Abstract
The contribution of ABCA1-mediated efflux of cellular phospholipid (PL) and cholesterol to human apolipoprotein A-I (apoA-I) to the formation of preβ1-HDL (or lipid-poor apoA-I) is not well defined. To explore this issue, we characterized the nascent HDL particles formed when lipid-free apoA-I was incubated with fibroblasts in which expression of the ABCA1 was upregulated. After a 2 h incubation, the extracellular medium contained small apoA-I/PL particles (preβ1-HDL; diameter = 7.5 ± 0.4 nm). The preβ1-HDL (or lipid-poor apoA-I) particles contained a single apoA-I molecule and three to four PL molecules and one to two cholesterol molecules. An apoA-I variant lacking the C-terminal α-helix did not form such particles when incubated with the cell, indicating that this helix is critical for the formation of lipid-poor apoA-I particles. These preβ1-HDL particles were as effective as lipid-free apoA-I molecules in mediating both the efflux of cellular lipids via ABCA1 and the formation of larger, discoidal HDL particles. In conclusion, preβ1-HDL is both a product and a substrate in the ABCA1-mediated reaction to efflux cellular PL and cholesterol to apoA-I. A monomeric apoA-I molecule associated with three to four PL molecules (i.e., lipid-poor apoA-I) has similar properties to the lipid-free apoA-I molecule.
Keywords: phospholipids, cholesterol, lipoprotein, reverse cholesterol transport, fibroblasts, high density lipoprotein, ATP binding cassette transporter A1, apolipoprotein A-I
There is a great deal of interest in understanding the biogenesis of HDL because this lipoprotein exerts antiatherogenic effects in vivo (1–3). Both the quantity and quality of HDL modulate its cardioprotective functions (4). The quality of HDL is significant because HDL comprises a heterogeneous collection of particles (5) that have different functionalities. It follows that it is important to understand the origins of the various types of HDL particles present in the circulation. ABCA1 is responsible for the production of HDL particles (6). The mechanism involves membrane phospholipid (PL) translocation via ABCA1 that induces bending of the membrane to create high-curvature sites to which apolipoproteins, such as apolipoprotein A-I (apoA-I), can bind and solubilize membrane PL and free (unesterified) cholesterol (FC) to create nascent HDL particles (7). In the case of apoA-I, the principal protein of HDL, the major nascent HDL products [lipoprotein A-I (LpA-I)] comprise discoidal particles containing two, three, or four apoA-I molecules per particle (8–11). These discoidal particles are the progenitors of spherical HDL2 and HDL3 particles that form the major fraction of circulating HDL in human plasma.
In addition to the HDL particles that each contain several apoA-I molecules, some 5% of the apoA-I in human plasma is present in a monomeric form (12–15). This species is variously called preβ1-HDL or “lipid-poor” apoA-I and has an apparent molecular mass of ∼70 kDa, although there is a great deal of variability in the reported lipid contents. Despite the low concentration, this pool of apoA-I is important because it is particularly effective at mediating cellular cholesterol efflux (14, 16). The origins of the preβ1-HDL remain unclear. ApoA-I molecules can cycle on and off HDL particles as the particle size changes as a result of remodeling by plasma factors such as lecithin:cholesterol acyltransferase, cholesteryl ester transfer protein, PL transfer protein, and lipases (14, 15, 17–19). However, the apoA-I molecules that dissociate from lipoprotein particles in this manner are essentially lipid-free (18, 19) and presumably account for the presence of lipid-free apoA-I in human plasma (20). The apoA-I molecules that form the preβ1-HDL pool presumably acquire their lipid via the ABCA1 pathway. Indeed, there is evidence that monomeric preβ1-HDL particles containing a little PL are formed when lipid-free apoA-I reacts with ABCA1 (11, 21).
Until now, understanding of the details of the production of preβ1-HDL has been hindered by a lack of knowledge about the mechanism of ABCA1-mediated formation of HDL particles. Characterization of the particles has also proved problematic because of the difficulty of obtaining sufficient preβ1-HDL. Here, we use the results of our prior studies of the ABCA1 mechanism (7, 10, 22) to address these issues and the relationship of preβ1-HDL to the larger discoidal LpA-I particles produced by ABCA1. When human apoA-I reacts with ABCA1-expressing fibroblasts, 7.5 nm preβ1-HDL particles that contain one apoA-I molecule, three to four PL molecules, and one to two FC molecules are formed. The C-terminal lipid binding domain of apoA-I plays a critical role in the solubilization of membrane lipids to create such particles. These preβ1-HDL particles react well with ABCA1, leading to their conversion into larger discoidal LpA-I containing two, three, or four apoA-I molecules per particle.
EXPERIMENTAL PROCEDURES
Materials
The isolation and purification of human apoA-I (Δ223–243) has been described previously (23). Porcine intestinal enteropeptidase was obtained from Sigma. The remaining materials used in these studies have been described (10).
Cell culture
The procedures for growing human skin fibroblasts (GM3468A) and human lung fibroblasts (WI38V13) have been described (10, 24). Expression of ABCA1 was upregulated by treatment of the cells with 22-hydroxycholesterol and 9-cis-retinoic acid before the efflux of cholesterol and choline-PL to apoA-I was measured (10). The efflux of cholesterol and choline-PL was monitored using established procedures (8, 10, 25).
Characterization of nascent HDL
The conditioned medium from ABCA1-expressing cells exposed to apoA-I was fractionated by gel filtration chromatography on Superdex 200 columns to obtain the various nascent HDL particles (8, 10). Two-dimensional nondenaturing gradient gel electrophoresis was also used to separate nascent apoA-I-containing HDL particles, and the gels were immunoblotted for apoA-I (10). The numbers of apoA-I molecules present in the HDL particles were determined using bis(sulfosuccinimidyl)suberate to chemically cross-link the apoA-I molecules within the particles (10). The susceptibility to proteolysis by enteropeptidase was assayed to distinguish between lipid-free and lipid-bound forms of apoA-I (26).
