Abstract
Several animal and human studies suggest pharmacological approaches may prevent steroid-induced osteonecrosis (ON). We asked whether the newly developed 3-hydroxymethyl-3-glutaryl-CoA (HMG-CoA) reductase inhibitor, pitavastatin, could prevent steroid-induced ON in rabbits. We injected 65 adult male Japanese white rabbits once with 20 mg/kg of methylprednisolone acetate into the right gluteus medius muscle. The rabbits were divided into two groups; one group of 35 rabbits received pitavastatins (PS), and the other group of 30 rabbits received no prophylaxis (CTR). Hematological examinations were performed just before the steroid injection (0 weeks) and at 1 and 2 weeks after steroid injection; both the femora and the humeri were histologically examined 2 weeks postinjection. The incidence of histologic changes consistent with early ON in the PS group (13 of 35; 37%) was lower in comparison to the CTR group (21 of 30; 70%). The size of the bone marrow fat cells in the PS group (56.6 ± 10 μm) was smaller than those in the CTR group (60 ± 4 μm). The data suggest pitavastatin has the potential to lower the incidence of steroid-induced ON in rabbits.
Introduction
Osteonecrosis (ON) of the femoral head frequently occurs (3% to 40%) in patients who receive corticosteroids as a treatment for underlying diseases such as systemic erythematosus (SLE), nephrotic syndrome, and renal transplantation [1, 13, 14, 19]. Once ON collapses the femoral head, most patients undergo surgery [13, 22, 27]. Therefore, preventing ON would be an ideal strategy for the treatment of this disease.
Several possible factors in the pathogenesis of ON have been suggested based on both human and animal studies, including coagulation abnormalities [11], hyperlipidemia [4, 10, 17, 18, 20, 31–33], and oxidative stress [8, 9]. Human studies suggest vascular occlusion may occur because of mechanical interruption by the thrombi or lipid emboli in the nutrient vessels [4, 31]. In the rabbit ON model, hyperlipidemia with associated abnormal thrombophilic coagulopathy has been linked to the development of ON [32, 33]. Based on these findings, several recent clinical and experimental studies have explored the effects of lipid-lowering agents on preventing ON [20, 23, 30]. Wang et al. [30] reported lovastatin prevented steroid-induced osteonecrosis in a chicken model.
3-hydroxymethyl-3-glutaryl-CoA (HMG-CoA) reductase inhibitors (statins) are potent inhibitors of cholesterol biosynthesis in the liver by blocking the conversion of HMG-CoA to mevalonate [6]. They have been widely used for the treatment of hyperlipidemia as well as preventing coronary artery diseases [25, 26]. Pitavastatin, a newly developed statin, is apparently a potent and prolonged inhibitor of sterol synthesis, lowering total cholesterol (TC), and affecting triglycerides (TG) by enhancing the hepatic low-density lipoprotein (LDL) receptor and suppressing very-low-density lipoprotein (VLDL) secretion. The cholesterol-reducing effect of pitavastatin is greater than that of the other statins [7].
We asked whether pitavastatin could reduce the risk of early histologic changes (bone marrow fat cell size) and the associated hyperlipidemia of steroid-induced ON in rabbits.
Materials and Methods
We compared the incidence of early histological changes of ON and the average sizes of the bone marrow fat cells in two groups, a steroid-only group (control group) and a group also treated with pitavastatin. The rabbit model of steroid-induced ON has been previously reported [33]. We studied 65 adult (with closed growth plates) male Japanese white rabbits (Kyudo, Tosu, Japan) ranging in age from 28 to 32 weeks. The rabbits were injected once with 20 mg/kg body weight of methylprednisolone acetate (MPSL, Upjohn, Tokyo, Japan) intramuscularly into the right gluteus medius muscle before the start of the investigation (week 0) [33]. The rabbits were divided into two groups, consisting of a pitavastatin group (PS group, n = 35), and a control group (CTR group, n = 30). Pitavastatin (Kowa Pharmaceutical, Nagoya, Japan) at a dose of 0.7 mg/kg body weight per day was intravenously administered in the PS group once daily for 4 weeks, starting from 2 weeks before the MPSL injection until 2 weeks after the injection. In the previous study we reported the incidence of ON in the rabbits which were injected once with 20 mg/kg body weight of MPSL was 70% and the incidence of ON in those with both MPSL injection and probucol treatment was 37% [20]. In order to detect whether probucol reduced the incidence of steroid-induced ON in rabbits, with a significance level (alpha) of 5% and a power of 80%, sample sizes of 31 were required for both groups. Because we expected pitavastatin would be more effective on the prevention of ON than probucol, we decided the sample size is over 30. The animals were housed at the Animal Center of Kyushu University. All of the experiments were conducted in accordance with the Guidelines for Animal Experiments of Kyushu University, the Law (no. 105), and the notification (no. 6) of the government and the Committee on Ethics in Japan.
