Abstract
An increased plasma triglyceride (TG) level is associated with coronary artery disease (CAD) and myocardial infarction (MI) and is a key characteristic of the metabolic syndrome. Here, we used a genome-wide linkage scan to identify a novel genetic locus that influences the plasma TG level. We genotyped 714 persons in 388 multiplex Caucasian families with premature CAD and MI with 408 polymorphic microsatellite markers that cover the entire human genome. The genome-wide scan identified positive linkage for the quantitative TG trait to a novel locus on chromosome 1p31-32 [peak single-point logarithm of odds (LOD) = 3.57, peak multipoint LOD = 3.12]. For single-point linkage analysis, two markers, D1S1728 and D1S551, showed LOD scores of 2.42 and 3.57, respectively. For multipoint linkage analysis, three markers, D1S3736, D1S1728, and D1S551, showed LOD scores of 2.43, 3.03, and 3.12, respectively. No other chromosomal regions showed a LOD score of >2.2. This study identifies a new genetic locus for TG on chromosome 1p31-32. Future studies of the candidate genes at this locus will identify a specific gene influencing the TG, which will provide insights into novel regulatory mechanisms of TG metabolism and may be important for the development of therapies to prevent CAD.
Keywords: genetics, linkage, coronary artery disease, myocardial infarction, metabolic syndrome
Atherosclerotic coronary artery disease (CAD) and its principal clinical complication of myocardial infarction (MI) are the leading causes of death in industrialized countries. Increased concentrations of plasma triglyceride (TG) is an independent risk factor for the development of CAD (1–4). High concentrations of TG and low HDL levels along with small, cholesteryl ester-depleted LDLs create an atherogenic lipid phenotype [Online Mendelian Inheritance in Man (MIM) 108725] that tends to cluster with other cardiovascular risk factors, including obesity, hypertension, and diabetes, in the metabolic syndrome, as described by the National Cholesterol Education Program's Adult Treatment Panel III (5). High plasma TG levels are also found in familial combined hyperlipidemia (MIM 144250), the most common genetic dyslipidemia (6).
Hypertriglyceridemia results from an abundance of TG-rich lipoproteins in the circulation: mainly chylomicrons, VLDLs, and intermediate density lipoproteins. TG-rich lipoproteins can accumulate in the circulation via increased production and/or decreased clearance, thereby increasing the risk for the development of CAD. One mechanism by which this occurs begins as plasma concentrations increase and TG-rich lipoproteins inappropriately transfer TG to LDLs, which become cholesterol-depleted. Small dense LDLs have an increased capacity to cross the endothelial basement membrane, cause damage to the endothelium, adhere to arterial wall proteoglycans, and undergo oxidation, and they are rapidly taken up by macrophage scavenger receptors, promoting atherosclerotic lesion development (7, 8).
The plasma TG concentration is a complex quantitative trait determined by many genes and by the interaction of those genes with one another and with the environment (i.e., diet, physical activity, smoking, alcohol intake). Genetic factors significantly affect plasma TG levels, with ∼40% of the variation in human populations attributable to genetic factors (9). Some gene variants have been shown to contribute to plasma TG levels, including LPL, APOE, APOA5, and the APOA1/C3/A4 locus (10–15), but those influencing much of the population variation have yet to be defined. Because of the importance of this lipid phenotype in the development of atherosclerosis, the identification of genes that influence its plasma concentration is important for the early prognosis and development of preventive therapies. In the present study, we describe a whole genome scan to identify chromosomal regions influencing TG levels in families with premature CAD and MI. We identified a chromosomal region on chromosome 1p31-32 that displayed positive linkage to the TG levels.
MATERIALS AND METHODS
Study population
We studied 714 individuals from 388 multiplex, Caucasian families with premature CAD and MI (GeneQuest) (16, 17). Families of other ethnic origins were excluded to avoid the confounding effects of population admixture. The clinical and demographic characteristics of the study population are shown in Table 1. Each family has at least two affected sibs with CAD and MI. “Premature” CAD was defined as any previous or current evidence of significant atherosclerotic CAD occurring to males of ⩽45 years of age and females of ⩽50 years of age (16, 17). Patients were recruited by cardiologists and data coordinators at the Cleveland Clinic Foundation over an ∼5 year period. Participants answered a health questionnaire, had anthropomorphic measures taken, and had fasted blood drawn for the measurement of serum markers and DNA extraction (16, 17). Plasma lipid levels were measured by standard laboratory procedures for 714 members of the GeneQuest population.
