapin F.S., III Responses to phosphorus of contrasting successional tree-seedlings species from the tropical deciduous forest of Mexico. Funct. Ecol. 1995b;9:760–766. doi:10.2307/2390249 [Google Scholar]
- Hughes R.F, Kauffman J.B, Jaramillo V.J. Biomass, carbon, and nutrient dynamics of secondary forests in a humid tropical region of Mexico. Ecology. 1999;80:1892–1907. doi:10.2307/176667 [Google Scholar]
- Ibarra-Manríquez G, Martínez-Ramos M. Landscape variation of liana communities in a Neotropical rain forest. Plant Ecol. 2002;160:91–112. doi:10.1023/A:1015839400578 [Google Scholar]
- Janzen D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970;104:501–528. doi:10.1086/282687 [Google Scholar]
- Janzen D.H. Management of habitat fragments in a tropical dry forest. Ann. Miss. Bot. Garden. 1988;75:105–116. doi:10.2307/2399468 [Google Scholar]
- Jepsen M.R. Above-ground carbon stocks in tropical fallows, Sarawak, Malaysia. Forest Ecol. Manage. 2006;225:287–295. doi:10.1016/j.foreco.2006.01.005 [Google Scholar]
- Johnson C.M, Zarin D.J, Johnson A.H. Post-disturbance aboveground biomass accumulation in global secondary forests. Ecology. 2000;81:1395–1401. doi:10.2307/177216 [Google Scholar]
- Justiniano M.J, Fredericksen T.S. Phenology of tree species in Bolivian dry forests. Biotropica. 2000;32:276–281. [Google Scholar]
- Kennard D.K. Secondary forest succession in a tropical dry forest: patterns of development across a 50-year chronosequence in lowland Bolivia. J. Trop. Ecol. 2002;18:53–66. [Google Scholar]
- Kennard D.K, Gould K, Putz F.E, Fredericksen T.S, Morales F. Effect of disturbance intensity on regeneration mechanisms in a tropical dry forest. Forest Ecol. Manage. 2002;162:197–208. doi:10.1016/S0378-1127(01)00506-0 [Google Scholar]
- Lang G.E, Knight D.H. Tree growth, mortality, recruitment, and canopy gap formation during a 10-year period in a tropical moist forest. Ecology. 1983;64:1075–1080. doi:10.2307/1937816 [Google Scholar]
- Lucas R.M, Honzak M, Amaral I.D, Curran P.J, Foody G.M. Forest regeneration on abandoned clearances in central Amazonia. Int. J. Remote Sensing. 2002;23:965–988. doi:10.1080/01431160110069791 [Google Scholar]
- Marod D, Kutintara U, Tanaka H, Nakashizuka T. The effects of drought and fire on seed and seedling dynamics in a tropical seasonal forest in Thailand. Plant Ecol. 2002;161:41–57. doi:10.1023/A:1020372401313 [Google Scholar]
- McLaren K.P, McDonald M.A. Coppice regrowth in a disturbed tropical dry limestone forest in Jamaica. Forest Ecol. Manage. 2003;180:99–111. doi:10.1016/S0378-1127(02)00606-0 [Google Scholar]
- Méndez-Bahena, A. 1999 Sucesión secundaria de la selva húmeda y conservación de recursos naturales en Marqués de Comillas, Chiapas. M.Sc. thesis, Departamento de Ecologia de los Recursos Naturales, Universidad Nacional Autónoma de México, Morelia, Mexico.