The body weight of each rabbit was measured before the experiment and at 1 and 2 weeks after the MPSL injection. Two weeks after the MPSL injection, both the femora and humeri were histologically examined for the presence of ON and the sizes of the bone marrow fat cells were examined morphologically. We used a camera that sent electronic images of the sections to an image processor. The diameter of bone marrow fat cells displayed on the video monitor was measured using an interactive mousepad-tracing instrument (NIH image software program) [18]. We (KN, TY, GM) determined the size of the bone marrow fat cells as the average of the greatest diameters of 100 fat cells in four randomly selected fields (1 field = 4 × 10−8 m2) from the viable areas. Based on previous reports, the diagnosis of ON was histologically confirmed 2 weeks after the steroid administration [9, 20].
Whole areas of the proximal third and distal condyles of both the femora and humeri (eight regions) were examined histologically for the presence of ON. A diagnosis of ON was made blindly and independently by three authors (KN, TY, GM). Based on the previously published criteria of ON, a diagnosis of ON was determined based on the presence of the accumulation of bone marrow cell debris, and the bone trabeculae with empty lacunae or pyknotic nuclei of osteocytes within the bone trabeculae, accompanied by surrounding bone marrow cell necrosis. The presence of repair tissue, comprising granulation tissue, infiltration of inflammatory cells and appositional bone formation, was also examined [32, 33]. If the diagnoses differed among the three examiners, a consensus was reached by discussing the histologic findings without knowledge of the group from which the sample was obtained. Rabbits with at least one osteonecrotic lesion among the eight areas examined were considered to have ON.
To evaluate the effect of pitavastatin as a lipid-lowering agent, we examined plasma lipid levels and the plasma LDL:HDL cholesterol ratio which is considered a potential risk factor for corticosteroid-induced ON in rabbits [17]. We collected blood samples from the auricular arteries while the animals were in a fasting state. The samples were obtained in the early morning just before the MPSL injection (week 0) and at weeks 1 and 2 after the MPSL injection. We measured total cholesterol, low-density lipoprotein, very-low-density lipoprotein, and triglyceride.
Data were expressed as the mean ± standard deviation. The size of the bone marrow fat cells in the two groups was compared using one-way analysis of variance (ANOVA) with Scheffe’s post hoc test. The hematologic data were compared by repeated-measures ANOVA with Scheffe’s post hoc test. Statistical analyses were performed using the Stat View j-0.5 software program (SAS Institute, Cary, NC).
Results
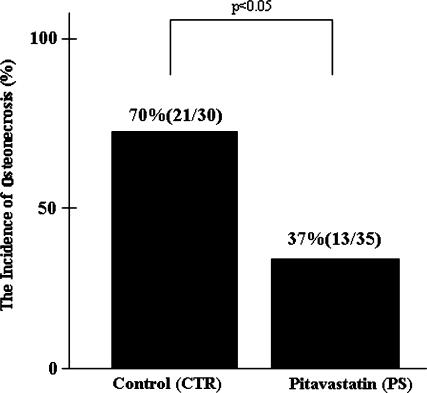
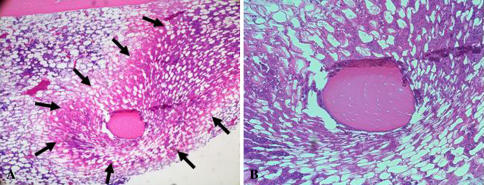
The incidence of early histological changes of ON in the PS group was lower (p = 0.008) in comparison to the CTR group: 21 of 30 (70%) rabbits in the control and 13 of 35 (37%) in the PS group (Fig. 1). We observed yellowish areas in the metaphysis and diaphysis. Histologically, the study rabbits demonstrated an accumulation of bone marrow cell debris, and the bone trabeculae had empty lacunae (Fig. 2A–B). These findings were consistent for all of the ON-positive rabbits.
Fig. 1.
The pitavastatin group had a lower (p = 0.008) incidence of ON (37%) than the control group (70%).
Fig. 2A–B.
