Abstract
Alcohol can induce adipogenesis by bone marrow stromal cells and may cause osteonecrosis of the femoral head. Currently, there are no medications available to prevent alcohol-induced osteonecrosis. We hypothesized puerarin, a Chinese herbal medicine with antioxidative and antithrombotic effects, can prevent alcohol-induced adipogenesis and osteonecrosis. Both bone marrow stromal cells (in vitro) and mice (in vivo) were treated either with ethanol or with ethanol and puerarin, with an untreated group serving as a control. In the in vitro study, the number of adipocytes, contents of triglycerides, and levels of PPARγ mRNA expression were decreased and alkaline phosphatase activity, contents of osteocalcin, and levels of osteocalcin mRNA expression were increased in cells treated with both alcohol and puerarin, compared with cells treated with alcohol only. In the in vivo study, marrow necrosis, fat cell hypertrophy and proliferation, thinner and sparse trabeculae, diminished hematopoiesis, and increased empty osteocyte lacunae in the subchondral region of the femoral head were observed in mice treated with alcohol. However, no such changes were seen in femoral heads of mice treated with alcohol and puerarin. The data suggest puerarin can inhibit adipogenic differentiation by bone marrow stromal cells both in vitro and in vivo and prevents alcohol-induced osteonecrosis in this model.
Introduction
Approximately one-third of patients with nontraumatic osteonecrosis (ON) have associated alcohol abuse [1, 5, 16–19, 36, 45]. Animal studies suggest alcohol can cause hypertrophy and proliferation of fat cells, fatty degeneration of osteocytes, and thinner and sparse trabeculae [20]. Several reports suggest primary marrow mesenchymal stromal cells (MSCs) and cloned bone marrow stem cells treated with alcohol resulted in adipogenic differentiation and decreased levels of alkaline phosphatase (ALP) and osteocalcin [10, 20, 28]. Adipose-specific gene 422(aP2) expression is enhanced and osteogenic gene Type I collagen expression decreased in MSCs exposed to alcohol [28]. These findings suggest the mechanism of alcohol-induced ON may be related to alcohol directly inducing adipogenesis and inhibiting osteogenesis in MSCs.
For thousands of years the traditional Chinese herbal medicine pueraria has been used to treat alcoholism [2, 25, 26, 32, 38, 41]. Recently, it was found puerarin, extracted from pueraria, is antioxidative, antithrombotic, and decreases cell injuries secondary to lipid peroxidation by protecting the stability of the cell membrane [4, 6, 7, 11, 12, 53, 55]. The findings indicate puerarin may have the potential to prevent alcohol-induced ON.
We therefore hypothesized puerarin inhibits alcohol-induced adipocytic differentiation of MSCs and fatty degeneration of osteocytes and may be able to prevent alcohol-induced osteonecrosis. We further hypothesized puerarin would reduce expression of PPARγ, a transcription factor important in adipogenesis.
Materials and Methods
In order to test our hypotheses, we conducted both in vitro and in vivo studies. Bone marrow stromal cells and mice were treated either with alcohol only or with both alcohol and puerarin, or received no treatment to serve as control. Preventive effects of puerarin on alcohol-induced adipogenesis and osteonecrosis were analyzed by examining the morphologic changes, specific gene expression, serology, and histology.
We obtained bone marrow cells from the midshafts of 6- to 8-week-old male and female mice femurs and plated at a density of 1.5 × 106 cells/cm2. The cells were maintained in Dulbecco’s modified Eagle medium (Gibco BRL, Gaithersburg, MD) containing 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), 50 mg/mL sodium ascorbate, and antibiotics (100 U/mL penicillin G and 100 mg/mL streptomycin) in a humidified atmosphere of 5% CO2 at 37° C. Medium was added to the wells at the same time as the cells were seeded, and was first changed after 72 hours, then every 48 hours thereafter. At the same time when cells were seeded, we established three groups: (1) cells treated with 0.09 mol/L ethanol, (2) cells treated with 0.09 mol/L ethanol and 0.01 mg/mL puerarin, and (3) cells without treatment as controls. Four replicates of each group were obtained.
After 21 days of culture, cells of each group were fixed in 75% ethanol for 8 minutes and stained with Sudan III for 30 minutes at 60° C and counterstained with hematoxylin. The number of adipocytes in 100-mm2 on each well in 24-well plates was counted under a microscope (Leitz, Allendale, NJ, USA) equipped with a color video camera and frame grabber. The average number of adipocytes in 24-well plates was calculated.
We assayed triglycerides using a kit (Sino-America, Luoyang, China). Confluent cells cultured for 21 days in each group were removed mechanically into 2 mL phosphate-buffered saline. The cells were lysed by freezing and thawing repeatedly and centrifuged at 700 × g for 10 minutes by Sorvall RMC 14 (Kendro, Newtown, CT, USA). The supernatants were used for the assay. A solution of chloroform and methanol (2:1, volume per volume) was mixed with the supernatants, placed at 37° C for 5 minutes, and then the chloroform-methanol phase was removed. Using the kit, the levels of the triglycerides were determined with a Hitachi 7150 Biochemistry analyzer (Hitachi, Tokyo, Japan).