Analytical procedures
The methods for determining the concentrations of protein, cholesterol, and phosphorus have been reported previously (10). The α-helix content of apoA-I was determined by circular dichroism spectroscopy (27). HDL PLs were separated and identified using an HPLC assay (28).
Solubilization of PL vesicles
The kinetics of solubilization of dimyristoyl phosphatidylcholine (DMPC) multilamellar vesicles (MLVs) by apoA-I and preβ-HDL at 24°C or 37°C to form small, discoidal apoA-I/phosphatidylcholine (PC) complexes were measured by monitoring the decrease in absorbance at 325 nm, as described (29). The solubilization by apoA-I and preβ-HDL at 37°C of MLVs prepared from a membrane PL mixture to create discoidal HDL particles was monitored in a similar manner. To mimic the mixture of membrane lipids incorporated into the nascent HDL particles created by the activity of ABCA1 in cells, the following lipids (purity > 99%) were purchased from Avanti Polar Lipids (Birmingham, AL) and used to prepare MLVs. The membrane lipid mixture comprised 55% (w/w total PL) bovine liver PC, 12% porcine brain sphingomyelin, 5% egg lyso-PC, 10% bovine liver phosphatidylethanolamine, 8% porcine brain phosphatidylserine, and 10% bovine liver phosphatidylinositol. Cholesterol was added at 8% (w/w) total PLs. This membrane lipid mixture was dissolved in chloroform, and after removal of the solvent, the lipids were dispersed as MLVs in deoxygenated Tris-buffered saline (10 nM Tris, 0.01% EDTA, 0.15 M NaCl, and 0.01% sodium azide, pH 7.4) by vortexing and stored at 4°C overnight under nitrogen before use in the solubilization assay. The incubations of MLVs (0.5 mg/ml) with apoA-I (0.2 mg/ml) were conducted under nitrogen (7).
RESULTS
Isolation of preβ-HDL
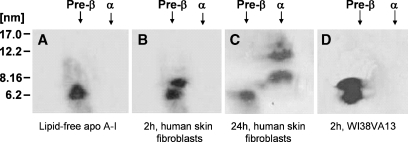
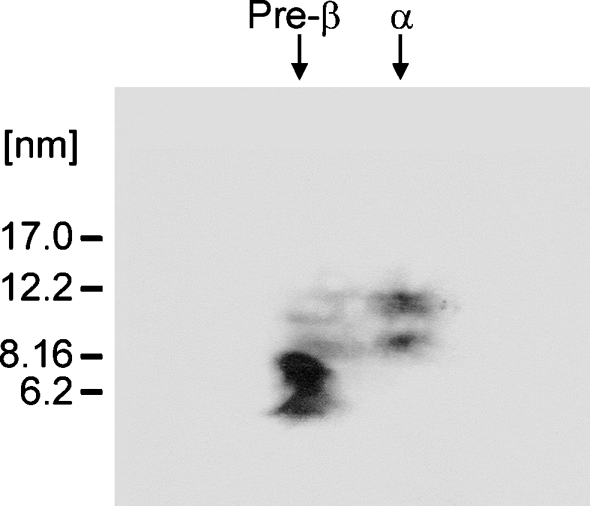
In previous studies, we characterized the nascent HDL particles created by the incubation of apoA-I with ABCA1-expressing J774 murine macrophages and GM3468 human skin fibroblasts (8, 10). After 8 or 24 h incubations, the predominant products were α-migrating discoidal HDL particles containing two, three, or four apoA-I molecules, and small preβ-HDL or lipid-poor apoA-I particles were not apparent. In addition, preβ-HDL was not observed in shorter 2 h incubations of either J774 or THP-1 macrophages with apoA-I (data not shown). However, as seen from the immunoblots of the two-dimensional gels shown in Fig. 1B, D, 2 h incubations of either GM3468 primary human skin fibroblasts or a transformed human embryonic fibroblast cell line, WI38VA13 (24), in which ABCA1 expression is upregulated, with apoA-I leads to the formation of detectable levels of preβ-HDL particles. Immunoblots of cell lysates indicate that the level of ABCA1 in J774 macrophages is some two times greater than that in the two types of fibroblasts (data not shown); this suggests that a greater availability of the transporter and lipid in macrophages reduces the incidence of preβ-HDL. The preβ-HDL particles migrate with an effective hydrodynamic diameter of 7.5 ± 0.4 nm and are distinct from the lipid-free apoA-I band, which has a diameter of 6.5 ± 0.7 nm (Fig. 1A). Lipid-free apoA-I in the monomeric state exists in a relatively expanded form because the hydrodynamic diameter of ∼6 nm is larger than that expected for a globular protein of the same molecular weight (8). The diameter of 7.5 nm for the preβ-HDL particles created by fibroblasts is consistent with the sizes of equivalent particles formed by other cell types (11, 21). The two-dimensional gel in Fig. 1C demonstrates that, after a 24 h incubation of apoA-I with fibroblasts, there is no apoA-I detected in the preβ-HDL position and the majority of the apoA-I is present as large α-migrating HDL particles (10); a similar effect also occurs with the WI38VA13 fibroblasts (data not shown). This result implies that the 7.5 nm preβ-HDL particles are converted into the larger 8 and 11 nm α-migrating particles.
Fig. 1.
