Abstract
Because there is no consensus regarding the factors predicting femoral head collapse in asymptomatic osteonecrosis of the hip, we studied the risk factors for collapse. Between 1990 and 2000, we used MRI to confirm asymptomatic osteonecrosis of the femoral head in 81 patients (81 hips) whose other hip had nontraumatic symptomatic osteonecrosis and we monitored them prospectively. The minimum followup was 5 years (mean, 8.3 years; range, 5–16 years). At the latest followup, 31 hips (38%) were symptomatic and 26 hips (32%) had collapsed. The mean interval between diagnosis and collapse was 4.1 years. We observed no correlation between femoral head collapse and patients’ age, gender, weight, presumed cause of osteonecrosis, or length of followup. With combined factors, only extent of large necrotic lesion (hazard ratio, 4.06; 95% confidence interval, 1.29–12.77) and location of Type C2 necrotic lesion (hazard ratio, 6.35; 95% confidence interval, 1.18–34.11) predicted collapse.
Level of Evidence: Level I, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
In patients with symptomatic osteonecrosis (ON) of the femoral head, the natural history has been well documented to progress to collapse and painful degenerative arthritis in almost all patients [1, 2, 4–6, 9, 11, 13, 14, 18, 21, 23, 26, 28, 29]. However, there is no consensus regarding the natural history in patients with asymptomatic ON of the femoral head. The reported rates of progression in these hips have ranged from as low as 17% to as high as 100% [4, 5, 7, 9, 11, 14, 18, 29].
Historically, investigators had difficulty evaluating the fate of asymptomatic ON because the disease could not be clearly detected in asymptomatic hips by simple radiographs. However, with the advent of MRI, it became easier to diagnose asymptomatic disease and to more accurately ascertain the extent, location, and stage of the necrotic lesion [2, 17, 28].
We asked whether the radiographic stage, extent of the necrotic lesion, and lesion location influenced the rate of collapse of the asymptomatic ON of the femoral head and if so, how long after symptom onset and initial diagnosis of ON. We then asked whether a number of potential demographic risk factors would predict collapse.
Materials and Methods
We identified 152 consecutive patients with established symptomatic atraumatic ON of the femoral head in one hip treated with THA or other surgical interventions such as osteotomy or core decompression between 1990 and 2000. Because of the high probability of bilateral involvement, both hips were evaluated with radiographs and MRI even when the contralateral hip was asymptomatic. Among the 152 patients, we identified 91 (59.9%) with asymptomatic ON of the femoral head in the contralateral hip. We monitored these 91 patients prospectively to detect collapse. We recorded the following potential risk factors: age, length of followup, weight, gender, presumed cause of necrosis, stage, extent of involvement, and location of the necrotic segment. We followed all patients a minimum of 5 years. All patients gave informed consent for participation, and our Institutional Review Board approved the protocol.
The necrotic lesions were confirmed by the band-like homogeneous and nonhomogeneous patterns shown on MRI [2]. Four patients (four hips) died from causes unrelated to surgery, and six patients (six hips) were lost to followup before the end of the minimum 5-year followup period; this left 81 patients (81 hips) as the subjects of our study. None of the 10 patients (12.3%) who died or were lost to followup monitoring showed collapse of the femoral head by the time of their final evaluation. There were 68 men and 13 women with a mean age of 50.5 years (range, 22–77 years). The left hip was studied in 36 patients and the right hip in 45. The presumed cause of ON was alcohol in 39 patients (48.1%), idiopathic in 30 (37%), and steroids in 12 (14.9%). Cases of posttraumatic ON were excluded. The minimum followup was 5 years (mean, 8.3 years; range, 5–16 years).
All patients underwent clinical and radiographic followup evaluation at 3-month intervals for the first 2 years and at yearly intervals thereafter until the time of collapse or for a minimum of 5 years. The initial examination included clinical evaluation, radiographs, and MRI images. Clinical information was obtained by means of an interview and physical examination conducted by two of the authors (BWM, KSS). Patients were asked to indicate the grade of hip pain as none, slight, mild, moderate, or severe using the same criteria as for the pain categories in the Harris hip score [8].
Radiographs and MRI images of all patients were analyzed by two independent readers (CHC, SML) who did not know the patients’ clinical and radiographic histories. If there was a disagreement, a third observer (KJL) interpreted the films and a unanimous decision was reached regarding the parameter. The coefficient of variations of estimation between the observers was 4.2%. Our analysis included various parameters, including Steinberg stage, extent of involvement, and location of the necrotic segment. Radiographic stage was assessed according to the classification system of Steinberg et al. [25]. Stage II was subdivided as Stage II sclerotic type and Stage II cystic type according to the method of Bozic et al. [3] (Table 1).