Alkaline phosphatase activity was determined using a kit for ALP (Changzheng Technological Inc, Shanghai, China). Confluent cells cultured for 12 days in each group were suspended mechanically in 1 mL phosphate-buffered saline for each well. The cells were lysed by freezing and thawing repeatedly and centrifuged at 700 × g for 10 minutes by Sorvall RMC 14 (Kendro, Newtown, CT). The supernatants were used for the assay. Absorption was measured at 410 nm on a spectrophotometer (Spectronic Instruments, Rochester, NY). The values were standardized by determining the total protein in the cell layers with the use of a Coomassie brilliant blue method. Culture media from cells cultured for 14 days in each group were collected and used to determine the levels of osteocalcin by radioimmunoassay.
We randomly divided 216 4-week-old Kunming mice (experimental animal center, Henan Province) into three groups: (1) model group: mice received spirits (20 mL/kg body weight) containing 46% ethanol intragastrically and normal saline (10 mL/kg) by intramuscular injection; (2) experimental group: received spirits (20 mL/kg body weight) containing 46% ethanol intragastrically and puerarin (0.5 g/kg body weight) by intramuscular injection; and (3) control group: received water (20 mL/kg) intragastrically and normal saline (10 mL/kg) by intramuscular injection daily. In addition, the animals were free to receive food and water at all times. Fifteen animals from each group were sacrificed using overdose anesthesia 4, 6, 8, and 10 months after treatment and specimens were processed for histology, and additional 12 animals were used at 10 months to detect gene expression. The study protocol was approved by the Animal Review Board of the University.
We collected blood samples after a 12-hour fasting period 4, 6, 8, and 10 months after treatment. Serum levels of total cholesterol (CHO), triglyceride (TG), and alkaline phosphatase activity (ALP) were determined by a method of biochemical assay using kits (Sino-America, Luoyang, China).
Liver specimens harvested from the right lobe each measuring about 5 × 5 × 3 mm were processed for frozen sectioning, and 5-μm sections were cut, stained with hematoxylin and eosin (HE) or Sudan III (Sigma Chemical, St. Louis, MO), and examined by light microscopy. Femoral head specimens were cut symmetrically along the coronal plane into two parts. Half of the specimens were fixed in 10% formalin for 24 hours and then decalcified in 10% ethylenediaminetetraacetic acid (EDTA) in Tris-HCl buffer. The tissues were embedded in paraffin. Five-μm sections were cut and stained with hematoxylin and eosin. The remaining specimens were sent for frozen section and stained with Sudan III. We examined five sections from each animal using a modification of the method described by Warner et al. [51]. Briefly, five fields within the zone of the subchondral area of the femoral head on each section were chosen. The first field was located at the approximate center of the subchondral bone at the weight-bearing area, and the remaining four fields were located at both sides of the first field with two fields at each side. The mean of the five fields from each section was determined to represent that section. The mean of the five sections from each animal was taken as the value for that animal. The following parameters were assessed: (1) 200 osteocyte lacunae in each established field were counted under a light microscope at ×200 magnification, and then the percentage of the empty osteocyte lacunae was determined; and (2) the average diameter of the largest fat cell was measured in each field using an ocular micrometer under the light microscope at ×200 magnification [49], and the average diameter for fat cells in each animal was determined.
After MSCs of each group were treated 6 days in vitro or animals of each group for 6 months in vivo, using β-actin as endocontrol, the expression levels of PPARγ mRNA and osteocalcin mRNA were analyzed by reverse transcription polymerase chain reaction. Bone marrow cells from all three groups were maintained in media for 10 days, treated 6 days with 0.09 mol/L ethanol, 0.09 mol/L ethanol and 0.01 mg/ml puerarin, or neither. The cells were digested with solution of 0.05% trypsin/0.02% EDTA, transferred into a centrifuge tube, and centrifuged at 1000 rpm for 10 minutes. The supernatants were removed. The remaining cells were lysed. Total RNA was isolated from cells using a Flash UNIQ-10 Spin Column Total RNA isolation kit (Sino-America, Luoyang, China). Animals from each group were sacrificed at 6 months after treatment. The femoral head specimens were placed into 1 mL TRIzol solution, ground completely, and rested for 5 minutes. Total RNA was isolated from ground femoral head using TRIzol methods. The expression levels of adipogenic transcription factor PPARγ (peroxisome proliferator-activated receptor-γ) mRNA and osteocalcin mRNA in cells and in animals were examined by reverse transcription polymerase chain reaction. The PPARγ and osteocalcin primer were designed by the biomolecular research center of University of Virginia. The β-actin primer was designed by Jikang Biotechnology Ltd. Co. of Shanghai. All primers were synthesized by Dingan Biotechnology Ltd. Co. of Shanghai. The sequences were:
PPARγ:
Forward primer-5′CTGGCCTCCCTGATGAATAA3′
Reverse primer-5′GGCGGTCTCCACTGAGAATA3′
Osteocalcin:
Forward primer-5′GAGCAGAGCTCCCTGAACTG3′
Reverse primer-5′GGTCGCCCTAGAGACAAGAA3′
β-actin:
Lower-5′CGACCAGAGGCATACAGG3′
Upper-5′GGTGTGATGGTGGGAATG3′
Products of β-actin, PPARγ and osteocalcin were 408 bp, 205 bp, and 200 bp oligonucleotides respectively. Reverse transcription of extracted RNA was performed to synthesize cDNA. The synthesized cDNA was subsequently amplified by the polymerase chain reaction (PCR) with specific primers. PCR reaction products were obtained by electrophoresis using 1.2% agarose gel. Semiquantitative analysis of PCR products was performed using a gel imaging scanning system. The ratios of osteocalcin or PPARγ absorbance value to β-actin absorbance value were viewed as absolute value of osteocalcin or PPARγ product respectively.