- Mesquita R.C.G, Ickes K, Ganade G, Williamson G.B. Alternative successional pathways in the Amazon Basin. J. Ecol. 2001;89:528–537. doi:10.1046/j.1365-2745.2001.00583.x [Google Scholar]
- Miller P.M, Kauffman J.B. Effects of slash and burn agriculture on species abundances and composition of a tropical deciduous forest. Forest Ecol. Manage. 1998;103:191–201. doi:10.1016/S0378-1127(97)00180-1 [Google Scholar]
- Milton K, Laca E.A, Demment M.W. Successional patterns of mortality and growth of large trees in a Panamanian lowland forest. J. Ecol. 1994;82:79–87. doi:10.2307/2261388 [Google Scholar]
- Mooney H.A, Bullock S.H, Medina E. Introduction. In: Bullock S.H, Mooney H.A, Medina E, editors. Seasonally dry tropical forests. Cambridge University Press; Cambridge, UK: 1995. pp. 1–8. [Google Scholar]
- Moran E.F, Brondizio E, Tucker J.M, da Silva-Fosberg M.C, McCracken S, Falesi I. Effects of soil fertility and land-use on forest succession in Amazônia. Forest Ecol. Manage. 2000;139:93–108. doi:10.1016/S0378-1127(99)00337-0 [Google Scholar]
- Murphy P.G, Lugo A.E. Ecology of tropical dry forest. Annu. Rev. Ecol. Syst. 1986;17:89–96. doi:10.1146/annurev.es.17.110186.000435 [Google Scholar]
- Nelson B.W, Mesquita R, Pereira J.L.G, de Souza S.G.A, Batista G.T, Couto L.B. Allometric regressions for improved estimate of secondary forest biomass in the central Amazon. Forest Ecol. Manage. 1999;117:149–167. doi:10.1016/S0378-1127(98)00475-7 [Google Scholar]
- Niklas K.J, Midgley J.J, Rand R.H. Tree size frequency distributions, plant density, age and community disturbance. Ecol. Lett. 2003;6:405–411. doi:10.1046/j.1461-0248.2003.00440.x [Google Scholar]
- Pascarella J.B, Aide T.M, Serrano M.I, Zimmerman J.K. Land-use history and forest regeneration in the Cayey Mountains, Puerto Rico. Ecosystems. 2000;3:217–228. doi:10.1007/s100210000021 [Google Scholar]
- Pascarella J.B, Aide T.M, Zimmerman J.K. Short-term response of secondary forests to hurricane disturbance in Puerto Rico, USA. Forest Ecol. Manage. 2004;199:379–393. [Google Scholar]
- Peet R.K, Christensen N.L. Competition and tree death. Bioscience. 1987;37:586–595. doi:10.2307/1310669 [Google Scholar]
- Peña-Claros M. Changes in forest structure and species composition during secondary forest succession in the Bolivian Amazon. Biotropica. 2003;35:450–461. [Google Scholar]
- Pickett S.T.A. Succession: an evolutionary interpretation. Am. Nat. 1976;110:107–119. doi:10.1086/283051 [Google Scholar]
- Pickett S.T.A, Collins S.L, Armesto J.J. Models, mechanisms and pathways of succession. Bot. Rev. 1987;53:335–371. [Google Scholar]
- Potts M.D, Ashton P.S, Kaufman L.S, Plotkin J.B. Habitat patterns in tropical rain forests: a comparison of 105 plots in northwest Borneo. Ecology. 2002;83:2782–2797. [Google Scholar]
- Pringle C.M. Geothermal waters surface at La Selva Biological Station, Costa Rica: volcanic processes introduce chemical discontinuities into lowland tropical streams. Biotropica. 1991;23:523–529. doi:10.2307/2388390 [Google Scholar]
- Ray G.J, Brown B.J. Seed ecology of woody species in a Caribbean dry forest. Restoration Ecol. 1994;2:156–163. doi:10.1111/j.1526-100X.1994.tb00063.x [Google Scholar]
- Read L, Lawrence D. Recovery of biomass following shifting cultivation in dry tropical forests of the Yucatan. Ecol. Appl. 2003;13:85–97. [Google Scholar]
- Rico-Gray V, García-Franco J. Vegetation and soil seed bank of successional stages in tropical lowland deciduous forests. J. Veg. Sci. 1992;3:617–624. doi:10.2307/3235828 [Google Scholar]
- Rincón E, Huante P. Growth responses of tropical deciduous tree seedlings to contrasting light conditions. Trees: Struct. Funct. 1993;7:202–207. [Google Scholar]
- Ruiz J, Fandino M.C, Chazdon R.L. Vegetation structure, composition, and species richness across a 56-year chronosequence of dry tropical forest on Providencia Island, Colombia. Biotropica. 2005;37:520–530. doi:10.1111/j.1744-7429.2005.00070.x [Google Scholar]
- Sabogal C. Regeneration of tropical dry forests in Central America, with examples from Nicaragua. J. Vegetation Sci. 1992;3:407–416. doi:10.2307/3235767 [Google Scholar]
- Saldarriaga J.G, West D.C, Tharp M.L, Uhl C. Long-term chronosequence of forest succession in the upper Rio Negro of Colombia and Venezuela. J. Ecol. 1988;76:938–958. doi:10.2307/2260625 [Google Scholar]
- Sanchez-Azofeifa G.A, et al. Research priorities for Neotropical Dry Forests. Biotropica. 2005;37:477–485. doi:10.1111/j.1744-7429.2005.00066.x [Google Scholar]
- Sanford R.L, Jr, Paaby P, Luvall J.C, Phillips E. Climate, geomorphology, and aquatic systems. In: McDade L.A, Bawa K.S, Hespenheide H.A, Hartshorn G.S, editors. La Selva: ecology and natural history of a neotropical rain forest. University of Chicago Press; Chicago, IL: 1994. pp. 19–33. [Google Scholar]