Histology suggesting early osteonecrotic lesions in the pitavastatin group is shown. (A) A lower magnified view exhibits an eosinophilic ON lesion (arrows) as compared with the normal area 2 weeks after the steroid injection (stain, hematoxylin and eosin; original magnification, ×40). (B) A higher magnified view demonstrates an accumulation of bone marrow cell debris and the bone trabeculae showing empty lacunae (stain, hematoxylin and eosin; original magnification, ×100).
The average sizes of the bone marrow fat cells were smaller (p = 0.002) in the PS group (56.6 ± 10 μm) than in the CTR group (60 ± 4 μm). In the CTR group, the average size of bone marrow fat cells was larger (p = 0.0001) in rabbits with early histological alterations (61.2 ± 2.7 μm) than in those (56.5 ± 2.5 μm).
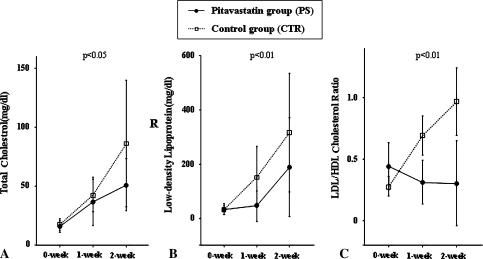
The levels of TC in the PS group were lower (p = 0.001) than those in the CTR group throughout the experimental period (Fig. 3A). The LDL cholesterol levels in the PS group remained at lower levels (p < 0.0001) than those in the CTR group throughout the experimental period (Fig. 3B). The average of the plasma LDL:HDL cholesterol ratio across the experimental period was lower (p < 0.0001) in the PS group than in the CTR group, although the plasma LDL:HDL cholesterol ratio was lower (p = 0.0002) in the CTR group than in the PS group at week 0 when the steroid had just been injected (Fig. 3C). However, the plasma lipid levels (VLDL, TG) did not differ between the two groups.
Fig. 3A–C.
Levels of total cholesterol (TC) and LDL and the ratio of LDL cholesterol to HDL cholesterol in the control and pitavastatin groups are shown. The timing just before the MPSL injection is indicated as 0-week. (A) The TC levels in the pitavastatin group were lower (p = 0.001) than those in the control group throughout the experimental period. (B) The levels of LDL cholesterol in the pitavastatin group remained lower (p < 0.0001) than those in the control group throughout the experimental period. (C) The pitavastatin group exhibited a lower (p < 0.0001) than average plasma LDL:HDL cholesterol ratio across the experimental period in comparison to the control group.
Discussion
Several animal and human studies suggest pharmacological approaches may prevent steroid-induced osteonecrosis (ON). We therefore tested the hypothesis that pitavastatin would prevent the development of steroid-induced ON in rabbits.
The major limitation of this study is that the duration of steroid treatment may be too short to confirm a diagnosis of ON. Previous studies suggest histopathologic occur 2–20 weeks after steroid administration [5, 8–12, 17, 18, 20, 31, 32, 33]. In this study, we examined the histological changes at 2 weeks, before any collapse and confirmation of ON could be observed. We presume our therefore represent the early changes of ON.
We measured only serum markers of adipogenesis, total cholesterol, low-density lipoprotein, very-low-density lipoprotein, and triglyceride, but we were not able to detect the direct mechanism of pitavastatin for the prevention of steroid-induced ON. It has been reported statins have favorable effects on the progression of atherosclerosis and plaque instability, independent of their lipid-lowering activity [16]. These pleiotropic effects of statins also include improvement of the endothelial function, antithrombotic actions, plaque stabilization, reduction of the vascular inflammatory process, and antioxidant function [28, 29]. Furthermore, in a recent study, statin was reported to inhibit both adipogenic and stimulated osteogenic differentiation [15]. These various effects of statin seem to have play an important role in the prevention of steroid-induced ON.
3-hydroxymethyl-3-glutaryl-CoA (HMG-CoA) reductase inhibitors (statins) are widely used for the treatment of hyperlipidemia as well as for the prevention of coronary artery disease [25, 26]. We selected pitavastatin among the various statins because pitavastatin has a stronger effect on LDL-cholesterol reduction than any of the other new statins, such as pravastatin, simvastatin, or atorvastatin [7, 24]. Another reason why pitavastatin was selected in this study is that the metabolism of pitavastatin by the cytochrome P450 (CYP) system is minimal, principally through CYP2C9, with little involvement of the CYP3A4 isoenzyme. Other new statins, simvastatin, lovastatin, atorvastatin, and cerivastatin are inhibitors of the CYP3A4 isoenzyme [21]. Therefore, the risk of a drug-drug interaction between statin and a steroid that is metabolized by the CYP 3A4 could be reduced by using pitavastatin.