The estimate of sample size was based on being able to detect at Month 10 a significant increase in largest fat cell size, number of empty osteocyte lacunae, and serum chemistry parameters in animals treated with alcohol only compared to mice that received both alcohol and puerarin and to mice that received no treatments. In computing sample size we assumed that each outcome represented a continuous random variable, taking on a value between 0 and 100%. We assumed the outcomes of study groups were distributed normally with means, and had a common standard deviation (σ) of 9.49. In our computations we also assumed a Type I error rate (α) of 0.05, and power (1-β) of 0.80 to detect a 30% change in the parameters. Based on these specifications we estimated that a total of 216 mice were required with an estimated drop of rate of 10%, with mice being randomly assigned in equal number to the three study groups. Data are presented as mean ± standard deviation (SD). Means of cholesterol, triglyceride, ALP, largest fat cell size, and gene expression were compared using one-way ANOVA, followed by the SNK multiple comparison procedure. Rates of empty osteocyte lacunae were compared using the multisample Kruskal-Wallis test.
Results
In Vitro Study
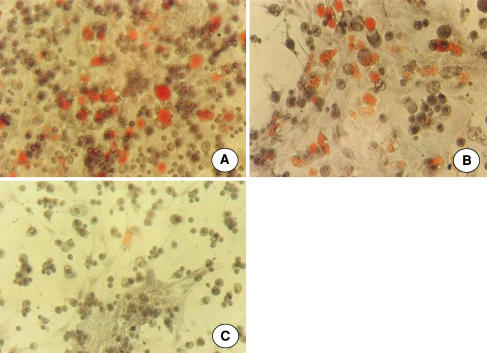
Puerarin inhibited adipogenesis while maintaining osteogenesis. In MSCs treated with alcohol for 2 weeks, cytoplasmic lipid droplets were observed under the inverted phase-contrast microscope. The size and number of fatty droplets increased with longer duration of culture. Few fatty drops were observed in the experimental and control group. At Day 21, staining with Sudan III showed a number of adipocytes in the MSCs of the model group were filled with reddish-orange lipid droplets (Fig. 1A), while fewer triglyceride vesicles appeared in the MSCs of the experimental group (Fig. 1B) and the control group (Fig. 1C). Adipocytes in the model group were 8.9-times (p < 0.001) and 15.6-times (p < 0.001) of that in the experimental group and control group respectively. The levels of triglycerides in the experimental group were decreased (p < 0.001) compared to the model group. As compared to the model group, the levels of ALP in the experimental group and control group were increased (p < 0.001). The levels of osteocalcin in the experimental group and control group were 2.2- and 5.29- times of that in the model group respectively (p < 0.001). No differences (p > 0.05) were seen between the experimental and control groups (Table 1).
Fig. 1A–C.
A number of reddish-orange triglyceride vesicles in the bone marrow stromal cells of (A) the model group treated with alcohol only for 21 days, while fewer triglyceride vesicles appear in the MSCs of (B) the experimental group treated with both alcohol and puerarin and in (C) the control group (Sudan III stain, original magnification, ×250).
Table 1.
Changes of triglyceride, ALP activity, and osteocalcin in MSCs of mice
| Groups | Adipocytes | Triglyceride (μg/well) | ALP activity (U/100 mg protein) | Osteocalcin (U/100 mg protein) |
|---|---|---|---|---|
| Model | 319.17 ± 19.92 | 11.55 ± 4.42 | 29.02 ± 13.37 | 4.95 ± 2.31 |
| Experimental | 335.92 ± 23.77 | 4.15 ± 1.92 | 57.06 ± 17.73 | 11.11 ± 4.57 |
| Control | 20.42 ± 12.15 | 3.42 ± 1.60 | 67.08 ± 18.64 | 13.43 ± 5.29 |
MSCs = marrow mesenchymal stromal cells. Analysis of variance: p < 0.001, model versus experimental or control; p > 0.05, experimental versus control. Model: cells treated with alcohol only. Experimental: cells treated with both alcohol and puerarin. Control: no treatment.