TABLE 1.
Clinical and demographic features of the study population
| Feature | All GeneQuest Individuals with TG Data | GeneQuest Probands with TG Data |
|---|---|---|
| No. of pedigrees | 388 | 388 |
| No. of family members | 714 | 388 |
| Gender (male/female) | 480/234 | 253/135 |
| Age (years) | 49.6 ± 7.8a | 47.6 ± 6.8a |
| Ethnicity | Caucasian | Caucasian |
| Smoking (%) | 81.5 | 79.1 |
| Body mass index (kg/m2) | 29.4 ± 5.7a | 29.6 ± 5.6a |
| Hypertension (%) | 46.7 | 47.9 |
| CAD | 694/714 (97%) | 388/388 (100%) |
| Myocardial infarction | 384/714 (54%) | 18/388 (5%) |
| Non-CAD | 20/714 (3%) | 0 |
| Non-insulin-dependent diabetes mellitus | 78/714 (11%) | 44/388 (11%) |
| Insulin-dependent diabetes mellitus | 31/714 (4%) | 18/388 (5%) |
| TG (mg/dl) | 230.0 ± 157.3a | 239.0 ± 220.6a |
CAD, coronary artery disease; TG, triglyceride.
These data are shown as means ± SD.
This study was approved by local institutional review boards on human subjects, and informed consent was obtained from the participants. Whole blood was drawn from each participant, and genomic DNA was isolated from the blood using standard protocols (Puregene Kits; Gentra).
Genotyping
Genotyping was funded by a grant to Q.K.W. from the National Heart, Lung, and Blood Institute and carried out by Mammalian Genotyping Services (directed by Dr. James L. Weber, Marshfield Clinic Center for Medical Genetics). A total of 408 polymorphic markers from Screening Set 11 were genotyped, and they span the human genome approximately every 10 centimorgan (cM). DNA samples from all 714 participants from 388 families were genotyped.
Genetic statistical analyses
A genome-wide linkage analysis was performed using the program Genehunter (Genehunter 2.1 package; Whitehead Institute, Cambridge, MA) using the sibs quantitative trait mapping function. TG values were log-transformed before analysis to normalize the distribution.
RESULTS
The characteristics of the study population included in the genome-wide scan are presented in Table 1. The study population consisted of 714 individuals from 388 Caucasian families. The mean TG value was 230.0 mg/dl for the entire cohort and 239.0 for the probands (Table 1). As the TG levels did not present with a normal distribution, TG values were log-transformed before analysis. The normalized TG values are shown in Fig. 1.
Fig. 1.
Distribution of normalized triglyceride (TG) levels in the GeneQuest population.
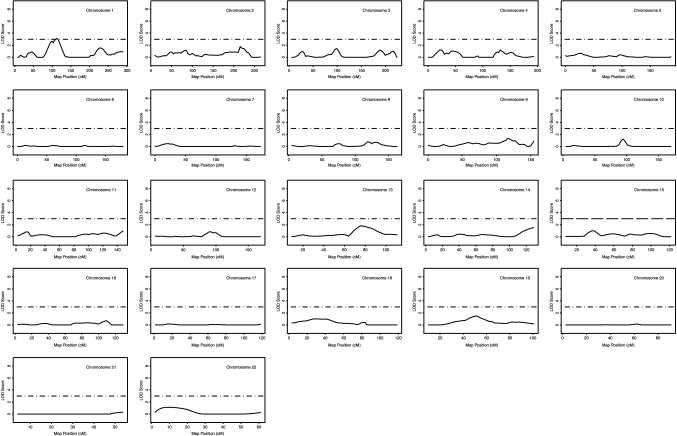
Multipoint and single-point univariate linkage analysis for TG concentrations was carried out across 22 chromosomes using 382 polymorphic markers that span the entire autosome approximately every 10 cM. Linkage results of the genome-wide scan are shown for TG in Fig. 2. We consider any chromosomal region with a logarithm of odds (LOD) score > 2.2 as a suggestive linkage, as recommended by Lander and Kruglyak (18).
Fig. 2.
Likelihood plots for TG quantitative trait loci (QTL). The y-axis of each plot represents the logarithm of odds (LOD) score, and the x-axis represents the marker map position. Solid lines represent the multipoint linkage analysis, and horizontal dashed lines indicate a LOD score of 3.0. cM, centimorgan.