Wang et al. [30] suggested lovastatin prevented steroid-induced osteonecrosis using a chicken model. We used a single high dose (20 mg/kg) which was higher than that used in several previous studies [4, 9, 30]. Abeles et al. [1] reported the high initial corticosteroid dosage in patients with systemic lupus erythematosus might induce ON of the femoral head.
Dexamethasone reportedly stimulates the differentiation of bone marrow stromal cells into adipocytes as well as the accumulation of fat in the marrow at the expense of expression of Type-1 collagen and osteocalcin mRNA [2]. This mechanism may explain steroid-induced hypertrophy and hyperplasia of fat cells in the bone marrow. On the other hand, a high LDL:HDL cholesterol ratio apparently reflects prominent lipid transport to the peripheral tissue, a potential risk factor for corticosteroid-induced osteonecrosis in rabbits [3, 17]. We observed a decrease in the LDL:HDL cholesterol ratio. In addition, pitavastatin reduced the size of the bone marrow fat cells. We therefore speculate the inhibitory effects of pitavastatin on the development of ON may be partly explained by the decrease in lipid deposition in the bone marrow fat cells.
Recent animal experimental studies suggest a combination treatment with warfarin plus probucol prevents the development of ON in steroid-treated rabbits [20]. In addition, Ichiseki et al. [9] reported oxidative stress plays a crucial role in the development of steroid-induced ON. These studies suggested steroid-induced ON has a multifactorial pathogenesis including hyperlipidemia, coagulation abnormalities, and oxidative stress. In a recent study, statins preserve endothelial integrity, reduce ischemia/reperfusion injury, and depress the interdependent inflammatory and coagulation cascades via pleiotropic properties [28]. We thus suppose that not only the LDL-lowering effects of pitavastatin but also these nonlipid effects contributed to the prevention of ON by reducing the formation of the thrombi and lipid emboli in the blood vessels.
Our preliminary data in this rabbit model suggest pitavastatin, a new HMG-CoA reductase inhibitor, may be useful to prevent steroid-induced ON.
Acknowledgments
We thank Naoko Kinukawa, Department of Medical Informatics, Kyushu University, Fukuoka, Japan, for her help and advice on the statistical analysis.
Footnotes
One of more of the authors (TY) received funding from a Grant-in-Aid in Scientific Research (No.18591665) from the Japan Society for the Promotion of Science.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abeles M, Urman JD, Rothfield NF. Aseptic necrosis of bone in systemic lupus erythematosus. Relationship to corticosteroid therapy. Arch Intern Med. 1978;138:750–754. [DOI] [PubMed]
- 2.Cui Q, Wang GJ, Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J Bone Joint Surg Am. 1997;79:1054–1063. [DOI] [PubMed]
- 3.Dobiasova M, Frohlich J. Understanding the mechanism of LCAT reaction may help to explain the high predictive value of LDL/HDL cholesterol ratio. Physiol Res. 1998;47:387–397. [PubMed]
- 4.Fisher DE. The role of fat embolism in the etiology of corticosteroid-induced avascular necrosis: clinical and experimental results. Clin Orthop Relat Res. 1978;130:68–80. [PubMed]
- 5.Gold EW, Fox OD, Weissfeld S, Curtiss PH. Corticosteroid-induced avascular necrosis: an experimental study in rabbits. Clin Orthop Relat Res. 1978;135:272–280. [PubMed]
- 6.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. [DOI] [PubMed]
- 7.Hayashi T, Rani P JA, Fukatsu A, Matsui-Hirai H, Osawa M, Miyazaki A, Tsunekawa T, Kano-Hayashi H, Iguchi A, Sumi D, Ignarro LJ. A new HMG-CoA reductase inhibitor, pitavastatin remarkably retards the progression of high cholesterol induced atherosclerosis in rabbits. Atherosclerosis. 2004;176:255–263. [DOI] [PubMed]
- 8.Ichiseki T, Kaneuji A, Katsuda S, Ueda Y, Sugimori T, Matsumoto T. DNA oxidation injury in bone early after steroid administration is involved in the pathogenesis of steroid-induced osteonecrosis. Rheumatology (Oxford). 2005;44:456–460. [DOI] [PubMed]
