Abstract
Major thrombophilic mutations have been identified as risk factors for nontraumatic osteonecrosis of the femoral head (ONFH) in Caucasians. We asked whether the genetic background of patients with ONFH in the Korean population was similar. We analyzed factor V G1691A mutation (factor V Leiden), prothrombin G20210A mutation, and methylenetetrahydrofolate reductase C677T and A1298C polymorphisms in 71 patients (53 men, 18 women) with ONFH. We classified these patients as 51 alcohol-induced, 18 idiopathic, one steroid-induced, and one dysbaric. We recruited 200 normal control subjects (128 men, 72 women). We used multiplex PCR/restriction fragment length polymorphism for each genotyping. We observed neither factor V Leiden nor prothrombin G20210A mutation. Although methylenetetrahydrofolate reductase A1298C genotypes were not associated with osteonecrosis, methylenetetrahydrofolate reductase C677T variant genotypes increased the risk of ONFH compared with 677CC. Odds ratios of 677CT and 677CT+TT were 2.00 (95% confidence interval, 1.05–3.81) and 1.96 (95% confidence interval, 1.07–3.59), respectively, compared with 677CC. Our data suggest methylenetetrahydrofolate reductase C677T polymorphism plays a role in the pathogenesis of osteonecrosis in the Korean population. It also implies the genetic risk profile of ONFH may differ among ethnic populations.
Level of Evidence: Level II, diagnostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteonecrosis of the femoral head (ONFH) is an ischemic injury, which results in necrosis of the subchondral bone, collapse of the femoral head, and degeneration of the hip [28]. The incidence of ONFH in Korea is relatively higher compared with that in other countries [11, 17]. ONFH is one of the most common diseases of the hip in Korea, occupying more than a half of the underlying causes of total hip arthroplasty, whereas it is relatively rare in the United States. Furthermore, the incidence of alcohol-induced ONFH is also higher in Korea [5].
Although ONFH can be caused by various conditions such as trauma, glucocorticoid therapy, alcoholism, storage diseases, and diseases resulting in vasculitis, except for traumatic conditions, the pathogenesis of osseous ischemia is not yet completely understood [3, 4, 28]. Recently, a number of authors have proposed intravascular coagulation as a pathogenetic mechanism of ONFH [10], with resulting interruption of the fine osseous blood supply. Several studies suggest a higher prevalence of coagulation abnormalities in patients with ONFH compared with control subjects [10, 15, 25].
Major genetic mutations related to coagulation abnormalities are factor V G1691A mutation (factor V Leiden), prothrombin G20210A mutation, and 5, 10-methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms [4, 9, 22]. The presence of these genetic variations is associated with a hypercoagulable state, and increases the risk of thromboembolic events. Such phenomena, however, have been observed mainly in Caucasians, and an association between genetic predisposition and thrombotic tendency may differ between ethnic groups [1, 13, 14, 16, 20]. Recent studies suggest these genetic predispositions play a role in the risk of ONFH [26, 27], but it is unclear whether they apply to non-Caucasian populations.
We therefore asked whether there was an association between major thrombophilic mutations (factor V Leiden, prothrombin G20210A mutation, and MTHFR C677T and A1298C polymorphisms) and the risk of ONFH in the Korean population, and whether the risk was related to the presumed etiology.
Materials and Methods
We recruited 271 individuals (181 men, 90 women): 71 consecutive patients with nontraumatic ONFH (53 men, 18 women) and 200 normal control subjects (128 men, 72 women) between September 2005 and November 2006. If the true change in the dependent variables is 0.20 standard deviations per one standard deviation change in the independent variable, this sample size would have approximately 90% power (alpha = 0.05, two-tail). We enrolled healthy individuals without any clinical disorders or history of thrombosis as normal control subjects. Their health status was decided by a physician by routine physical checkup consisting of clinical, radiographic, and laboratory evaluations. Alcohol or medication history was checked by questionnaire and interview, and the presence of pelvic or hip lesions by plain radiograph. The control group was used for estimation of the frequency of factor V Leiden, prothrombin G20210A mutation, and MTHFR C677T and A1298C polymorphisms in the general population. The median ages of patients with ONFH and normal control subjects were 55 years (range, 18–80 years) and 34 years (range, 21–63 years), respectively. We obtained prior approval of our Institutional Review Board, and informed consent on genetic analysis from the subjects at enrollment.