Table 1.
Modified classification system of Steinberg et al. [25]
| Stage | Radiographic findings | MRI findings |
|---|---|---|
| Stage I | Normal | Abnormal |
| Stage II* | Abnormal | Abnormal |
| Sclerotic type | Sclerotic change in necrotic segments | Low signal intensity in necrotic segment in T1-weighted and T2-weighted images |
| Cystic type | Cystic change in necrotic segments | Low signal intensity in necrotic segment in T1 and high signal intensity in necrotic segment in T2 |
* Stage II was subdivided as Stage II sclerotic type and Stage II cystic type according to a method of Bozic et al. [3].
The extent of the necrotic lesion was quantified using the method of Steinberg et al. [25] in which hips were evaluated by simple visual estimate of lesion size. Hips evaluated by visual estimate were grouped by lesion size: small (less than 15% of head involvement); medium (15% to 30% involvement); and large (greater than 30%).
The location of necrosis on T1-weighted midcoronal images was classified as Type A, B, C1, or C2 using the criteria described by Sugano et al. [27]. Type A lesions occupy the medial third or less of the weightbearing area of the femoral head; Type B lesions the medial two-thirds or less; Type C1 lesions more than the medial two-thirds but not extending laterally to the acetabular edge; and Type C2 lesions more than the medial two-thirds and extending laterally to the acetabular edge.
We determined differences in clinical data between hips in which collapse developed and those in which it did not using the Kruskal-Wallis test (age, length of followup, weight) and chi square test (gender, presumed cause of necrosis). Clinical failure was defined as the occurrence of pain, and radiographic failure was defined as occurrence of segmental collapse. With the collapse of the femoral head as seen on a radiograph as the end point, survival for all enrolled patients was calculated using the Kaplan-Meier method and plotted. The differences in the survival distributions were tested with the log-rank test. We performed multivariate regression analysis using the Cox proportional hazards model to identify the independent factors with regard to the collapse of the head. We used SPSS software (version 12.0; SPSS Inc, Chicago, IL).
Results
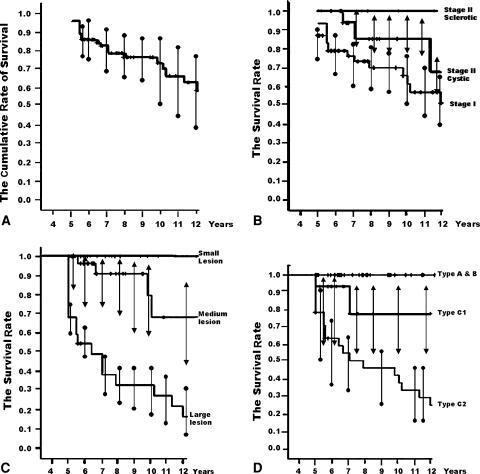
At the latest followup examination, 31 of 81 hips (38%) became symptomatic and 26 hips (32%) demonstrated collapse. With occurrence of collapse as the end point, the cumulative rates of survival (with 95% confidence intervals) were 91.4% at 5 years, 76.5% at 8 years, and 70% at 10 years (Fig. 1A). Five hips that were symptomatic had only slight pain and did not collapse. The mean interval between diagnosis and collapse was 4.1 years (range, 1.2–11.9 years). The mean interval between diagnosis and pain onset was 3.4 years (range, 0.7–8.9 years). Twelve of the 26 hips (46%) demonstrated collapse after 4 years; the longest delay between diagnosis and collapse was 11.9 years. When collapse occurred, it was always preceded by pain of an average 8 months’ duration (range, 1–36 months).
Fig. 1A–D.
The Kaplan-Meier survivorship curve. (A) The cumulative rates of survival (with 95% confidence intervals) are 91.4% at 5 years, 76.5% at 8 years, and 70% at 10 years with collapse of the femoral head as the end point. Error bars show the 95% confidence interval. (B) Survival rates according to initial Steinberg stages of osteonecrosis. There were no differences (p = 0.060, log-rank test) in survival among hips classified as Steinberg stages. Error bars show the 95% confidence interval. (C) Survival rates according to initial extent of the osteonecrosis by MRI using the method of Steinberg et al. [25]. The times to collapse were different (p = 0.000, log-rank test) among the three groups. Error bars show the 95% confidence interval. (D) Survival rates according to the location of osteonecrosis. The times to collapse were different (p = 0.000, log-rank test) among the three groups. Error bars show the 95% confidence interval.