- 9.Ichiseki T, Matsumoto T, Nishino M, Kaneuji A, Katsuda S. Oxidative stress and vascular permeability in steroid-induced osteonecrosis model. J Orthop Sci. 2004;9:509–515. [DOI] [PubMed]
- 10.Irisa T, Yamamoto T, Miyanishi K, Yamashita A, Iwamoto Y, Sugioka Y, Sueishi K. Osteonecrosis induced by a single administration of low-dose lipopolysaccharide in rabbits. Bone. 2001;28:641–649. [DOI] [PubMed]
- 11.Jones JP Jr. Intravascular coagulation and osteonecrosis. Clin Orthop Relat Res. 1992;277:41–53. [PubMed]
- 12.Kawai K, Tamaki A, Hirohata K. Steroid-induced accumulation of lipid in the osteocytes of the rabbit femoral head. A histochemical and electron microscopic study. J Bone Joint Surg Am. 1985 Jun;67:755–763. [PubMed]
- 13.Koo KH, Kim R. Quantifying the extent of osteonecrosis of the femoral head. A new method using MRI. J Bone Joint Surg Br. 1995;77:875–880. [PubMed]
- 14.Landmann J, Renner N, Gächter A, Thiel G, Harder F. Cyclosporin A and osteonecrosis of the femoral head. J Bone Joint Surg Am. 1987;69:1226–1228. [PubMed]
- 15.Li X, Cui Q, Kao C, Wang GJ, Balian G. Lovastatin inhibits adipogenic and stimulates osteogenic differentiation by suppressing PPARgamma2 and increasing Cbfa1/Runx2 expression in bone marrow mesenchymal cell cultures. Bone. 2003;33:652–659. [DOI] [PubMed]
- 16.Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest. 2002;110:285–288. [DOI] [PMC free article] [PubMed]
- 17.Miyanishi K, Yamamoto T, Irisa T, Yamashita A, Jingushi S, Noguchi Y, Iwamoto Y. A high low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a potential risk factor for corticosteroid-induced osteonecrosis in rabbits. Rheumatology (Oxford). 2001;40:196–201. [DOI] [PubMed]
- 18.Miyanishi K, Yamamoto T, Irisa T, Yamashita A, Jingushi S, Noguchi Y, Iwamoto Y. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002;30:185–190. [DOI] [PubMed]
- 19.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–474. [DOI] [PubMed]
- 20.Motomura G, Yamamoto T, Miyanishi K, Jingushi S, Iwamoto Y. Combined effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits. Arthritis Rheum. 2004;50:3387–3391. [DOI] [PubMed]
- 21.Mukhtar RY, Reid J, Reckless JP. Pitavastatin. Int J Clin Pract. 2005;59:239–252. [DOI] [PubMed]
- 22.Ohzono K, Saito M, Takaoka K, Ono K, Saito S, Nishina T, Kadowaki T. Natural history of nontraumatic avascular necrosis of the femoral head. J Bone Joint Surg Br. 1991;73:68–72. [DOI] [PubMed]
- 23.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001;386:173–178. [DOI] [PubMed]
- 24.Saito Y, Yamada N, Teramoto T, Itakura H, Hata Y, Nakaya N, Mabuchi H, Tushima M, Sasaki J, Goto Y, Ogawa N. Clinical efficacy of pitavastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, in patients with hyperlipidemia. Dose-finding study using the double-blind, three-group parallel comparison. Arzneimittelforschung. 2002;52:251–255. [DOI] [PubMed]
- 25.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [DOI] [PubMed]
- 26.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, Macfarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. [DOI] [PubMed]
- 27.Shimizu K, Moriya H, Akita T, Sakamoto M, Suguro T. Prediction of collapse with magnetic resonance imaging of avascular necrosis of the femoral head. J Bone Joint Surg Am. 1994;76:215–223. [DOI] [PubMed]
- 28.Sowers J. Effects of statins on the vasculature: implications for aggressive lipid management in the cardiovascular metabolic syndrome. Am J Cardiol. 2003;91:14–22. [DOI] [PubMed]
- 29.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Atheroscler Thromb Vasc Biol. 2001;21:1712–1719. [DOI] [PubMed]
- 30.Wang GJ, Cui Q, Balian G. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000;370:295–310. [DOI] [PubMed]
- 31.Wang GJ, Sweet DE, Reger SI, Thompson RC. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am. 1977;59:729–735. [PubMed]
- 32.Yamamoto T, Hirano K, Tsutsui H, Sugioka Y, Sueishi K. Corticosteroid enhances the experimental induction of osteonecrosis in rabbits with Shwartzman reaction. Clin Orthop Relat Res. 1995;316:235–243. [PubMed]
- 33.Yamamoto T, Irisa T, Sugioka Y, Sueishi K. Effects of pulse methylprednisolone on bone and marrow tissues: corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum. 1997;40:2055–2064. [DOI] [PubMed]