Each patient underwent complete clinical, radiographic and laboratory evaluations. In all the 71 patients (102 hips), the diagnosis of ONFH depended on the combination of clinical symptoms and both plain radiographs and MRI. According to the Association Research Circulation Osseous (ARCO) classification [8], 23 hips were classified as stage II, 34 hips as stage III, and 45 hips as stage IV. In the clinical evaluation, we paid special attention to corticosteroid medication, alcohol abuse, and history of thromboembolic events, and established a presumed etiology for the osteonecrosis in each patient. To be considered as having alcohol-induced osteonecrosis, patients had to have had an estimated regular alcohol consumption of more than 400 mL per week before developing symptoms [5, 12]. To be considered as having corticosteroid-induced osteonecrosis, patients had to have been taking continuous corticosteroid medication of more than 20 mg prednisone per day for a minimum of two months before developing symptoms [9]. We considered patients with no obvious underlying etiology as having idiopathic osteonecrosis. Fifty-one patients had alcohol-induced osteonecrosis, one had steroid-induced, one had dysbaric osteonecrosis, and 18 had idiopathic (Table 1).
Table 1.
Demographic data of patients with osteonecrosis of the femoral head
| Cause | Number of patients | Age* (years, range) | Gender | Involvement | ||
|---|---|---|---|---|---|---|
| Male | Female | Unilateral | Bilateral | |||
| Idiopathic | 18 | 54 (18–76) | 3 | 15 | 12 | 6 |
| Alcohol-induced | 51 | 55 (29–80) | 49 | 2 | 28 | 23 |
| Steroid-induced | 1 | 41 | 0 | 1 | 0 | 1 |
| Dysbarism | 1 | 46 | 1 | 0 | 0 | 1 |
| Total | 71 | 55 (18–80) | 53 | 18 | 40 | 31 |
*Age expressed in median value.
Peripheral blood samples were used for molecular analysis. DNA was extracted using proteinase K treatment followed by phenol-chloroform extraction and ethanol precipitation [21].
For factor V Leiden and prothrombin G20210A mutations, the previously described multiplex polymerase chain reaction (PCR) method was modified [23]. Primers for factor V Leiden were FV1 (5′-tgcccagtgcttaacaagacca-3′) and FV2 (5′-tgttatcacactggtgctaa-3′), and those for prothrombin G20210A mutation were PT1 (5′-tctagaaacagttgcctggc-3′) and PT2 (5′-atagcactgggagcattgaagc-3′). PCR temperature cycling parameters were: 94° C for 15 minutes followed by 40 cycles of 94° C for 30 seconds, 55° C for 30 seconds, and 72° C for 30 seconds. After final extension at 72° C for 10 minutes, two amplification products (345-bp band for factor V Leiden and 267-bp band for prothrombin G20210A) were simultaneously digested with Mnl I and Hind III restriction enzymes. Informative bands for both factor V Leiden (wild type, 272-bp band; heterozygote, 272- and 249-bp bands; mutant type, 249-bp band) and prothrombin G20210A (wild type, 163-bp band; heterozygote, 200- and 163-bp bands; mutant type, 200-bp band) were identified by electrophoresis on a 2.5% agarose gel.
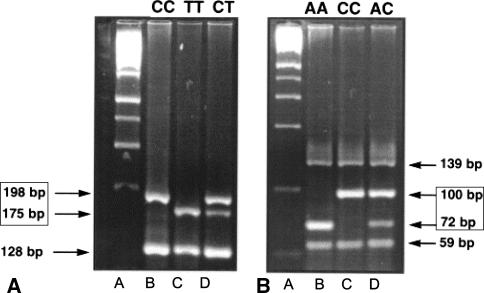
For simultaneous detection of MTHFR C677T and A1298C polymorphisms, another multiplex PCR was performed following the previously described method [24]. Primers for C677T genotyping were 677F (5′-tgaaggagaaggtgtctgcggga-3′) and 677R (5′-aggacggtgcggtgagagtg-3′), and those for A1298C were 1298F (5′-caaggaggagctgctgaaga-3′) and 1298R (5′-ccactccagcatcactcact-3′). After 40 cycles of PCR, amplification products were individually digested with Hinf I and Mbo II restriction enzymes for C677T and A1298C genotypings, respectively. Informative bands for both C677T genotyping (wild type, single 198-bp band; heterozygote, 198- and 175-bp bands; homozygote variant, single 175-bp band) and A1298C genotyping (wild type, single 72-bp band; heterozygote, 72- and 100-bp bands; homozygote variant, single 100-bp band) were identified by electrophoresis on a 4% NuSieve GTG agarose gel (Cambrex BioScience Rockland, Rockland, ME) (Fig. 1).
Fig. 1A–B.
Restriction fragment length polymorphism analysis of MTHFR polymorphisms is shown. (A) Shown are gels for Hinf I digestion for C677T genotyping. Informative bands were 175-bp and 198-bp bands. A, molecular marker; B, CC type; C, TT type; D, CT type. (B) Shown are gels for Mbo II digestion for A1298C genotyping. Informative bands were 72-bp and 100-bp bands. A, molecular marker; B, AA type; C, CC type; D, AC type. MTHFR, methylenetetrahydrofolate reductase.