In Vivo Study
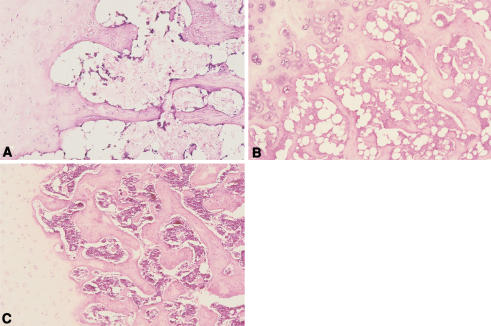
Similar to the in vitro results, puerarin inhibited adipogenesis while maintaining a relative normal level of lipid metabolism and osteogenesis, and thus prevented osteonecrosis. After 6 months of treatment, levels of total cholesterol in serum in mice treated with alcohol was increased (p < 0.01) as well as the triglyceride level (p < 0.001) compared to the experimental and control groups. In contrast, ALP values were decreased (p < 0.01) (Table 2). Fatty livers were found in animals that were treated with alcohol but not seen in the experimental or control group mice. Marrow and bone necrosis, diminished hematopoiesis, increased fat, and an increased percentage of empty osteocyte lacunae occurred in the subchondral region of the femoral head 6 months after treatment with alcohol (Fig. 2A–C). But no such changes were observed in the experimental group. While the percentage of empty osteocyte lacunae increased (p < 0.05) in animals treated with alcohol, the percentage of empty osteocyte lacunae in animals treated with both alcohol and puerarin did not increase (p > 0.05) compared to the control group (Table 3). The average diameter of the largest fat cells in the model group was increased (p < 0.05) compared to the other two groups (Table 4).
Table 2.
Changes of cholesterol, triglyceride, and ALP activity levels in serum of mice 6 months after treatment
| Groups | Cholesterol (mmol/L) | Triglyceride (mmol/L) | ALP activity (International Units) |
|---|---|---|---|
| Model | 6.39 ± 0.49c | 1.01 ± 0.15c | 161.6 ± 32.44b |
| Experimental | 3.19 ± 0.11a | 0.71 ± 0.13a | 196.5 ± 31.52a |
| Control | 2.83 ± 0.36 | 0.68 ± 0.22 | 203.4 ± 22.83 |
ALP = alkaline phosphatase. Analysis of variance = a: p > 0.05, b: p < 0.05, c: p < 0.01. Model: animals treated with alcohol only. Experimental: animals treated with both alcohol and puerarin. Control: no treatment.
Fig. 2A–C.
Fat cell hypertrophy, empty osteocyte lacunae, bone and marrow necrosis in subchondral area of the femoral head noticed in (A) the model group treated with alcohol only, while less adipocytes and no marrow necrosis were found in (B) the experimental group treated with both alcohol and puerarin, compared to normal in (C) the control group (original magnification ×100).
Table 3.
Changes of empty osteocyte lacuna in the femoral heads of mice
| Time (months) | Empty osteocyte lacuna (%) | ||
|---|---|---|---|
| Model group | Experimental group | Control group | |
| 4 | 11.2 ± 3.2a | 10.5 ± 1.8a | 10.8 ± 2.3 |
| 6 | 13.5 ± 1.6b | 9.8 ± 2.2a | 10.3 ± 2.7 |
| 8 | 15.8 ± 3.4c | 10.7 ± 3.1a | 11.1 ± 2.9 |
| 10 | 19.5 ± 4.1c | 11.6 ± 3.1a | 12.0 ± 3.1 |
Analysis of variance = a: p > 0.05, b: p < 0.05, c: p < 0.01.
Table 4.
Changes of largest fat cell diameter in the femoral heads of mice
| Time (months) | Largest fat cell diameter (μm) | ||
|---|---|---|---|
| Model group | Experimental group | Control group | |
| 4 | 38.69 ± 4.14a | 38.89 ± 2.25a | 38.65 ± 3.26 |
| 6 | 40.02 ± 3.25b | 39.15 ± 3.67a | 38.51 ± 3.09 |
| 8 | 42.67 ± 2.66c | 38.76 ± 3.41a | 39.12 ± 2.85 |
| 10 | 45.38 ± 3.02c | 39.61 ± 3.94a | 40.13 ± 2.63 |
Analysis of variance = a: p > 0.05, b: p < 0.05, c: p < 0.01.