- Slocum M.G, Aide T.M, Zimmerman J.K, Navarro L. Natural regeneration of subtropical montane forest after clearing fern thickets in the Dominican Republic. J. Trop. Ecol. 2004;20:483–486. doi:10.1017/S0266467404001646 [Google Scholar]
- Sheil D. A half-century of permanent plot observations in Budongo forest, Uganda: histories, highlights, and hypotheses. In: Dallmeier F, Comiskey J.A, editors. Forest biodiversity research, monitoring, and modeling: conceptual background and old world case studies. UNESCO; Paris, France: 1998. pp. 399–428. [Google Scholar]
- Sheil D. Developing tests of successional hypotheses with size-structured populations, and an assessment using long-term data from a Ugandan rain forest. Plant Ecol. 1999;140:117–127. doi:10.1023/A:1009729108668 [Google Scholar]
- Sheil D. Long-term observations of rain forest succession, tree diversity and responses to disturbance. Plant Ecol. 2001;155:183–199. doi:10.1023/A:1013243411819 [Google Scholar]
- Siebe, C., Martínez-Ramos, M., Segura-Warnholtz, G., Rodríguez-Velázquez, J., Sánchez-Béltran, S. 1996 Soil and vegetation patterns in the tropical rainforest at Chajul, southeast Mexico. In Proceedings of the International Congress on Soil of Tropical Forest Ecosystems 3rd Conference on Forest Soils (ISSS-AISS-IBG) (ed. D. Sigmarangkir), pp. 40–58. Indonesia: Maluwarman University Press.
- Silver W.L, Ostertag R, Lugo A.E. The potential for carbon sequestration through reforestation of abandoned tropical agricultural and pasture lands. Restoration Ecol. 2000;8:394–407. doi:10.1046/j.1526-100x.2000.80054.x [Google Scholar]
- Slocum M.G, Horvitz C.C. Seed arrival under different genera of trees in a Neotropical pasture. Plant Ecol. 2000;149:51–62. doi:10.1023/A:1009892821864 [Google Scholar]
- Sollins P, Sancho R.M, Sanford R.L. Soils and soil process research. In: McDade L.A, Bawa K.S, Hespenheide H.A, Hartshorn G.S, editors. La Selva: ecology and natural history of a Neotropical rainforest. University of Chicago; Chicago, IL: 1994. [Google Scholar]
- Steininger M.K. Secondary forest structure and biomass following short and extended land-use in central and southern Amazonia. J. Trop. Ecol. 2000;16:689–708. doi:10.1017/S0266467400001656 [Google Scholar]
- Suazo Ortuño, I. 1998 Aspectos ecológicos de Pteridium aquilinum (Polypodiaceae) en la región de Chajul, Chiapas. M.Sc. thesis. Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, México.
- Swaine M.D. Characteristics of dry forests in West Africa and the influence of fire. J. Vegetation Sci. 1992;3:365–374. doi:10.2307/3235762 [Google Scholar]
- Terborgh J, Foster R.B, Nuñez V.P. Tropical tree communities: a test of the nonequilibrium hypothesis. Ecology. 1996;77:561–567. doi:10.2307/2265630 [Google Scholar]
- Toledo M, Salick J. Secondary succession and indigenous management in semi-deciduous forest fallows of the Amazon Basin. Biotropica. 2006;38:161–170. doi:10.1111/j.1744-7429.2006.00120.x [Google Scholar]
- Uhl C, Clark K, Clark H, Murphy P. Early plant succession after cutting and burning in the upper Rio Negro of the Amazon Basin. J. Ecol. 1981;69:631–649. doi:10.2307/2259689 [Google Scholar]
- Uhl C, Buschbacker R, Serrão E.A.S. Abandoned pastures in eastern Amazonia. I. Patterns of plant succession. J. Ecol. 1988;75:663–681. [Google Scholar]
- Uriarte M, Condit R, Canham C.D, Hubbell S.P. A spatially explicit model of sapling growth in a tropical forest: does the identity of neighbours matter? J. Ecol. 2004;92:348–360. doi:10.1111/j.0022-0477.2004.00867.x [Google Scholar]
- Uriarte M, Canham C.D, Thompson J, Zimmerman J.K, Brokaw N. Seedling recruitment in a hurricane-driven tropical forest: light limitation, density-dependence and the spatial distribution of parent trees. J. Ecol. 2005;93:291–304. doi:10.1111/j.0022-0477.2005.00984.x [Google Scholar]
- Valencia R, Foster R.B, Villa G, Condit R, Svenning J.C, Hernandez C, Romoleroux K, Losos E, Magard E, Balslev H. Tree species distributions and local habitat variation in the Amazon: large forest plot in eastern Ecuador. J. Ecol. 2004;92:214–229. doi:10.1111/j.0022-0477.2004.00876.x [Google Scholar]
- Vandermeer J, de la Cerda I.G, Boucher D, Perfecto I, Ruiz J. Hurricane disturbance and tropical tree species diversity. Science. 2000;290:788–791. doi: 10.1126/science.290.5492.788. doi:10.1126/science.290.5492.788 [DOI] [PubMed] [Google Scholar]
- Vester H. F. M. 1997 The trees and the forest. The role of tree architecture in canopy development; a case study in secondary forests (Araracuara, Colombia). Ph.D. thesis, University of Amsterdam.