Western blot analysis of two-dimensional native gel electrophoresis of apolipoprotein A-I (apoA-I)-containing nascent HDL particles generated by incubation of human skin fibroblasts and human lung fibroblasts with human apoA-I. After incubation of human skin fibroblasts and human lung fibroblasts (WI38V13) in which expression of ABCA1 was upregulated with apoA-I (15 μg/ml) for 2 h at 37°C, media were collected and prepared as described in Experimental Procedures. A total of 5 μg of apoA-I-containing particles from each medium was electrophoresed in the first dimension on a 0.7% agarose gel followed by electrophoresis in the second dimension on a 2–36% concave polyacrylamide gel. The nascent HDL bands from the two-dimensional gel were transferred onto a nitrocellulose membrane via a semidry blot system and probed with a polyclonal anti-apoA-I antibody. A: Lipid-free human apoA-I. B: Conditioned medium after 2 h of incubation with human skin fibroblasts. C: Twenty-four hour-conditioned medium from human skin fibroblasts. D: Two hour-conditioned medium from human lung fibroblasts. Molecular size markers (diameter in nanometers) are indicated. The HDL particle diameters are derived from the Rf values of the centers of the various bands as described (10).
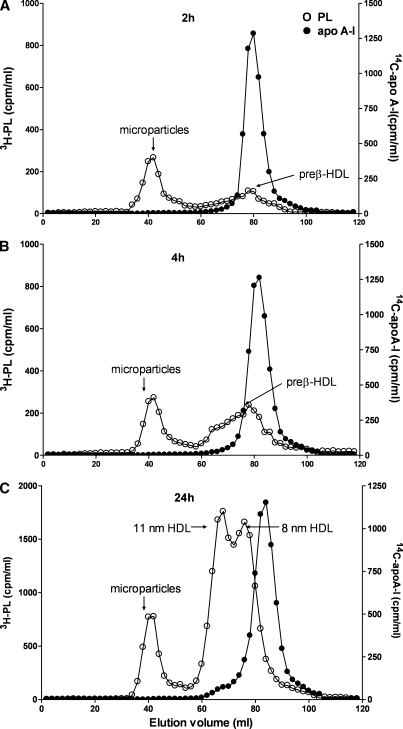
We resorted to preparative gel filtration chromatography to isolate sufficient preβ-HDL for further characterization. The elution profiles depicted in Fig. 2 are consistent with the two-dimensional gels shown in Fig. 1. The preβ-HDL formed with cellular PL after a 2 h incubation of apoA-I with the fibroblasts has an elution volume of ∼80 ml (Fig. 2A). The unreacted lipid-free apoA-I elutes at a similar position, whereas cellular microparticles elute in the void volume (40 ml) (8, 10). It is apparent from Fig. 2B that, after a 4 h incubation, some of the PL released from the cells also elutes in the 60–70 ml range, consistent with the formation of larger nascent HDL particles [a two-dimensional gel confirmed the presence of larger α-migrating HDL particles (data not shown)]. The elution profile of 24 h-conditioned medium (Fig. 2C) is consistent with our prior work (10) in showing the presence of 8 and 11 nm nascent HDL particles (elution volumes of 77 and 67 ml, respectively). A peak of radiolabeled cellular PL is no longer visible at the elution position of preβ-HDL; this is in agreement with the data in Fig. 1C showing that no apoA-I is present in a preβ-HDL band. Similar elution profiles to those depicted in Fig. 2 were also obtained when the cells were labeled with [3H]cholesterol rather than [3H]choline (data not shown). The fractions corresponding to the preβ-HDL peak were pooled and used for further characterization of the particle.
Fig. 2.
Gel filtration elution profiles of medium collected after 2, 4, and 24 h incubations of [3H]choline-labeled and ABCA1-stimulated human skin fibroblasts with [14C]human apoA-I. Human skin fibroblasts were labeled with [3H]choline and treated with 9-cis-retinoic acid and 22-hydroxycholesterol as described in Experimental Procedures. [14C]human plasma apoA-I (15 μg/ml) was added to the cells. After 2 h (A), 4 h (B), and 24 h (C) incubations at 37°C, media were collected and subjected to Superdex 200 gel filtration chromatography. Fractions were collected and radioactivity was determined by liquid scintillation counting. Open circles, [3H]choline-phospholipid; closed circles, [14C]human plasma apoA-I. One representative profile is shown out of three independent experiments. PL, phospholipid.
Characterization of preβ-HDL
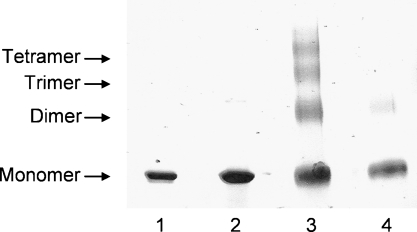
As we have done previously for nascent HDL particles (10), we used chemical cross-linking to assess the number of apoA-I molecules present in a preβ-HDL particle. As expected, SDS-PAGE analysis of lipid-free apoA-I and preβ-HDL in the absence of cross-linking reveals bands corresponding to apoA-I monomer (molecular weight = 28,000) (Fig. 3, lanes 1 and 2). The well-known ability of lipid-free apoA-I to self-associate is demonstrated by lane 3 of Fig. 3, in which the cross-linked sample exhibits additional bands corresponding to dimer, trimer, and tetramer (10, 30). Importantly, apoA-I in a preβ-HDL sample behaves differently under the same experimental conditions; after cross-linking, the apoA-I is primarily in the monomer state, with only minor dimer formation evident (Fig. 3, lane 4). The finding that apoA-I in preβ-HDL is monomeric is consistent with prior reports (11, 12, 15, 21, 31).
Fig. 3.