We observed no differences in survival (p = 0.060, log-rank test) among hips classified as Steinberg stages (Fig. 1B). The survival rate for Stage I hips was 21.9% (95% confidence interval [CI], 10%–54%). The survival rate for Stage II cystic-type hips was 17% (95% CI, 13%–47%). Only eight hips (10%) were classified as Stage II sclerotic type and neither had pain nor demonstrated collapse. The survival rate for those hips was 100% (Table 2).
Table 2.
Fate of asymptomatic hips according to Steinberg stage [25]
| Stage | Hips | Pain | Collapse |
|---|---|---|---|
| Stage I | 58 (68%) | 23 (42%) | 20 (36%) |
| Stage II* | |||
| Cystic type | 18 (22%) | 8 (44%) | 6 (33%) |
| Sclerotic type | 8 (10%) | 0 (0%) | 0 (0%) |
* Stage II was subdivided as Stage II sclerotic type and Stage II cystic type according to a method of Bozic et al. [3].
Lesion size predicted survival likelihood and duration. The survival rate for hips with small lesions was 100%. The survival rate for hips with medium lesions was 36.4% (95% CI, 0%–73%). However, the survival rate for hips with large lesions was 0% (Table 3). The log-rank test showed longer durations (p = 0.000) of survival for hips with small or medium lesions than for hips with large lesions (Fig. 1C).
Table 3.
Fate of asymptomatic hips according to extent of the necrotic lesion*
| Extent | Hips | Pain | Collapse |
|---|---|---|---|
| Small (greater than 15%) | 30 (37%) | 0 (0%) | 0 (0%) |
| Medium (15% to 30%) | 29 (36%) | 9 (31%) | 6 (21%) |
| Large (greater than 30%) | 22 (27%) | 22 (100%) | 20 (91%) |
* The extent of the necrotic lesion was quantified with using the method of Steinberg et al. [25] in which hips were evaluated by simple visual estimate of lesion size.
The log-rank test showed longer durations (p = 0.000) of survival for the hips with Type A, B, or C1 necrotic lesions than for those with Type C2 lesion (Fig. 1D). The survival rate for hips with Type A or B (lesions occupying less than the medial two-thirds) was 100%. The survival rate for hips with Type C1 (lesions occupying more than the medial two-thirds but not extending laterally to the acetabular edge) was 77.8% (95% CI, 48%–100%). The extent of necrosis in these two hips was classified as large. The survival rate for Type C2 lesions (lesions occupying more than the medial two-thirds and extending laterally to the acetabular edge) was 4.2% (95% CI, 0%–12%) (Table 4).
Table 4.
Fate of asymptomatic hips according to location of the necrotic lesion*
| Location | Hips | Pain | Collapse |
|---|---|---|---|
| Type A | 3 (3%) | 0 (0%) | 0 (0%) |
| Type B | 35 (43%) | 1 (3%) | 0 (0%) |
| Type C1 | 15 (19%) | 2 (13%) | 2 (13%) |
| Type C2 | 28 (35%) | 28 (100%) | 24 (86%) |
* The location of the necrosis on T1-weighted midcoronal images was classified as Type A, B, C1, or C2 using the criteria described by Sugano et al. [27].
We observed no correlation between femoral head collapse and patients’ age, gender, weight, presumed cause of ON, or length of followup (Table 5). When all possible factors were analyzed together using a Cox model, the extent of large necrotic lesions (hazard ratio, 4.06; 95% CI, 1.29–12.77; p = 0.016) and location of Type C2 necrotic lesions (hazard ratio, 6.35; 95% CI, 1.18–34.11; p = 0.031) were risk factors for collapse.
Table 5.
Relationship between the final status of femoral head and various clinical and radiographic parameters for patients with asymptomatic osteonecrosis of the femoral head
| Patient characteristics | No collapse | Collapse | p Value |
|---|---|---|---|
| Number of hips: 81 | 55 hips (68%) | 26 hips (32%) | |
| Age at surgery (years) | 49.2 ± 12.3 | 53.2 ± 10.2 | 0.154 |
| Gender | 0.592 | ||
| Male | 47 | 21 | |
| Female | 8 | 5 | |
| Length of followup (years) | 8.4 ± 3.0 | 8.3 ± 3.6 | 0.167 |
| Weight (kg) | 63.5 ± 6.7 | 60.4 ± 8.8 | 0.086 |
| Presumed cause of necrosis | 0.146 | ||
| Alcohol | 24 | 15 | |
| Idiopathic | 20 | 10 | |
| Steroid | 11 | 1 |
All values are presented as the mean ± standard deviation.