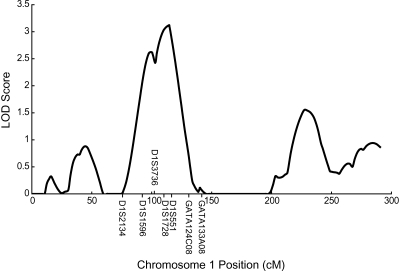
The strongest evidence of linkage for TG was observed on chromosome 1p (Figs. 2, 3, Table 2). Peak linkage was detected at chromosome 1p31 (Table 2, Fig. 3) (multipoint LOD = 3.12, single-point LOD = 3.57 at the closest marker, D1S551, 114 cM). At the 1p31 TG locus, two markers showed a single-point LOD score of >2.2 (2.42 for D1S1728 and 3.57 for D1S551), whereas three markers showed a multipoint LOD score of >2.2 (2.43 for D1S3736, 3.03 for D1S1728, and 3.12 for D1S551). No other chromosomal regions showed a LOD score of >2.2, although chromosomal regions 1q, 13q, and 19q showed LOD scores of 1.5–1.9 (Fig. 2).
Fig. 3.
Identification of a QTL for the TG level on chromosome 1p31-32. The solid line represents LOD scores from the multipoint linkage analysis for TG levels. The y-axis represents the LOD score, and the x-axis represents the marker map position.
TABLE 2.
Summary of markers linked to the TG levels
| Chromosome and Marker | Cytogenetic Location | Genetic Position (centimorgan) | Physical Position (bp) | Single-Point LOD | Multipoint LOD |
|---|---|---|---|---|---|
| D1S1596 | 1p32.1 | 89 | 59,502,822 | 1.37 | 1.73 |
| D1S3736 | 1p31.1 | 102 | 73,956,946 | 1.63 | 2.43 |
| D1S1728 | 1p31.1 | 109 | 81,771,186 | 2.42 | 3.03 |
| D1S551 | 1p31.1 | 114 | 82,667,761 | 3.57 | 3.12 |
LOD, logarithm of odds. The cytogenetic location, genetic position, and physical position of markers were all from the University of California at Santa Cruz database (http://genome.ucsc.edu/cgi-bin/hgGateway).
DISCUSSION
The present study describes results from a genome-wide scan for TG quantitative trait loci in a sample of premature CAD and MI families. We identified positive linkage to TG on chromosome 1p31-32 (maximum single-point LOD = 3.57, maximum multipoint LOD = 3.12). Previously, several studies detected linkage for plasma TG concentrations in human populations (reviewed in Ref. 19). These loci include chromosome 2q14 and 9p21 in a large Hutterite pedigree (20), chromosome 19q13.2 in families with type 2 diabetes (21), and chromosome 3p12.1-3q13.3 in Caucasian type 2 diabetics (22). Other loci include one on 15q21 in the National Heart, Lung, and Blood Institute Family Heart Study (23), one on 3p12.1-3q13.3 in Caucasian type 2 diabetics (22), and one on 12q23-q24, which was found to have pleiotrophic effects on TG and HDL-C in the HERITAGE Family Study (24). Our study augments the current comprehension of genetic loci that influence plasma TG levels in humans.
At the chromosome 1p31-32 TG locus, the ±1-LOD drop interval spans a region from 89 to 119 cM and the ±2-LOD drop interval includes the region from ∼85.5 to 127.0 cM for TG (Fig. 3). The ±2-LOD interval contains 375 known genes. Three strong candidate genes that reside within this ±2-LOD interval include angiopoietin-like 3 (ANGPTL3; MIM 604774), leptin receptor (LEPR; MIM 601007), and sterol carrier protein 2 (SCP2; MIM 184755). The ANGPTL3 gene is located within the 1-LOD drop interval (physical location, 62,835,775–62,843,768, from the University of California at Santa Cruz Genome Browser database: http://genome.ucsc.edu/cgi-bin/hgGateway). ANGPTL3 is a target of the liver X receptor whose protein product has been shown to inhibit lipoprotein lipase and endothelial lipase (25–28). In mice, a mutation resulting in the truncation of ANGPTL3 reduced plasma TG concentrations, whereas overexpression of ANGPTL3 increased circulating plasma TG concentrations (29). The LEPR gene is also located within the 1-LOD drop interval (physical location, 65,764,016–65,873,699) and encodes a receptor for the hormone leptin, which is an important regulator of body weight and lipid balance. Recently, the Gln223Arg polymorphism in the leptin receptor was found to be associated with familial combined hyperlipidemia, a disorder that is characterized by increased levels of total cholesterol, TG, and apolipoprotein B (30).