Chemical cross-linking with bis(sulfosuccinimidyl)suberate (BS3) of apoA-I in preβ-HDL particles created from ABCA1-expressing human skin fibroblasts. Fast-protein liquid chromatography fractions corresponding to elution volumes of 76–82 ml (see Fig. 2A) were pooled and dialyzed overnight against 0.1 M phosphate buffer, pH 7.4, concentrated to 1–2 mg apoA-I/ml using an Amicon Ultra-centrifugal filter, and then incubated with BS3 (10 mM) at room temperature. The cross-linked apoA-I samples were analyzed by 4–20% gradient SDS-PAGE. Lane 1, lipid-free apoA-I (applied 3 μg of apoA-I in 5 μl); lane 2, preβ-HDL particles (applied 5 μg of apoA-I in 5 μl); lane 3, lipid-free apoA-I plus BS3 (applied 15 μg of apoA-I in 15 μl); lane 4, preβ-HDL particles plus BS3 (applied 10 μg of apoA-I in 15 μl). Positions of monomer, dimer, trimer, and tetramer apoA-I were assigned according to their calculated molecular weights.
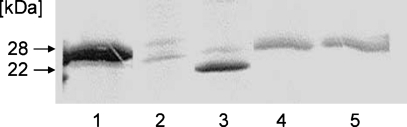
To confirm that the apoA-I molecules in preβ-HDL are indeed associated with lipid, we used the proteolytic methods of Safi, Maiorano, and Davidson (26) to distinguish between lipid-free and lipid-bound apoA-I. Comparison of lanes 1 and 3 in Fig. 4 shows that, as expected, enteropeptidase cleaves lipid-free apoA-I to give a 22 kDa fragment. Comparison of lanes 4 and 5 indicates that apoA-I in preβ-HDL is not susceptible to hydrolysis by the enteropeptidase under the same conditions. This result establishes qualitatively that the monomeric apoA-I molecules in the preβ-HDL are lipidated to some extent and are in a lipid-poor rather than a lipid-free state (26). Direct analysis of the PL and FC contents of the preβ-HDL confirms that there are three to four PL and one to two FC molecules associated with each apoA-I molecule (Table 1). This PL/apoA-I ratio agrees with reports of preβ-HDL composition using other cell types (11, 21), reconstituted particles (26, 31), and human plasma preβ-HDL (12). However, the reported FC contents of preβ-HDL particles vary widely. In agreement with the PL compositions of plasma preβ-HDL (14) and larger nascent HDL particles released from cells (10), the predominant (87%) PL classes are the choline-containing species PC, sphingomyelin, and lyso-PC (Table 1). The 8% content of acidic phosphatidylserine and phosphatidylinositol molecules apparently does not increase the particle negative charge sufficiently to cause α-electrophoretic mobility.
Fig. 4.
Protection of apoA-I in preβ-HDL particles from proteolysis. As described in Experimental Procedures, 50 μg of lipid-free apoA-I and 50 μg of preβ-HDL particles were incubated with enteropeptidase (0.13 U/μg apoA-I) for 4 h at 37°C and analyzed by 4–20% SDS-PAGE. Lane 1, 3 μg of lipid-free apoA-I; lane 2, 3 μg of enteropeptidase alone; lane 3, 5 μg of lipid-free apoA-I digested by enteropeptidase; lane 4, 5 μg of preβ-HDL particles alone; lane 5, 5 μg of preβ-HDL particles digested by enteropeptidase. Lanes 1–3 were visualized by Brilliant Colloidal G stain, whereas lanes 4, 5 were visualized by immunoblotting with anti-human apoA-I antibody.
TABLE 1.
Characteristics of preβ-HDL formed by incubation of apoA-I with ABCA1-expressing human skin fibroblasts
| PL Contentc
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrodynamic Diametera | Composition [PL/free (unesterified) cholesterol/apoA-I]b | PC | Sphingomyelin | Lyso-PC | Phosphatidylethanolamine | Phosphatidylserine | Phosphatidylinositol | ApoA-I Numberd | α-Helix Contente | |||||
| nm | mol/mol | % total PL | mol/particle | % | ||||||||||
| 7.5 ± 0.4 | 3–4:1–2:1 | 45 ± 5 | 26 ± 5 | 16 ± 4 | 5 ± 2 | 5 ± 2 | 3 ± 2 | 1 | 48 ± 3 | |||||
ApoA-I, apolipoprotein A-I; PC, phosphatidylcholine; PL, phospholipid.
Particle diameter of an equivalent globular protein was derived as described in Experimental Procedures. Mean ± SD (n = 3).
The composition of the pooled fractions of the preβ-HDL peak from the fast-protein liquid chromatography profile (Fig. 2A) was determined using mass analysis of lipids and apoA-I (see Experimental Procedures). Mean ± SD (n = 3).
Percentages of total PL were obtained using HPLC and phosphorus analysis. Mean ± SD (n = 4).
Obtained by cross-linking apoA-I with bis(sulfosuccinimidyl)suberate (cf. Fig. 3).
The α-helix content was obtained from circular dichroism spectra (see Experimental Procedures). Mean ± SD (n = 3).
The apoA-I molecules in the preβ-HDL are ∼50% α-helical (Table 1), which is similar to the levels seen in plasma and reconstituted preβ-HDL (12, 31) and in lipid-free apoA-I in dilute solution (23). This helix content is significantly lower than the value of ∼70% seen when apoA-I is associated with greater amounts of PL in discoidal HDL particles or bound to the surface of PL vesicles (32). It follows that the association of three to four PL molecules with an isolated apoA-I molecule is insufficient to induce an increase in α-helix content, unlike the situation when apoA-I is present at a PL-water interface.