Discussion
There is no consensus regarding the factors predicting femoral head collapse in asymptomatic ON of the hip, although considerable evidence relates predictive factors (eg, size and location of lesion) to outcomes in symptomatic ON. We therefore asked whether the radiographic stage, extent of the necrotic lesion, and lesion location influenced the rate of collapse of the asymptomatic ON of the femoral head and if so, how long after symptom onset and initial diagnosis of ON. Because of the potential influence of demographic factors on collapse, we then asked whether these would predict collapse.
Our study had certain limitations, including the small size of certain radiographic subgroups and the heterogeneity of patient demographics other than presumed causes of ON. We also did not use quantitative volumetric measurements to determine lesion size by digital image analysis, a method that has been proven more accurate than angular measurement [15, 24, 25]. In addition, we exclusively included patients with asymptomatic hips on one side and symptomatic hips on the other side. The outcome of patients with both hips asymptomatic might be different from what we observed. Asymptomatic hips will more likely have progressive collapse or symptoms when there the have symptoms in the contralateral hip [10].
We found 31 of 81 (38%) hips became symptomatic and 26 hips (32%) demonstrated collapse. The collapse was related to the extent and location of the necrotic lesion on MRI. The mean interval between diagnosis of the asymptomatic ON and collapse was 4.1 years. The clinical and radiographic failure rates in our study are lower than those of previous reports (Table 6). Discrepancies in disease prognosis between previous studies and our study arise from several sources such as differences in study populations, in length of followup periods, and in techniques for measuring the extent or location of necrotic lesions. Jergessen and Khan [14] reported 14 of 19 asymptomatic hips with Steinberg Stage II disease had progression of the disease and collapse. However, they did not use MRI images for diagnosis and measuring the extent of necrotic lesions and may have excluded hips that in fact had ON that subsequently did not collapse, thus biasing their group. This throws into question the older literature using only radiographs. Bradway and Morrey [4] also reported all 15 hips in their study of asymptomatic hips eventually collapsed. In a prospective study of 40 asymptomatic hips with very small osteonecrotic lesions, Hernigou et al. [11] reported 88% became symptomatic and 73% showed collapse. Recently, Hernigou et al. [9] reported pain developed in 91% and collapse had occurred in 77% in asymptomatic ON associated with sickle cell disease. However, several investigators [5, 17, 21] have shown using MRI certain small lesions spontaneously heal and decrease in size over time.
Table 6.
Review of the literature regarding outcomes in patients with untreated asymptomatic osteonecrosis of the femoral head
| Authors | Number of hips | Length of followup (years)* | Symptomatic progression (%) | Collapse (%) |
|---|---|---|---|---|
| Hernigou et al. [9] | 121 | 14 | 91 | 77 |
| Kopecky et al. [17] | 25 | 1.3 | 28 | 28 |
| Takatori et al. [29] | 32 | 1.7 | Not available | 43.8 |
| Jergessen and Khan [14] | 19 | > 5 | 73.7 | 71.4 |
| Hernigou et al. [11] | 40 | 11 | 88 | 73 |
| Bradway and Morrey [4] | 15 | Not available | 100 | 100 |
* All values are presented as the mean.
A number of factors may influence the rate of progression of ON of the femoral head [11, 13, 14–17, 19, 23–26]. The extent of the necrotic lesion is generally considered the principal factor in determining the risk of collapse [11, 16, 29]. Takatori et al. [29] reported a close relationship between the size of the necrotic lesion and collapse. Koo and Kim [16] reported collapse in 97% of the medium and large lesions but in only 12.5% of smaller lesions. Our findings support the finding that the more extensive the necrotic lesion, the higher the risk of collapse.