- Vieira D.L.M, Scariot A. Principles of natural regeneration of tropical dry forests for restoration. Restoration Ecol. 2006;14:11–20. doi:10.1111/j.1526-100X.2006.00100.x [Google Scholar]
- Vieira D.L.M, Scariot A, Sampaio A.B, Holl K.D. Tropical dry-forest regeneration from root suckers in Central Brazil. J. Trop. Ecol. 2006;22:353–357. doi:10.1017/S0266467405003135 [Google Scholar]
- Walker L.R. Tree damage and recovery from Hurricane Hugo in Luquillo Experimental Forest, Puerto Rico. Biotropica. 1991;23:379–385. doi:10.2307/2388255 [Google Scholar]
- White J, Harper J.L. Correlated changes in plant size and number in plant populations. J. Ecol. 1970;58:467–485. doi:10.2307/2258284 [Google Scholar]
- Whitmore, T. C. 1974 Change with time and the role of cyclones in tropical rain forest on Kolombangara, Solomon Islands. Commonwealth Forestry Institute Paper 46. Oxford, UK: Commonwealth Forestry Institute.
- Whitmore T.C. An introduction to tropical rainforests. Oxford University Press; Clarendon, UK: 1990. [Google Scholar]
- Whitmore T.C, Burslem D.F.R.P. Major disturbances in tropical rainforests. In: Newbery D.M, Prins H.H.T, Brown N.D, editors. Dynamics of tropical communities. Blackwell Science Ltd; Oxford, UK: 1998. pp. 549–565. [Google Scholar]
- Whittaker R.H. Recent evolution of ecological concepts in relation to the eastern forests of North America. In: Egerton Frank., editor. History of American ecology. Arno Press; New York, NY: 1972. [Google Scholar]
- Wijdeven S.M.J, Kuzee M.E. Seed availability as a limiting factor in forest recovery processes in Costa Rica. Restoration Ecol. 2000;8:414–424. doi:10.1046/j.1526-100x.2000.80056.x [Google Scholar]
- Wills C, Condit R, Foster R.B, Hubbell S.P. Strong density- and diversity-related effects help to maintain tree species diversity in a Neotropical forest. Proc. Natl Acad. Sci. USA. 1997;94:1252–1257. doi: 10.1073/pnas.94.4.1252. doi:10.1073/pnas.94.4.1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills C, et al. Nonrandom processes maintain diversity in tropical forests. Science. 2006;311:527–530. doi: 10.1126/science.1117715. doi:10.1126/science.1117715 [DOI] [PubMed] [Google Scholar]
- Wunderle J.M. The role of animal seed dispersal in accelerating native forest regeneration on degraded tropical lands. Forest Ecol. Manage. 1997;99:223–235. doi:10.1016/S0378-1127(97)00208-9 [Google Scholar]
- Yih K, Boucher D.H, Vandermeer J.H, Zamora N. Recovery of the rain forest of southeastern Nicaragua after destruction by Hurricane Joan. Biotropica. 1991;23:106–113. doi:10.2307/2388295 [Google Scholar]
- Zarin D.J, Ducey M.J, Tucker J.M, Salas W.A. Potential biomass accumulation in Amazonian regrowth forests. Ecosystems. 2001;4:658–668. doi:10.1007/s10021-001-0035-y [Google Scholar]
- Zarin D.J, et al. Legacy of fire slows carbon accumulation in Amazonian forest regrowth. Front. Ecol. Environ. 2005;3:365–369. [Google Scholar]
- Zimmerman J.K, Pascarella J.B, Aide T.M. Barriers to forest regeneration in an abandoned pasture in Puerto Rico. Restoration Ecol. 2000;8:350–360. doi:10.1046/j.1526-100x.2000.80050.x [Google Scholar]