Gene Expression
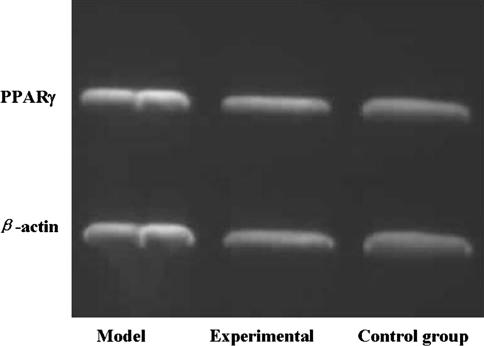
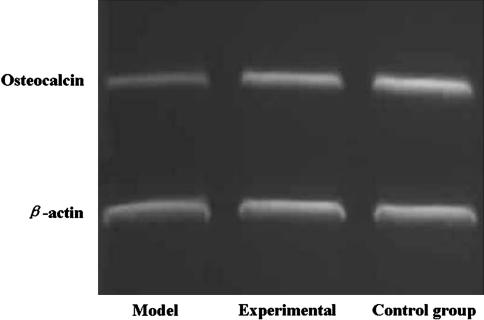
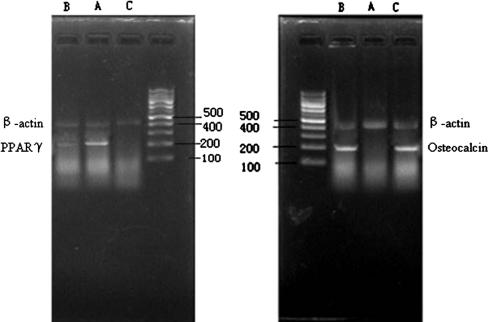
The expression of PPARγ mRNA in the cells or animals of both the experimental group and control group was lower (p < 0.05) than that in the cells or animals of model group, and there was no difference (p > 0.05) between the experimental and control groups. The expression of osteocalcin mRNA in the cells or animals of both the experimental group and control group was higher (p < 0.01) than that in the cells or animals of the model groups, and there were no differences (p > 0.05) between the experimental and control groups (Figs. 3, 4, 5).
Fig. 3.
Expression of PPARγ mRNA in the cells of both the experimental group and control group was lower than that in the cells of the model group, and there was no difference between the experimental and control group.
Fig. 4.
Expression of osteocalcin mRNA in the cells of both experimental group and control group was higher (p < 0.01) than of the model group, and there was no difference (p > 0.05) between the experimental and control group.
Fig. 5.
Electrophoresis of PPARγ and osteocalcin mRNA in the femoral head of mice shows expression of PPARγ mRNA in the tissue of both (B) the experimental group and (C) the control group was lower (p < 0.05) than that in the tissue of (A) the model group. Expression of osteocalcin mRNA in the tissue of both the experimental group and control group was higher (p < 0.01) than the model group, and there was no difference (p > 0.05) between the experimental and control group.
Discussion
Although a number of studies document alcoholism in 10% to 74% of patients with nontraumatic ON of the femoral head, the mechanisms of alcohol-induced ON remain unknown and presently there is no effective treatment for the disease [1, 5, 16–19, 21, 23, 36, 45, 47]. Hypertrophy and proliferation of fat cells, diminished hematopoiesis, lipid deposition in osteocytes, fatty degeneration of osteocytes, marrow necrosis, and thinner and sparse trabeculae are histopathologic changes occurring in the early stages of steroid- and alcohol-induced ON of the femoral head [9, 19, 22, 23, 36, 47, 50, 57]. Several hypotheses have been proposed in the literature, including the increased size and number of fatty cells, increased intraosseous pressure, fatty degeneration of osteocytes, fat embolism, and extraosseous arterial occlusion due to abnormal changes in histologic features, hemodynamics, metabolism, and biochemical features within the femoral head [1, 10, 17, 23, 24, 35, 39, 48]. Studies on primary and cloned MSCs have demonstrated differentiation into a large number of adipocytes increased while levels of ALP activity and osteocalcin decreased with longer durations of exposure and with higher concentrations of steroid or ethanol [8, 10, 28, 56]. These findings indicated steroids or alcohol can directly induce adipogenesis and reduce osteogenesis in bone marrow stroma, and produce intracellular lipid deposits leading to death of osteocytes, which may be associated with the development of ON [20, 23, 27, 44], especially in patients with long-term and excessive use of steroids or alcohol. Puerarin, a Chinese herbal medicine, has antioxidative and antithrombotic effects. We therefore hypothesized puerarin inhibits alcohol-induced adipocytic differentiation of MSCs and fatty degeneration of osteocytes and may be able to prevent alcohol-induced osteonecrosis. We further hypothesized puerarin would reduce expression of PPARγ, a transcription factor important in adipogenesis.
A major limitation of the study is that while marrow adipocyte proliferation and necrosis were observed, no femoral head collapse was noted during the study. These pathologic changes simulate early stage human form of ON but do not reflect the late stage of ON. Therefore, even if the treatment is effective in this animal model, it may not be useful in humans.
At present, there are no effective treatments for early-stage ON. To some extent, this is because the pathogenesis of ON is unknown. However, recent advances in cell and molecular biology have enabled researchers to identify some of the key factors contributing to the development of ON. It is now well known MSCs can differentiate into osteoblasts, adipocytes, chondrocytes, and even myoblasts [3, 40]. There is evidence for a considerable degree of plasticity in the differentiation of these stromal cell lines [14, 52]. Most MSCs can differentiate into osteoblasts and osteocytes, and a few into adipocytes, under normal culture conditions in vitro [8, 20, 56]. Alcohol can induce the differentiation of MSCs into adipocytes and inhibit their osteogenic differentiation, which may be a triggering step causing the onset of ON and the pathogenesis of alcohol-induced ON [10, 20, 28, 50]. Because the differentiation of MSCs into adipocytes might be one of the most important reasons leading to steroid- or alcohol-induced ON, the proper therapy should be directed at inhibiting the differentiation of MSCs into fat cells. Lovastatin prevents steroid-induced adipogenesis and onset of ON [9, 29, 30]. However, few studies are associated with treatment of alcohol-induced ON.