Surprisingly, we found a higher survival rate for Stage II sclerotic hips than for Stage I and Stage II cystic hips. Most researchers have reported ON of the femoral head generally has a progressive course of collapse once changes are apparent radiographically [1, 12, 24]. However, asymptomatic hips in the early stage of ON have variable paths of disease progression [13, 14, 17, 22, 24, 29]. Ito et al. [13] observed there was no difference in occurrence of progression between hips with Steinberg Stage I disease and hips with Stage II disease. Shimizu et al. [23] reported survival rates for the asymptomatic hips with normal radiographic findings were better than for hips with abnormal radiographic findings. When we classified hips by radiographic stage, there was no difference in occurrence of collapse between Stage I and Stage II (36% and 23%, respectively). However, six of 18 hips with Stage II cystic type demonstrated collapse, and none of eight hips with Stage II sclerotic type showed collapse when we subdivided Stage II. This finding is consistent with the observations of a previous report [3]. Bozic et al. [3] reported cystic changes in the femoral head as seen on plain radiographs were associated with a more than fourfold increase in the rate of failure after core decompression. Previous studies demonstrate hips with large necrotic lesions have a high possibility of collapse [13, 16, 23, 24, 29]. In our series large necrotic lesions were not observed in eight hips with Stage II sclerotic disease but were in five of 18 hips with Stage II cystic disease. Some investigators have suggested sclerotic changes in necrotic lesions indicate a repair process that inhibits bone resorption mechanism and provides structural integrity in the femoral head [13, 18]. On the other hand, cystic changes within necrotic lesions may represent poor trabeculae and extensive bone resorption by osteoclastic resorption with greater potential for subsequent collapse [7, 13].
The location of the necrotic lesion also influences the rate of collapse [22, 24–29]. Sugano et al. [28] originally proposed an MRI classification system that includes Types A, B, and C lesions; they reported Type A lesions are medial and rarely progress, Type B lesions are central and have intermediate progression, and Type C lesions have lateral involvement of the head and the worst prognosis. However, Ito et al. [13] reported the location of the necrotic lesion was not a major factor. In 2001, Japanese investigators [27] revised the classification system of location and subdivided Type C lesions into Subtypes C1 and C2 because the incidence of progressive collapse of the femoral head varies considerably between them [20, 27]. Our data also suggest the risk of collapse is higher in Type C2 lesions (86%) than in Type C1 lesions (14%). We believe this explains the lack of observed difference in the risk of collapse between Type B and Type C lesions in previous reports [13, 21] that do not differentiate C1 and C2 lesions.
Our study confirmed two important prognostic factors for collapse: extent and location of the necrotic lesion. Although it was more likely to occur in hips with large lesions, collapse also occurred in medium-sized lesions if they were localized to the lateral area of the femoral head. Six of eight hips with medium-sized lesions but with C2 location (lateral area of the femoral head) developed collapse.
A question frequently asked by our patients with ON of the femoral head is how long the surgery on their symptomatic hip will last until they need a second operation for the asymptomatic contralateral hip. We found it took an average of 4.1 years (but with a wide range of 1.2–11.9 years) from MRI diagnosis to collapse with nearly 50% of the collapse occurring as soon as 4 years after diagnosis.
The occurrence of pain in the asymptomatic hip was a good predictor of collapse in our study. We recommend evaluating these patients immediately after pain onset because pain usually precedes collapse by an average of 8 months.
Footnotes
Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patient/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aaron RK, Lennox D, Bunce GE, Ebert T. The conservative treatment of osteonecrosis of the femoral head. A comparison of core decompression and pulsing electromagnetic fields. Clin Orthop Relat Res. 1989;249:209–218. [PubMed]
- 2.Bassett LW, Gold RH, Reicher M, Bennett LR, Tooke SM. Magnetic resonance imaging in the early diagnosis of ischemic necrosis of the femoral head. Clin Orthop Relat Res. 1987;214:237–248. [PubMed]