The other candidate gene, SCP2 (physical location, 53,253,150–53,289,870), is located outside the 1-LOD drop interval but within the 2-LOD drop interval. SCP2 is an enzyme that plays a role in intracellular cholesterol trafficking and metabolism. It is involved in peroxisomal β-oxidation of 2-methyl-branched chain fatty acids and β-oxidation of the steroid side chains in the conversion of cholesterol into bile acids (31, 32). Interestingly, the LRP8 gene (physical location, 53,488,950–53,506,858) is also located outside the 1-LOD drop interval but within the 2-LOD drop interval of the TG locus identified here. We recently showed that the single nucleotide polymorphism (SNP) R952Q (rs5174) in the LRP8 gene on the short arm of chromosome 1 is associated with increased platelet activation and the risk of premature familial CAD and MI but not with sporadic and nonfamilial CAD and MI (16). The LRP8 gene encodes low density lipoprotein receptor-related protein 8 and is also known as the apolipoprotein E receptor 2 (ApoER2). It belongs to the low density lipoprotein receptor family (33) and can bind and internalize apolipoprotein E-containing lipid particles. Future association studies with SNPs in these candidate genes and/or other candidate genes at the locus may identify the susceptibility gene(s) that influence plasma TG levels.
After submission of the results from this study for publication, Kathiresan et al. (34) reported the identification of a significant association of SNP rs12130333 on chromosome 1p31 with TG levels through a genome-wide SNP association study. SNP rs12130333 is an intergenic SNP (physical location, 62,964,365) located in the 1-LOD drop interval of our 1p31-32 TG locus. As rs12130333 is not in a gene, the specific gene influencing plasma TG levels at this locus is still unknown. SNP rs12130333 is located within the 1-LOD drop interval and close to the LEPR and ANGPTL3 candidate genes for TG discussed above. It is notable that our 1p31-32 TG locus completely overlaps with the TG locus (rs12130333) identified by the genome-wide SNP association study, suggesting that both family-based linkage analysis and genome-wide SNP association studies are equally effective approaches for identifying genetic loci for a complex quantitative trait.
In conclusion, the present study identified a new locus for TG levels on chromosome 1p31-32, the first step in the positional identification of the underlying genetic variant(s) for this complex lipid trait. Identification of the genetic variant(s) contributing to the 1p31-32 locus may elucidate novel molecular mechanisms regulating plasma TG levels.
Abbreviations
ANGPTL3, angiopoietin-like 3
CAD, coronary artery disease
cM, centimorgan
LEPR, leptin receptor
LOD, logarithm of odds
MI, myocardial infarction
MIM, Online Mendelian Inheritance in Man
SCP2, sterol carrier protein 2
SNP, single nucleotide polymorphism
TG, triglyceride
Published, JLR Papers in Press, January 31, 2008.
Footnotes
This research was supported by a National Heart, Lung, and Blood Institute Genotyping Service grant, by National Institutes of Health Grants P50 HL-77107 and P50 HL-81011, by the China National 863 Scientific Plan No. 2006AA02Z476, and by the National Basic Research Program of China (973 Program), Grants 2007CB512000, 2007CB512001, and 2007CB512002 (all to Q.K.W.).