Consistent with previous observations [10, 20, 24, 28, 46, 50], our data suggest alcohol can induce adipogenesis both in vitro and in vivo, a change found in early stage ON. Adipogenic differentiation is a complex process regulated by many factors. PPARγ is closely involved in the induction of fatty differention [29, 33]. It is an adipogenic regulator and belongs to the nucleus hormone receptor subgroup. Its activity is regulated by ligands like most members of nucleus receptor families [33]. PPARγ mRNA appears before activation of many other adipocyte genes in adipogenic differentiation of 3T3-L1 and 3T3-F442A cells [43]. Our study suggests the high level of expression of PPARγ occurred only in model groups treated with alcohol, indicating alcohol may be a factor for these bone marrow cells to change from a primarily osteogenic nature to adipogenic phenotype. Although the regulation of adipogenesis may involve complex mechanisms, the data suggest fat cell hypertrophy and hyperplasia in bone marrow may be a direct result of treatment with alcohol. Other mechanisms may also be involved in the development of alcohol-induced ON. Consumption of alcohol can elevate serum lipid peroxides and reduce superoxide dismutase activity [25, 26]. Lipid peroxidation may cause cytomembrane injury and induce degeneration of arterioles and arteriolosclerosis, which eventually leads to ischemia in the target organ, including the femoral head. Furthermore, the direct cytotoxicity of lipid peroxidation, caused by alcohol and its metabolites, might further insult ischemic osteocytes, resulting in an irreversible state of injury leading to cell death and finally ON. In addition, alcohol consumption produces a fatty liver and alcoholic liver injury [17, 31, 37]. Serum triglyceride and cholesterol levels increase, fat degeneration is induced, and liver cells are injured. These changes, as our data indicate, may produce fatty vesicles in the circulation, leading to embolism and ischemia in the subchondral region of the femoral head.
Puerarin inhibited alcohol-induced osteonecrosis in our preliminary studies. The traditional Chinese medicine pueraria has been used to treat alcoholism for thousands of years. Recently, many observations demonstrate puerarin extracted from pueraria is antioxidative, can prevent injuries to cells due to lipid peroxidation, and can maintain the stability of the cell membrane structure by removing the oxyradicals from tissues and cells [4, 6, 12, 13]. The protective effects of puerarin on cells are also related to calcium ion rivalry and β-receptor blockage [54]. In addition, puerarin can decompose acetaldehyde, suppress alcohol absorption, accelerate metabolism and excretion of alcohol in blood, decrease blood viscosity, and inhibit blood platelet aggregation [7, 15, 34, 42]. Thus, these effects of puerarin can prevent the development of a fatty liver, hyperlipidemia, and capillary vessel wall injuries, and hypercoagulation. Most importantly, the data suggest puerarin can decrease alcohol-induced adipogenic gene expression and thus diminish fat marrow changes, while maintaining the cell’s osteogenic differentiation. The inhibitive effects of puerarin on bone-marrow adipogenesis and its concomitant enhancement of osteogenesis may provide a novel approach to the prevention and treatment of alcohol-induced ON.
Footnotes
Investigation performed at Orthopaedic Institute of Zhengzhou University, Zhengzhou, Henan, China.
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Arlet J. Nontraumatic avascular necrosis of the femoral head. Past, present, and future. Clin Orthop Relat Res. 1992;277:12–21. [PubMed]
- 2.Benlhabib E, Baker JI, Keyler DE, Singh AK. Effects of purified puerarin on voluntary alcohol intake and alcohol withdrawal symptoms in P rats receiving free access to water and alcohol. J Med Food. 2004;7:180–186. [DOI] [PubMed]
- 3.Bianco P, Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–1668. [DOI] [PMC free article] [PubMed]
- 4.Cervellati R, Renzulli C, Guerra MC, Speroni E. Evaluation of antioxidant activity of some natural polyphenolic compounds using the Briggs-Rauscher reaction method. J Agric Food Chem. 2002;50:7504–7509. [DOI] [PubMed]