Requirements for the formation of preβ-HDL
It is apparent from the above results that lipid-free apoA-I is converted into the lipid-poor form (preβ-HDL) by interaction with ABCA1-expressing fibroblasts. The acquisition of cellular lipid by apoA-I involves the binding of apoA-I to an exovesiculated domain of the plasma membrane created by the PL translocase activity of ABCA1, followed by solubilization of the PL bilayer in the vesiculated domain to form HDL particles (7). In agreement with this mechanism, apoA-I can spontaneously solubilize MLVs formed from a mixture of membrane lipids to create discoidal HDL particles with diameters in the range 9–12 nm (7). Figure 5 shows a two-dimensional gel of the products of such a reaction, and it is clear that, as expected, two species of α-migrating particles with diameters in the 9–12 nm range are present. Importantly, there are also two populations of apoA-I-containing particles that exhibit preβ mobility. Comparison of the two-dimensional gels in Fig. 1A, B with Fig. 5 indicates that the larger species migrating with an apparent hydrodynamic diameter of 7.5 nm is preβ-HDL. It follows that ABCA1 activity is not essential for preβ-HDL particle formation; rather, the particles are formed as a consequence of the ability of apoA-I to solubilize PL bilayers that are in the appropriate physical state. One of the products of the latter process is a monomeric apoA-I molecule associated with a few PL molecules (i.e., lipid-poor apoA-I or preβ-HDL). Interestingly, we could not detect preβ-HDL particles when apoA-I was reacted with DMPC MLVs at either 24°C or 37°C for 1 h; the reason for this is perhaps that the solubilization reaction was relatively rapid, so that the level of monomeric, lipid-poor apoA-I was low.
Fig. 5.
Western blot analysis of two-dimensional native gel electrophoresis of apoA-I-containing particles generated by incubation of membrane lipid multilamellar vesicles (MLVs) with apoA-I. The MLVs were prepared with a mixture of natural phosphatidylcholine (PC), sphingomyelin, lyso-PC, phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol, and cholesterol and were incubated with human apoA-I for 6 h as described in Experimental Procedures. The particles were separated by two-dimensional gel electrophoresis as described for Fig. 1 and detected by immunoblotting using an antibody against apoA-I.
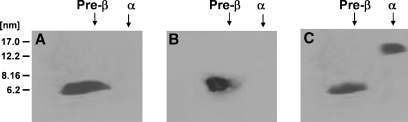
The C-terminal α-helix of apoA-I plays a critical role in the solubilization of membrane lipid MLVs, because deletion of this helix greatly inhibits both this reaction (7) and the ability of apoA-I to mediate lipid efflux from ABCA1-expressing cells (25). The experiments summarized in Fig. 6 were performed to determine whether the apoA-I C-terminal α-helix is required for the formation of preβ-HDL. Comparison of the two-dimensional gels in Figs. 6B and 1B indicates that, unlike wild-type apoA-I, the variant apoA-I (Δ223–243) lacking the C-terminal α-helix does not form preβ-HDL particles when incubated with fibroblasts for 2 h. Furthermore, extending the incubation time to 24 h leads to the formation of only a larger diameter (∼14 nm) α-migrating HDL particle (Fig. 6C). The lack of formation of 8 and 11 nm discoidal particles and the presence of only larger particles is consistent with prior gel filtration chromatographic analysis with this apoA-I variant (8). In conclusion, the C-terminal α-helix of apoA-I is required for the formation of preβ-HDL particles, implying that this helix is involved in forming the binding site for the few lipid molecules associated with the monomeric apoA-I molecule (Table 1).
Fig. 6.
Western blot analysis of two-dimensional native gel electrophoresis of apoA-I-containing HDL particles generated by incubation of ABCA1-expressing human skin fibroblasts with human apoA-I (Δ223–243). Human skin fibroblasts were incubated with apoA-I (Δ223–243) (15 μg/ml) for 2 h at 37°C, and HDL particles in the media were separated by two-dimensional gel electrophoresis as described for Fig. 1. A: Lipid-free apoA-I (Δ223–243). B: Two hour-conditioned medium from human skin fibroblasts. C: Twenty-four hour-conditioned medium from human skin fibroblasts.
Properties of preβ-HDL
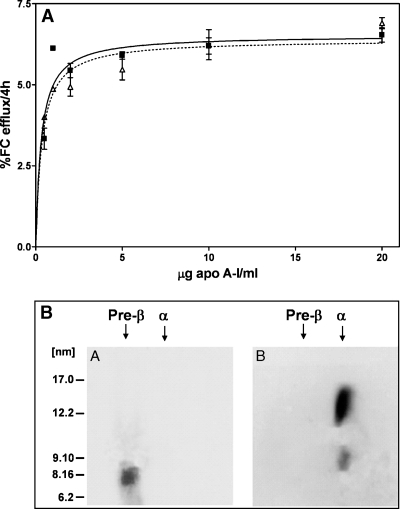
Because preβ-HDL particles are apparent after 2 h incubations of apoA-I with ABCA1-expressing fibroblasts but not after 24 h incubations (Figs. 1, 2), it seems that the preβ-HDL particles are themselves substrates for the ABCA1 reaction. To examine this possibility, we compared the abilities of lipid-free apoA-I and lipid-poor apoA-I (preβ-HDL) to mediate the efflux of cellular lipids via ABCA1. Figure 7A shows that FC efflux to different concentrations of lipid-free apoA-I exhibits a hyperbolic velocity-substrate curve, as reported previously (25). The results also show that the lipid-poor apoA-I molecules in preβ-HDL are equally efficient in supporting FC efflux. This conclusion is consistent with one recent study of the efflux capabilities of preβ-HDL (21) but not with another that showed decreased efflux with preβ-HDL (11). Given that preβ-HDL can support cellular lipid efflux via ABCA1, it is to be expected that the 7.5 nm preβ-HDL particles become larger as they acquire lipid. The two-dimensional gels in Fig. 7B demonstrate that after a 24 h incubation with fibroblasts, the preβ-HDL particles are converted into 9 and 12 nm α-migrating HDL particles.
Fig. 7.