- 3.Bozic KJ, Zurakowski D, Thornhill TS. Survivorship analysis of hips treated with core decompression for nontraumatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 1999;81:200–209. [DOI] [PubMed]
- 4.Bradway JK, Morrey BF. The natural history of the silent hip in bilateral atraumatic osteonecrosis. J Arthroplasty. 1993;8:383–387. [DOI] [PubMed]
- 5.Cheng EY, Thongtrangan I, Laorr A, Saleh KJ. Spontaneous resolution of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2004;86:2594–2599. [DOI] [PubMed]
- 6.Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. [DOI] [PubMed]
- 7.Glimcher MJ, Kenzora JE. The biology of osteonecrosis of the human femoral head and its clinical implications: II. The pathologic changes in the femoral head as an organ and in the hip joint. Clin Orthop Relat Res. 1979;139:283–312. [PubMed]
- 8.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 9.Hernigou P, Habibi A, Bachir D, Galacteros F. The natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell disease. J Bone Joint Surg Am. 2006;88:2565–2572. [DOI] [PubMed]
- 10.Hernigou P, Lambotte JC. Bilateral hip osteonecrosis: influence of hip size on outcome. Ann Rheum Dis. 2000;59:817–821. [DOI] [PMC free article] [PubMed]
- 11.Hernigou P, Poigrand A, Nogier A, Manicom O. Fate of very small asymptomatic stage-I osteonecrotic lesions of the hip. J Bone Joint Surg Am. 2004;86:2589–2593. [DOI] [PubMed]
- 12.Hungerford DS, Zizic TM. The treatment of ischemic necrosis of bone in systemic lupus erythematosus. Medicine. 1980;59:143–148. [DOI] [PubMed]
- 13.Ito H, Matsuno T, Kaneda K. Prognosis of early stage avascular necrosis of the femoral head. Clin Orthop Relat Res. 1999;358:149–157. [DOI] [PubMed]
- 14.Jergessen HE, Khan AS. The natural history of untreated asymptomatic hips in patients who have non-traumatic osteonecrosis. J Bone Joint Surg Am. 1997;79:359–363. [DOI] [PubMed]
- 15.Kerboul M, Thomine J, Postel M, Merle d’Aubigne R. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. J Bone Joint Surg Br. 1974;56:291–296. [PubMed]
- 16.Koo KH, Kim R. Quantifying the extent of osteonecrosis of the femoral head. A new methods using MRI. J Bone Joint Surg Br. 1995;77:875–880. [PubMed]
- 17.Kopecky KK, Braunstein EM, Brandt KD, Filo RS, Leapman SB, Capello WN, Klatte EC. Apparent avascular necrosis of the hip: appearance and spontaneous resolution of MR findings in renal allograft recipients. Radiology. 1991;179:523–527. [DOI] [PubMed]
- 18.Lee CK, Hansen HT, Weiss AB. The ‘silent hip’ of idiopathic ischemic necrosis of the femoral head in adults. J Bone Joint Surg Am. 1980;62:795–800. [PubMed]
- 19.Mont MA, Jones LC, Pacheo I, Hungerford DS. Radiographic predictors of outcome of core decompression for hips with osteonecrosis stage III. Clin Orthop Relat Res. 1998;354:159–168. [DOI] [PubMed]
- 20.Nishi T, Sugano N, Miki H, Hashimoto J, Yoshiokawa H. Does alendronate prevent collapse in osteonecrosis of the femoral head? Clin Orthop Relat Res. 2006;443:273–279. [DOI] [PubMed]
- 21.Nishi T, Sugano N, Ohzono K, Sakai T, Haraguchi K, Yoshikawa H. Progression and cessation of collapse in osteonecrosis of the femoral head. Clin Orthop Relat Res. 2002;400:149–157. [DOI] [PubMed]
- 22.Ohzono K, Saito M, Takaoka K, Ono K, Saito S, Nishina T, Kadowaki T. Natural history of nontraumatic avascular necrosis of the femoral head. J Bone Joint Surg Br. 1991;73:68–72. [DOI] [PubMed]
- 23.Shimizu K, Moriya H, Sakamoto M, Suguro T. Prediction of collapse with magnetic resonance imaging of avascular necrosis. J Bone Joint Surg Am. 1994;76:215–223. [DOI] [PubMed]
- 24.Steinberg DR, Steinberg ME, Garino JP, Dalinka M, Udupa JK. Determining lesion size in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):27–34. [DOI] [PubMed]
- 25.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed]
- 26.Stulberg BN, Davis AW, Bauer TW, Levine M, Easley K. Osteonecrosis of the femoral head. A prospective randomized treatment protocol. Clin Orthop Relat Res. 1991;268:140–151. [PubMed]
- 27.Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7:601–605. [DOI] [PubMed]
- 28.Sugano N, Ohzono K, Masuhara K, Takaoka K, Ono K. Prognostication of osteonecrosis of the femoral head with systemic lupus erythematosus by magnetic resonance imaging. Clin Orthop Relat Res. 1994;305:190–199. [DOI] [PubMed]
- 29.Takatori Y, Kokubo T, Hinomiya S, Nakamura S, Morimoto S, Kusaba I. Avascular necrosis of the femoral head. Natural history and magnetic resonance imaging. J Bone Joint Surg Br. 1993;75:217–221. [DOI] [PubMed]