- 5.Chakkalakal DA. Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res. 2005;29:2077–2090. [DOI] [PubMed]
- 6.Cherdshewasart W, Sutjit W. Correlation of antioxidant activity and major isoflavonoid contents of the phytoestrogen-rich Pueraria mirifica and Pueraria lobata tubers. Phytomedicine. 2007 Sept 21; [Epub ahead of print]. [DOI] [PubMed]
- 7.Choo MK, Park EK, Yoon HK, Kim DH. Antithrombotic and antiallergic activities of daidzein, a metabolite of puerarin and daidzin produced by human intestinal microflora. Biol Pharm Bull. 2002;25:1328–1332. [DOI] [PubMed]
- 8.Cui Q, Wang GJ, Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J Bone Joint Surg Am. 1997;79:1054–1063. [DOI] [PubMed]
- 9.Cui Q, Wang GJ, Su CC, Balian G. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997;344:8–19. [DOI] [PubMed]
- 10.Cui Q, Wang Y, Saleh KJ, Wang GJ, Balian G. Alcohol-induced adipogenesis in a cloned bone-marrow stem cell. J Bone Joint Surg Am. 2006;88 Suppl 3:148–154. [DOI] [PubMed]
- 11.Fan LL, Sun LH, Li J, Yue XH, Yu HX, Wang SY. The protective effect of puerarin against myocardial reperfusion injury. Study on cardiac function. Chin Med J (Engl). 1992;105:11–17. [PubMed]
- 12.Guerra MC, Speroni E, Broccoli M, Cangini M, Pasini P, Minghett A, Crespi-Perellino N, Mirasoli M, Cantelli-Forti G, Paolini M. Comparison between chinese medical herb Pueraria lobata crude extract and its main isoflavone puerarin antioxidant properties and effects on rat liver CYP-catalysed drug metabolism. Life Sci. 2000;67:2997–3006. [DOI] [PubMed]
- 13.Han RM, Tian YX, Becker EM, Andersen ML, Zhang JP, Skibsted LH. Puerarin and conjugate bases as radical scavengers and antioxidants: molecular mechanism and synergism with beta-carotene. J Agric Food Chem. 2007;55:2384–2391. [DOI] [PubMed]
- 14.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. [DOI] [PubMed]
- 15.Heyman GM, Keung WM, Vallee BL. Daidzin decreases ethanol consumption in rats. Alcohol Clin Exp Res. 1996;20:1083–1087. [DOI] [PubMed]
- 16.Hirota Y, Hirohata T, Fukuda K, Mori M, Yanagawa H, Ohno Y, Sugioka Y. Association of alcohol intake, cigarette smoking, and occupational status with the risk of idiopathic osteonecrosis of the femoral head. Am J Epidemiol. 1993;137:530–538. [DOI] [PubMed]
- 17.Hungerford DS, Lennox DW. The importance of increased intraosseous pressure in the development of osteonecrosis of the femoral head: implications for treatment. Orthop Clin North Am. 1985;16:635–654. [PubMed]
- 18.Hungerford DS, Zizic TM. Alcoholism associated ischemic necrosis of the femoral head. Early diagnosis and treatment. Clin Orthop Relat Res. 1978:144–153. [PubMed]
- 19.Jacobs B. Epidemiology of traumatic and nontraumatic osteonecrosis. Clin Orthop Relat Res. 1978;130:51–67. [PubMed]
- 20.Jin WS, Tan YY, Chen YG, Wang Y. Determination of puerarin, daidzin and daidzein in root of Pueraria lobata of different origin by HPLC [in Chinese]. Zhongguo Zhong Yao Za Zhi. 2003;28:49–51. [PubMed]
- 21.Jones JP Jr. Intravascular coagulation and osteonecrosis. Clin Orthop Relat Res. 1992;277:41–53. [PubMed]
- 22.Jones JP Jr. Fat embolism, intravascular coagulation, and osteonecrosis. Clin Orthop Relat Res. 1993;292:294–308. [PubMed]
- 23.Jones JP Jr. Alcoholism, hypercortisonism, fat embolism and osseous avascular necrosis. 1971. Clin Orthop Relat Res. 2001;393:4–12. [DOI] [PubMed]
- 24.Kawai K, Tamaki A, Hirohata K. Steroid-induced accumulation of lipid in the osteocytes of the rabbit femoral head. A histochemical and electron microscopic study. J Bone Joint Surg Am. 1985;67:755–763. [PubMed]
- 25.Keung WM. Biochemical studies of a new class of alcohol dehydrogenase inhibitors from Radix puerariae. Alcohol Clin Exp Res. 1993;17:1254–1260. [DOI] [PubMed]
- 26.Keung WM, Vallee BL. Daidzin and its antidipsotropic analogs inhibit serotonin and dopamine metabolism in isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95:2198–2203. [DOI] [PMC free article] [PubMed]
- 27.Leach RE, Baskies A. Alcoholism and its effect on the human hip. Clin Orthop Relat Res. 1973;90:95–99. [PubMed]
- 28.Li J, Wang Y, Li Y, Xu J, Xiong T. Alcohol induced regulation of adipogenic and osteogenic gene expression of marrow stromal cells. Chin J Orthop. 2003;23:493–495.