Cellular cholesterol efflux capabilities of preβ-HDL. A: Preβ-HDL particles were isolated from 2 h-conditioned medium of human fibroblasts by fast-protein liquid chromatography (see Fig. 2) and compared with lipid-free apoA-I in their abilities to mediate cholesterol efflux from ABCA1-expressing human skin fibroblasts. FC, free (unesterified) cholesterol. Error bars represent mean ± SD. B: Pure preβ-HDL particles (gel A) were incubated at 15 μg apoA-I/ml with ABCA1-expressing human skin fibroblasts for 24 h and analyzed by native two-dimensional gel electrophoresis as described for Fig. 1 (gel B).
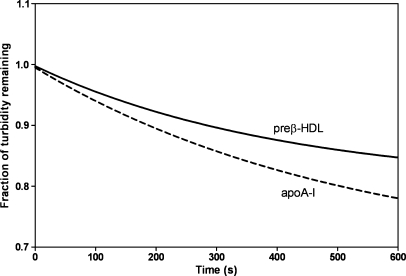
Because the lipidation of apoA-I by ABCA1 involves PL bilayer solubilization (7), the fact that preβ-HDL can be converted into larger HDL particles by supporting cellular lipid efflux implies that preβ-HDL can solubilize PL bilayers effectively. The results in Fig. 8 indicate that this is indeed the case; like lipid-free apoA-I, the poorly lipidated apoA-I molecules in preβ-HDL can solubilize MLVs of DMPC.
Fig. 8.
Solubilization of dimyristoyl phosphatidylcholine (DMPC) MLVs by apoA-I and preβ-HDL. Representative time courses for the clearance of MLVs at 24°C are shown. The DMPC MLV concentration was 0.25 mg/ml, and the apoA-I concentration was 0.1 mg/ml.
DISCUSSION
The interest in preβ1-HDL has arisen because of the possible importance of this lipoprotein particle in reverse cholesterol transport (14, 33). The pool of preβ1-HDL in plasma probably contains both lipid-free apoA-I and lipid-poor apoA-I (15). Consequently, it is necessary to define the structure-function relationships of apoA-I in both physical states to obtain a more complete understanding of preβ1-HDL. Although there is a large literature describing the properties of lipid-free apoA-I (reviewed in Refs. 34–36), the properties of lipid-poor apoA-I are less well studied because of the difficulty of obtaining it in sufficient quantity.
The results in Table 1 show that lipid-poor, monomeric apoA-I (three to four PL molecules per molecule of apoA-I) is formed when apoA-I is incubated with ABCA1-expressing fibroblasts. The human apoA-I molecule in the lipid-free state adopts a two domain tertiary structure comprising an N-terminal helix bundle domain and a separate hydrophobic C-terminal domain (23, 36). It seems that the addition of three to four PL molecules does not perturb this structure much, because the α-helix content remains ∼50% (Table 1). Indeed, although the presence of three to four PL molecules increases the hydrodynamic diameter somewhat (Table 1), the remaining properties are very similar to those of the lipid-free apoA-I molecule. The C-terminal domain of human apoA-I plays a critical role in the binding to a PL-water interface (32) and is apparently also important for stabilizing the lipid-poor state. This conclusion is based on the observation that deletion of the C-terminal α-helix, which initiates lipid interaction (36), prevents the formation of lipid-poor apoA-I (preβ1-HDL) (Fig. 6). Because deletion of either the C-terminal domain or the C-terminal α-helix greatly reduces the binding of the hydrophobic 8-anilino-1-naphthalenesulfonic acid molecule (23), it can be inferred that the hydrophobic PL molecules bind similarly, probably in a pocket formed between the hydrophobic C-terminal domain and the N-terminal helix bundle domain. Consistent with this idea, residue 188 is close to the boundary between the N- and C-terminal domains, and cleavage at this site by enteropeptidase (26) is prevented by the presence of the few PL and FC molecules in lipid-poor apoA-I (Fig. 4).
Incubation of apoA-I with ABCA1-expressing fibroblasts for 2 h enables preβ1-HDL particles to form (Fig. 1B, D). Because larger, discoidal nascent HDL particles are not formed in this time frame, it seems that lipid-poor apoA-I molecules can be formed directly during the efflux of cellular PL via ABCA1 (21). These lipid-poor apoA-I molecules are apparently intermediates in the formation of larger discoidal HDL particles, because, upon longer incubation with the cells, they are converted into discs that contain 100–200 PL molecules (10). However, the experiments described in Fig. 5 show that both lipid-poor and discoidal particles are created when apoA-I solubilizes MLVs formed from membrane lipids. This concurrent creation of lipid-poor apoA-I molecules and discoidal HDL implies that lipid-poor apoA-I molecules are by-products formed during PL bilayer solubilization. In this situation, the lipidated apoA-I products can contain apoA-I molecules in the monomeric, dimeric, trimeric, and tetrameric states.
The monomeric, lipid-poor apoA-I associated with three to four PL molecules behaves like lipid-free apoA-I with respect to both its participation in ABCA1-mediated FC efflux (Fig. 7) and its ability to solubilize PL bilayers (Fig. 8). Because apoA-I molecules carrying their full complement of PL molecules in a discoidal particle cannot participate in the ABCA1-mediated release of cellular PL, it follows that there is a transition point at which the addition of PL molecules inactivates lipid-poor apoA-I (11). Thus, the efflux properties of lipid-poor apoA-I (preβ1-HDL) formed with different cells may be different as a result of variations in the amount of associated PL. The latter parameter is likely to be sensitive to the PL composition of the membrane microenvironment in which ABCA1 is located. The FC content of such membrane domains presumably influences the number of FC molecules that are solubilized and incorporated into the lipid-poor apoA-I particle that is produced. This reasoning suggests that cellular FC released via ABCA1 is located in a preβ1-HDL product of the reaction (cf. Ref. 37).