Two sets of multiplex PCR were conducted in a Peltier Thermal Cycler-200 (MJ Research, Inc, Waltham, MA), and all restriction enzymes were purchased from Promega (Madison, WI).
Odds ratio (OR) as an estimate of relative risk and 95% confidence interval (CI) were calculated using MedCalc software (MedCalc Software, version 9.30; Mariakerke, Belgium). If the value 1 was not in the range of CI, we determined there was an increased relative risk in one group compared with the other.
Results
For MTHFR C677T polymorphism, MTHFR C677T variant genotypes increased the risk of ONFH compared with 677CC genotype. The presence of the 677CT genotype was associated with twofold increase (95% CI, 1.05–3.81) in risk of ONFH compared with the CC genotype. The presence of combined CT and TT genotypes also increased the risk of ONFH (OR, 1.96; 95% CI, 1.07–3.59) compared with the CC genotype. None of the 271 subjects (71 patients with ONFH, 200 normal control subjects) was a carrier of factor V Leiden and prothrombin G20210A mutations. For MTHFR A1298C, the presence of variant genotypes, 1298AC, 1298CC, or both, showed no difference in the risk of ONFH when using 1298AA as a reference (Table 2).
Table 2.
MTHFR polymorphisms and the risk of ONFH
| Genotypes | Number of patients with ONFH (%) | Control subjects (%) (N = 200) | Odds ratio (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Total (N = 71) | Idiopathic (N = 18) | Alcohol-induced (N = 51) | Total versus control subjects | Idiopathic versus control subjects | Alcohol-induced versus control subjects | ||
| MTHFR C677T | |||||||
| CC | 18 (25.4) | 4 (22.2) | 13 (25.5) | 80 (40) | 1* | 1* | 1* |
| CT | 36 (50.7) | 8 (44.4) | 28 (54.9) | 80 (40) | 2.00 (1.05–3.81)† | 2.00 (0.58– 6.91) | 2.15 (1.04–4.46)† |
| TT | 17 (23.9) | 6 (33.3) | 10 (19.6) | 40 (20) | 1.89 (0.88–4.05) | 3.00 (0.80–11.24) | 1.54 (0.62–3.81) |
| CT + TT | 53 (74.6) | 14 (77.8) | 38 (74.5) | 120 (60) | 1.96 (1.07–3.59)† | 2.33 (0.74–7.34) | 1.95 (0.98–3.89) |
| MTHFR A1298C | |||||||
| AA | 49 (69) | 10 (55.6) | 37 (72.5) | 116 (58) | 1* | 1* | 1* |
| AC | 22 (31) | 8 (44.4) | 14 (27.5) | 78 (39) | 0.67 (0.37–1.19) | 1.19 (0.45–3.15) | 0.56 (0.29–1.11) |
| CC | 0 (0) | 0 (0) | 0 (0) | 6 (3) | ND | ND | ND |
| AC + CC | 22 (31) | 8 (44.4) | 14 (27.5) | 84 (42) | 0.62 (0.35–1.10) | 1.10 (0.42–2.92) | 0.53 (0.27–1.03) |
Two patients with steroid therapy (n = 1) and dysbarism (n = 1) were included in total patients with ONFH; *reference category (odds ratio, 1.0); †statistically significant; MTHFR = methylenetetrahydrofolate reductase; ONFH = osteonecrosis of the femoral head; CI = confidence interval; ND = not determined.
When patients were divided into idiopathic (n = 18) and alcohol-induced groups (n = 51), only in the alcohol-induced group was the presence of the 677CT genotype related to the 2.15-fold increase (95% CI, 1.04–4.46) in the risk of ONFH compared with the CC genotype (Table 2).
Discussion
Major thrombophilic mutations are apparent risk factors for nontraumatic osteonecrosis of the femoral head (ONFH) in Caucasians but these have not been confirmed in other populations. We asked whether there was any association between major thrombophilic mutations (Factor V Leiden, prothrombin G20210A mutation and MTHFR polymorphisms) and the occurrence of ONFH in the Korean population. Because current knowledge on the thrombophilic genetic background of ONFH was mainly obtained from the Caucasian populations, we questioned whether any difference exists in other ethnic groups. Our second question was whether the risk was related to the presumed etiology, considering the higher prevalence of an alcohol-induced ONFH in Korea [5].