- 29.Li X, Cui Q, Kao C, Wang GJ, Balian G. Lovastatin inhibits adipogenic and stimulates osteogenic differentiation by suppressing PPARgamma2 and increasing Cbfa1/Runx2 expression in bone marrow mesenchymal cell cultures. Bone. 2003;33:652–659. [DOI] [PubMed]
- 30.Li X, Jin L, Cui Q, Wang GJ, Balian G. Steroid effects on osteogenesis through mesenchymal cell gene expression. Osteoporos Int. 2005;16:101–108. [DOI] [PubMed]
- 31.Lieber CS, Savolainen M. Ethanol and lipids. Alcohol Clin Exp Res. 1984;8:409–423. [DOI] [PubMed]
- 32.Lin RC, Li TK. Effects of isoflavones on alcohol pharmacokinetics and alcohol-drinking behavior in rats. Am J Clin Nutr. 1998;68:1512S–1515S. [DOI] [PubMed]
- 33.Lowell BB. PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999;99:239–242. [DOI] [PubMed]
- 34.Luo ZR, Zheng B. Effect of Puerarin on platelet activating factors CD63 and CD62P, plasminogen activator inhibitor and C-reactive protein in patients with unstable angia pectoris [in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2001;21:31–33. [PubMed]
- 35.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med. 1992;326:1473–1479. [DOI] [PubMed]
- 36.Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am. 2006;88:1117–1132. [DOI] [PubMed]
- 37.Niemela O, Parkkila S, Pasanen M, Iimuro Y, Bradford B, Thurman RG. Early alcoholic liver injury: formation of protein adducts with acetaldehyde and lipid peroxidation products, and expression of CYP2E1 and CYP3A. Alcohol Clin Exp Res. 1998;22:2118–2124. [DOI] [PubMed]
- 38.Overstreet DH, Lee YW, Rezvani AH, Pei YH, Criswell HE, Janowsky DS. Suppression of alcohol intake after administration of the Chinese herbal medicine, NPI-028, and its derivatives. Alcohol Clin Exp Res. 1996;20:221–227. [DOI] [PubMed]
- 39.Prasain JK, Jones K, Brissie N, Moore R, Wyss JM, Barnes S. Identification of puerarin and its metabolites in rats by liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 2004;52:3708–3712. [DOI] [PubMed]
- 40.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. [DOI] [PubMed]
- 41.Rezvani AH, Overstreet DH, Perfumi M, Massi M. Plant derivatives in the treatment of alcohol dependency. Pharmacol Biochem Behav. 2003;75:593–606. [DOI] [PubMed]
- 42.Shi RL, Zhang JJ. Protective effect of puerarin on vascular endothelial cell apoptosis induced by chemical hypoxia in vitro [in Chinese]. Yao Xue Xue Bao. 2003;38:103–107. [PubMed]
- 43.Smas CM, Sul HS. Control of adipocyte differentiation. Biochem J. 1995;309 (Pt 3):697–710. [DOI] [PMC free article] [PubMed]
- 44.Solomon L. Drug-induced arthropathy and necrosis of the femoral head. J Bone Joint Surg Br. 1973;55:246–261. [PubMed]
- 45.Solomon L. Mechanisms of idiopathic osteonecrosis. Orthop Clin North Am. 1985;16:655–667. [PubMed]
- 46.Suh KT, Kim SW, Roh HL, Youn MS, Jung JS. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2005;431:220–225. [DOI] [PubMed]
- 47.Turner RT, Evans GL, Zhang M, Sibonga JD. Effects of parathyroid hormone on bone formation in a rat model for chronic alcohol abuse. Alcohol Clin Exp Res. 2001;25:667–671. [DOI] [PubMed]
- 48.Wang GJ, Dughman SS, Reger SI, Stamp WG. The effect of core decompression on femoral head blood flow in steroid-induced avascular necrosis of the femoral head. J Bone Joint Surg Am. 1985;67:121–124. [PubMed]
- 49.Wang GJ, Sweet DE, Reger SI, Thompson RC. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am. 1977;59:729–735. [PubMed]
- 50.Wang Y, Mao K, Li Y, Xu Z. Alcohol induced animal model of osteonecrosis of the femoral head. Chin Exper Surg. 1998;15:182–183.
- 51.Warner JJ, Philip JH, Brodsky GL, Thornhill TS. Studies of nontraumatic osteonecrosis. Manometric and histologic studies of the femoral head after chronic steroid treatment: an experimental study in rabbits. Clin Orthop Relat Res. 1987;225:128–140. [PubMed]
- 52.Wolf NS, Penn PE, Rao D, McKee MD. Intraclonal plasticity for bone, smooth muscle, and adipocyte lineages in bone marrow stroma fibroblastoid cells. Exp Cell Res. 2003;290:346–357. [DOI] [PubMed]
- 53.Wu L, Qiao H, Li Y, Li L. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine. 2007;14:652–658. [DOI] [PubMed]
- 54.Xiao L, Luo W, Su H, Cheng X, Wu Q, Ye C. The influence of puerarin on the NO and ACE activity secreted by blood vessel endothelial cells cultured under hyperpressure condition. New J Traditional Chin Med. 2002;42:31–33.
- 55.Xu X, Zhang S, Zhang L, Yan W, Zheng X. The Neuroprotection of puerarin against cerebral ischemia is associated with the prevention of apoptosis in rats. Planta Med. 2005;71:585–591. [DOI] [PubMed]
- 56.Yin L, Li YB, Wang YS. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: mechanism of steroid-induced osteonecrosis. Chin Med J (Engl). 2006;119:581–588. [PubMed]
- 57.Zhang W, Yu S, Zheng L. Experimental study on IL-1 regulating differentiation of osteoblast. Chin J Stomatol. 1996;31:288–291. [PubMed]