In summary, preβ1-HDL is formed by the ABCA1-mediated conversion of lipid-free apoA-I into lipid-poor apoA-I. The sources of the lipid-free apoA-I for this process are 1) apoA-I molecules that cycle on and off HDL particles during remodeling of the particle (15, 17–19) and 2) pro-apoA-I, which is secreted by hepatocytes and enterocytes and cleaved in the plasma compartment. When lipid-free apoA-I participates in the ABCA1 reaction, it can either directly acquire a few PL molecules and become lipid-poor apoA-I or be incorporated directly into discoidal HDL during the rate-limiting cell membrane solubilization step (7). During the ABCA1 reaction, apoA-I molecules mostly acquire lipids by the solubilization of exovesiculated membrane domains, but the possibility that apoA-I molecules bound directly to ABCA1 (22) acquire a few PL molecules to form preβ1-HDL cannot be excluded. The monomeric, lipid-poor apoA-I molecules that are by-products can participate further in ABCA1-mediated cellular lipid efflux and be converted into larger discoidal HDL particles that contain two, three, or four apoA-I molecules.
Acknowledgments
The authors thank Heidi Collins, Sarah Hayes, and Margaret Nickel for expert technical assistance.
Abbreviations
apoA-I, apolipoprotein A-I
DMPC, dimyristoyl phosphatidylcholine
FC, free (unesterified) cholesterol
LpA-I, lipoprotein A-I
MLV, multilamellar vesicle
PC, phosphatidylcholine
PL phospholipid
Published, JLR Papers in Press, February 5, 2008.
Footnotes
This work was supported by National Institutes of Health Grant HL-22633.
References
- 1.Linsel-Nitschke P., and A. R. Tall. 2005. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 4 193–206. [DOI] [PubMed] [Google Scholar]
- 2.Rader D. J. 2006. Molecular regulation of HDL metabolism and function: implications for novel therapies. J. Clin. Invest. 116 3090–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barter P. J., and K. A. Rye. 2006. Relationship between the concentration and antiatherogenic activity of high-density lipoproteins. Curr. Opin. Lipidol. 17 399–403. [DOI] [PubMed] [Google Scholar]
- 4.Kontush A., and M. J. Chapman. 2006. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 58 342–374. [DOI] [PubMed] [Google Scholar]
- 5.Lund-Katz S., L. Liu, S. T. Thuahnai, and M. C. Phillips. 2003. High density lipoprotein structure. Front. Biosci. 8 d1044–d1054. [DOI] [PubMed] [Google Scholar]
- 6.Lee J-Y., and J. S. Parks. 2005. ATP-binding cassette transporter AI and its role in HDL formation. Curr. Opin. Lipidol. 16 19–25. [DOI] [PubMed] [Google Scholar]
- 7.Vedhachalam C., P. T. Duong, M. Nickel, D. Nguyen, P. Dhanasekaran, H. Saito, G. H. Rothblat, S. Lund-Katz, and M. C. Phillips. 2007. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J. Biol. Chem. 282 25123–25130. [DOI] [PubMed] [Google Scholar]
- 8.Liu L., A. E. Bortnick, M. Nickel, P. Dhanasekaran, P. V. Subbaiah, S. Lund-Katz, G. H. Rothblat, and M. C. Phillips. 2003. Effects of apolipoprotein A-I on ATP-binding cassette transporter A1-mediated efflux of macrophage phospholipid and cholesterol. J. Biol. Chem. 278 42976–42984. [DOI] [PubMed] [Google Scholar]
- 9.Krimbou L., H. H. Hassan, S. Blain, S. Rashid, M. Denis, M. Marcil, and J. Genest. 2005. Biogenesis and speciation of nascent apoA-I-containing particles in various cell lines. J. Lipid Res. 46 1668–1677. [DOI] [PubMed] [Google Scholar]
- 10.Duong P. T., H. L. Collins, M. Nickel, S. Lund-Katz, G. H. Rothblat, and M. C. Phillips. 2006. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-I. J. Lipid Res. 47 832–843. [DOI] [PubMed] [Google Scholar]
- 11.Mulya A., J-Y. Lee, A. K. Gebre, M. J. Thomas, P. L. Colvin, and J. S. Parks. 2007. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler. Thromb. Vasc. Biol. 27 1828–1836. [DOI] [PubMed] [Google Scholar]
- 12.Kunitake S. T., K. J. La Sala, and J. P. Kane. 1985. Apolipoprotein A-I-containing lipoproteins with pre-beta electrophoretic mobility. J. Lipid Res. 26 549–555. [PubMed] [Google Scholar]
- 13.Ishida B. Y., J. Frolich, and C. J. Fielding. 1987. Prebeta-migrating high density lipoprotein: quantitation in normal and hyperlipemic plasma by solid phase radioimmunoassay following electrophoretic transfer. J. Lipid Res. 28 778–786. [PubMed] [Google Scholar]
- 14.Fielding C. J., and P. E. Fielding. 1995. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 36 211–228. [PubMed] [Google Scholar]
- 15.Rye K. A., and P. J. Barter. 2004. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 24 1–8. [DOI] [PubMed] [Google Scholar]
- 16.Castro G. R., and C. J. Fielding. 1988. Early incorporation of cell-derived cholesterol into pre-β-migrating high-density lipoprotein. Biochemistry. 27 25–29. [DOI] [PubMed] [Google Scholar]