References
- 1.Hokanson J. E., M. A. Austin, A. Zambon, and J. D. Brunzell. 1993. Plasma triglyceride and LDL heterogeneity in familial combined hyperlipidemia. Arterioscler. Thromb. 13 427–434. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins P. N., G. Heiss, R. C. Ellison, M. A. Province, J. S. Pankow, J. H. Eckfeldt, and S. C. Hunt. 2003. Coronary artery disease risk in familial combined hyperlipidemia and familial hypertriglyceridemia: a case-control comparison from the National Heart, Lung, and Blood Institute Family Heart Study. Circulation. 108 519–523. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins P. N., L. L. Wu, S. C. Hunt, and E. A. Brinton. 2005. Plasma triglycerides and type III hyperlipidemia are independently associated with premature familial coronary artery disease. J. Am. Coll. Cardiol. 45 1003–1012. [DOI] [PubMed] [Google Scholar]
- 4.Jeppesen J., H. O. Hein, P. Suadicani, and F. Gyntelberg. 1998. Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation. 97 1029–1036. [DOI] [PubMed] [Google Scholar]
- 5.Adult Treatment Panel III. 2002. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106: 3143–3421. [PubMed]
- 6.Cantor R. M., T. de Bruin, N. Kono, S. Napier, A. van Nas, H. Allayee, and A. J. Lusis. 2004. Quantitative trait loci for apolipoprotein B, cholesterol, and triglycerides in familial combined hyperlipidemia pedigrees. Arterioscler. Thromb. Vasc. Biol. 24 1935–1941. [DOI] [PubMed] [Google Scholar]
- 7.Eckel R. H. 2007. Mechanisms of the components of the metabolic syndrome that predispose to diabetes and atherosclerotic CVD. Proc. Nutr. Soc. 66 82–95. [DOI] [PubMed] [Google Scholar]
- 8.Krauss R. M. 1995. Dense low density lipoproteins and coronary artery disease. Am. J. Cardiol. 75 53B–57B. [DOI] [PubMed] [Google Scholar]
- 9.Shearman A. M., J. M. Ordovas, L. A. Cupples, E. J. Schaefer, M. D. Harmon, Y. Shao, J. D. Keen, A. L. DeStefano, O. Joost, P. W. Wilson, et al. 2000. Evidence for a gene influencing the TG/HDL-C ratio on chromosome 7q32.3-qter: a genome-wide scan in the Framingham Study. Hum. Mol. Genet. 9 1315–1320. [DOI] [PubMed] [Google Scholar]
- 10.Dallongeville J., S. Lussier-Cacan, and J. Davignon. 1992. Modulation of plasma triglyceride levels by apoE phenotype: a meta-analysis. J. Lipid Res. 33 447–454. [PubMed] [Google Scholar]
- 11.Groenendijk M., R. M. Cantor, T. W. de Bruin, and G. M. Linga-Thie. 2001. The apoAI-CIII-AIV gene cluster. Atherosclerosis. 157 1–11. [DOI] [PubMed] [Google Scholar]
- 12.Isaacs A., F. A. Sayed-Tabatabaei, O. T. Njajou, J. C. Witteman, and C. M. van Duijn. 2004. The -514 C->T hepatic lipase promoter region polymorphism and plasma lipids: a meta-analysis. J. Clin. Endocrinol. Metab. 89 3858–3863. [DOI] [PubMed] [Google Scholar]
- 13.Pennacchio L. A., M. Olivier, J. A. Hubacek, J. C. Cohen, D. R. Cox, J. C. Fruchart, R. M. Krauss, and E. M. Rubin. 2001. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 294 169–173. [DOI] [PubMed] [Google Scholar]
- 14.Pennacchio L. A., and E. M. Rubin. 2003. Apolipoprotein A5, a newly identified gene that affects plasma triglyceride levels in humans and mice. Arterioscler. Thromb. Vasc. Biol. 23 529–534. [DOI] [PubMed] [Google Scholar]
- 15.Wittrup H. H., A. Tybjaerg-Hansen, and B. G. Nordestgaard. 1999. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease. A meta-analysis. Circulation. 99 2901–2907. [DOI] [PubMed] [Google Scholar]
- 16.Shen G. Q., L. Li, D. Girelli, S. B. Seidelmann, S. Rao, C. Fan, J. E. Park, Q. Xi, J. Li, Y. Hu, et al. 2007. An LRP8 variant is associated with familial and premature coronary artery disease and myocardial infarction. Am. J. Hum. Genet. 81 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q., S. Rao, G. Q. Shen, L. Li, D. J. Moliterno, L. K. Newby, W. J. Rogers, R. Cannata, E. Zirzow, R. C. Elston, et al. 2004. Premature myocardial infarction novel susceptibility locus on chromosome 1P34-36 identified by genomewide linkage analysis. Am. J. Hum. Genet. 74 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lander E., and L. Kruglyak. 1995. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat. Genet. 11 241–247. [DOI] [PubMed] [Google Scholar]