- 17.Kunitake S. T., C. M. Mendel, and L. K. Hennessy. 1992. Interconversion between apolipoprotein A-I-containing lipoproteins of pre-beta and alpha electrophoretic mobilities. J. Lipid Res. 33 1807–1816. [PubMed] [Google Scholar]
- 18.Liang H. Q., K. A. Rye, and P. J. Barter. 1994. Dissociation of lipid-free apolipoprotein A-I from high density lipoproteins. J. Lipid Res. 35 1187–1199. [PubMed] [Google Scholar]
- 19.Liang H. Q., K. A. Rye, and P. J. Barter. 1995. Cycling of apolipoprotein A-I between lipid-associated and lipid-free pools. Biochim. Biophys. Acta. 1257 31–37. [DOI] [PubMed] [Google Scholar]
- 20.Asztalos B. F., and P. S. Roheim. 1995. Presence and promotion of ‘free apolipoprotein A-I-like’ particles in human plasma. Arterioscler. Thromb. Vasc. Biol. 15 1419–1423. [DOI] [PubMed] [Google Scholar]
- 21.Chau P., Y. Nakamura, C. J. Fielding, and P. E. Fielding. 2006. Mechanism of prebeta-HDL formation and activation. Biochemistry. 45 3981–3987. [DOI] [PubMed] [Google Scholar]
- 22.Vedhachalam C., A. B. Ghering, W. S. Davidson, S. Lund-Katz, G. H. Rothblat, and M. C. Phillips. 2007. ABCA1-induced cell surface binding sites for apoA-I. Arterioscler. Thromb. Vasc. Biol. 27 1603–1609. [DOI] [PubMed] [Google Scholar]
- 23.Saito H., P. Dhanasekaran, D. Nguyen, P. Holvoet, S. Lund-Katz, and M. C. Phillips. 2003. Domain structure and lipid interaction in human apolipoproteins A-I and E: a general model. J. Biol. Chem. 278 23227–23232. [DOI] [PubMed] [Google Scholar]
- 24.Duong M-N., H. L. Collins, W. Jin, I. Zanotti, E. Favari, and G. H. Rothblat. 2006. Relative contributions of ABCA1 and SR-BI to cholesterol efflux to serum from fibroblasts and macrophages. Arterioscler. Thromb. Vasc. Biol. 26 541–547. [DOI] [PubMed] [Google Scholar]
- 25.Vedhachalam C., L. Liu, M. Nickel, P. Dhanasekaran, G. M. Anantharamaiah, S. Lund-Katz, G. Rothblat, and M. C. Phillips. 2004. Influence of apo A-I structure on the ABCA1-mediated efflux of cellular lipids. J. Biol. Chem. 279 49931–49939. [DOI] [PubMed] [Google Scholar]
- 26.Safi J. W., N. Maiorano, and W. S. Davidson. 2001. A proteolytic method for distinguishing between lipid-free and lipid-bound apolipoprotein A-I. J. Lipid Res. 42 864–872. [PubMed] [Google Scholar]
- 27.Sparks D. L., S. Lund-Katz, and M. C. Phillips. 1992. The charge and structural stability of apolipoprotein A-I discoidal and spherical recombinant high density lipoprotein particles. J. Biol. Chem. 267 25839–25847. [PubMed] [Google Scholar]
- 28.Weibel G. L., E. T. Alexander, M. R. Joshi, D. J. Rader, S. Lund-Katz, M. C. Phillips, and G. H. Rothblat. 2007. Wild-type apoA-I and the Milano variant have similar abilities to stimulate cellular lipid mobilization and efflux. Arterioscler. Thromb. Vasc. Biol. 27 2022–2029. [DOI] [PubMed] [Google Scholar]
- 29.Segall M. L., P. Dhanasekaran, F. Baldwin, G. M. Anantharamaiah, K. Weisgraber, M. C. Phillips, and S. Lund-Katz. 2002. Influence of apoE domain structure and polymorphism on the kinetics of phospholipid vesicle solubilization. J. Lipid Res. 43 1688–1700. [DOI] [PubMed] [Google Scholar]
- 30.Swaney J. B. 1986. Use of cross-linking reagents to study lipoprotein structure. Methods Enzymol. 128 613–626. [DOI] [PubMed] [Google Scholar]
- 31.Sparks D. L., P. G. Frank, S. Braschi, T. A. M. Neville, and Y. L. Marcel. 1999. Effect of apolipoprotein A-I lipidation on the formation and function of pre-beta and alpha-migrating LpA-I particles. Biochemistry. 38 1727–1735. [DOI] [PubMed] [Google Scholar]
- 32.Saito H., P. Dhanasekaran, D. Nguyen, E. Deridder, P. Holvoet, S. Lund-Katz, and M. C. Phillips. 2004. Alpha-helix formation is required for high affinity binding of human apolipoprotein A-I to lipids. J. Biol. Chem. 279 20974–20981. [DOI] [PubMed] [Google Scholar]
- 33.Curtiss L. K., D. T. Valenta, N. J. Hime, and K. A. Rye. 2006. What is so special about apolipoprotein AI in reverse cholesterol transport? Arterioscler. Thromb. Vasc. Biol. 26 12–19. [DOI] [PubMed] [Google Scholar]
- 34.Brouillette C. G., G. M. Anantharamaiah, J. A. Engler, and D. W. Borhani. 2001. Structural models of human apolipoprotein A-I: a critical analysis and review. Biochim. Biophys. Acta. 1531 4–46. [DOI] [PubMed] [Google Scholar]
- 35.Marcel Y. L., and R. S. Kiss. 2003. Structure-function relationships of apolipoprotein A-I: a flexible protein with dynamic lipid associations. Curr. Opin. Lipidol. 14 151–157. [DOI] [PubMed] [Google Scholar]
- 36.Saito H., S. Lund-Katz, and M. C. Phillips. 2004. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog. Lipid Res. 43 350–380. [DOI] [PubMed] [Google Scholar]
- 37.Sviridov D., O. Miyazaki, K. Theodore, A. Hoang, I. Fukamachi, and P. Nestel. 2002. Delineation of the role of pre-beta 1-HDL in cholesterol efflux using isolated pre-beta 1-HDL. Arterioscler. Thromb. Vasc. Biol. 22 1482–1488. [DOI] [PubMed] [Google Scholar]