- 19.Seda O. 2004. Comparative gene map of hypertriglyceridaemia. Folia Biol. (Praha). 50 43–57. [PubMed] [Google Scholar]
- 20.Newman D. L., M. Abney, H. Dytch, R. Parry, M. S. McPeek, and C. Ober. 2003. Major loci influencing serum triglyceride levels on 2q14 and 9p21 localized by homozygosity-by-descent mapping in a large Hutterite pedigree. Hum. Mol. Genet. 12 137–144. [DOI] [PubMed] [Google Scholar]
- 21.Elbein S. C., and S. J. Hasstedt. 2002. Quantitative trait linkage analysis of lipid-related traits in familial type 2 diabetes: evidence for linkage of triglyceride levels to chromosome 19q. Diabetes. 51 528–535. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra A., and J. K. Wolford. 2005. Analysis of quantitative lipid traits in the genetics of NIDDM (GENNID) study. Diabetes. 54 3007–3014. [DOI] [PubMed] [Google Scholar]
- 23.Arnett D. K., M. B. Miller, H. Coon, R. C. Ellison, K. E. North, M. Province, M. Leppert, and J. H. Eckfeldt. 2004. Genome-wide linkage analysis replicates susceptibility locus for fasting plasma triglycerides: NHLBI Family Heart Study. Hum. Genet. 115 468–474. [DOI] [PubMed] [Google Scholar]
- 24.Feitosa M. E., T. Rice, I. B. Borecki, T. Rankinen, A. S. Leon, J.S. Skinner, J. P. Despres, J. Blangero, C. Bouchard, and D. C. Rao. 2006. Pleiotropic QTL on chromosome 12q23-q24 influences triglyceride and high-density lipoprotein cholesterol levels: the HERITAGE Family Study. Hum. Biol. 78 317–327. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto K., R. Koishi, T. Shimizugawa, and Y. Ando. 2006. Angptl3-null mice show low plasma lipid concentrations by enhanced lipoprotein lipase activity. Exp. Anim. 55 27–34. [DOI] [PubMed] [Google Scholar]
- 26.Inaba T., M. Matsuda, M. Shimamura, N. Takei, N. Terasaka, Y. Ando, H. Yasumo, R. Koishi, M. Makishima, and I. Shimomura. 2003. Angiopoietin-like protein 3 mediates hypertriglyceridemia induced by the liver X receptor. J. Biol. Chem. 278 21344–21351. [DOI] [PubMed] [Google Scholar]
- 27.Li C. 2006. Genetics and regulation of angiopoietin-like proteins 3 and 4. Curr. Opin. Lipidol. 17 152–156. [DOI] [PubMed] [Google Scholar]
- 28.Shimamura M., M. Matsuda, H. Yasumo, M. Okazaki, K. Fujimoto, K. Kono, T. Shimizugawa, Y. Ando, R. Koishi, T. Kohama, et al. 2007. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 27 366–372. [DOI] [PubMed] [Google Scholar]
- 29.Koishi R., Y. Ando, M. Ono, M. Shimamura, H. Yasumo, T. Fujiwara, H. Horikoshi, and H. Furukawa. 2002. Angptl3 regulates lipid metabolism in mice. Nat. Genet. 30 151–157. [DOI] [PubMed] [Google Scholar]
- 30.van der Vleuten G. M., L. A. Kluijtmans, A. Hijmans, H. J. Blom, A. F. Stalenhoef, and J. de Graaf. 2006. The Gln223Arg polymorphism in the leptin receptor is associated with familial combined hyperlipidemia. Int. J. Obes. (Lond). 30 892–898. [DOI] [PubMed] [Google Scholar]
- 31.Kannenberg F., P. Ellinghaus, G. Assmann, and U. Seedorf. 1999. Aberrant oxidation of the cholesterol side chain in bile acid synthesis of sterol carrier protein-2/sterol carrier protein-x knockout mice. J. Biol. Chem. 274 35455–35460. [DOI] [PubMed] [Google Scholar]
- 32.Seedorf U., M. Raabe, P. Ellinghaus, F. Kannenberg, M. Fobker, T. Engel, S. Denis, F. Wouters, K. W. Wirtz, R. J. Wanders, et al. 1998. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes Dev. 12 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riddell D. R., D. V. Vinogradov, A. K. Stannard, N. Chadwick, and J. S. Owen. 1999. Identification and characterization of LRP8 (apoER2) in human blood platelets. J. Lipid Res. 40 1925–1930. [PubMed] [Google Scholar]
- 34.Kathiresan, S., O. Melander, C. Guiducci, A. Surti, N. P. Burtt, M. J. Rieder, G. M. Cooper, C. Roos, B. F. Voight, A. S. Havulinna, et al. 2008. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 40: In press. [DOI] [PMC free article] [PubMed]