Our data are, however, limited in that they were obtained from a relatively small study population. In particular, the tendency of increased risk in the idiopathic ONFH group (n = 18) might have been significant with a larger sample size (Table 2). When allele frequencies were analyzed in MTHFR C677T polymorphism, although the presence of T allele tended to increase the risk of ONFH, it did not reach a statistical significance (OR, 1.46; 95% CI, 0.99–2.14). Accordingly, the modest statistical significance of MTHFR C677T variant genotypes with about twofold increased risk of ONFH should be verified in the future large-scale studies. The possibility of underestimation of alcohol abuse in both patients and control subjects may be another limitation, considering the difficulties in detecting and confirming the degree of alcohol consumption.
Although the exact pathophysiology of nontraumatic ONFH is still unclear, an increased tendency for intravascular coagulation has been recently proposed as a pathogenetic mechanism leading to the interruption of the osseous blood supply and osteonecrosis. Several authors propose that if thrombosis occurs, it is followed by a sequential process of obstruction of the venous drainage, progressive rise of venous pressure, impairment of arterial perfusion, and osseous necrosis [9, 10, 28].
Factor V Leiden generates coagulation factor V, which is less effectively degraded by activated protein C resulting in a hypercoagulable state, and has been established as an important and unequivocal risk factor for venous thrombosis [2, 7]. Prothrombin G20210A mutation leads to higher levels of prothrombin, increased generation of thrombin, and thrombophilia. Factor V Leiden and prothrombin G20210A mutations are the most common genetic risk factors predisposing to thrombosis in Caucasians, and their role in the occurrence of ONFH has been also identified in several studies. One study reported that either mutation was present in 22.2% of patients and in 7.3% of control subjects, and their presence was related to osteonecrosis with an OR of 3.6 [28]. Such a higher prevalence of factor V Leiden and prothrombin G20210A mutations in patients with ONFH than in control subjects, however, was observed only in Caucasians, leaving room for further investigation in the other ethnic groups.
We found neither factor V Leiden nor prothrombin G20210A mutation occurred in the study population. This implies factor V Leiden and prothrombin G20210A mutations are not genetic risk factors for ONFH in Asians, or at least in the Korean population. Our finding confirms previous reports, demonstrating the absence of these mutations in the Korean and Chinese populations [16, 18]. It provides more evidence that the genetic risk profile of ONFH, likewise those of the other hypercoagulable diseases including deep vein thrombosis or pulmonary embolism, may be variable in different ethnic populations.
Hyperhomocysteinemia is an established risk factor for thrombosis. MTHFR polymorphisms, C677T and A1298C, decrease the enzyme activity regulating the intracellular metabolism of homocysteine and thereby mildly elevate the plasma homocysteine level [6, 22]. In the present study, MTHFR A1298C genotypes were not associated with the risk of ONFH. However, MTHFR C677T variant genotypes increased the risk of ONFH compared with 677CC. When patients were further divided into etiologic groups, such a statistical significance was observed only in the alcohol-induced group (Table 2). Most of our patients (51 of 71 patients, 71.8%) had alcohol-induced ONFH in contrast to studies of Caucasians, in which most patients had idiopathic or steroid-induced ONFH [4, 26–28]. Ours may be the largest genetic study on alcohol-induced ONFH. Our data suggests alcohol-induced ONFH is also related to coagulation abnormalities likewise the other etiologic types of ONFH, although the genetic risk profile may be different. Taken together, variable incidences and causes of ONFH may be implicated with an ethnic difference or a sociocultural difference, which may affect the pathogenesis of ONFH.
In one small case-control study of Korean subjects, several thrombotic (protein C activity, protein S activity, antithrombin, anticardiolipin antibody, and immunoglobulins), and fibrinolytic factors (tissue plasminogen activator, plasminogen activator inhibitor-1, lipoprotein[a], and plasminogen) were compared among 24 patients with nontraumatic ONFH and their age- and gender-matched control subjects [19]. There were no differences in the levels of these factors, and data could not confirm an etiologic role for thrombotic and fibrinolytic disorders in East Asian patients with nontraumatic ONFH. However, homocysteine levels were not measured in that study, and the role of thrombophilia in the risk of ONFH cannot be exactly elucidated with the measurement of coagulation profile alone, which may be affected by a variety of acquired conditions.
We found neither factor V Leiden nor prothrombin G20210A mutation in our Korean study population. Although MTHFR A1298C genotypes were not associated with the risk of ONFH, MTHFR C677T variant genotypes increased the risk of ONFH compared with the 677CC wild genotype. The data suggest the MTHFR C677T polymorphism may play a role in the pathogenesis of ONFH in the Korean population, especially in that of alcohol-induced ONFH. It also implies the genetic risk profile of ONFH may be variable in different ethnic populations. Further studies in various ethnic groups are awaited to support the present findings.
Acknowledgments
We thank Tae Young Kang, MT, for his excellent technical assistance.
Footnotes
One or more of the authors (J-DC) have received funding from a research grant at Hallym University Medical Center (01-2006-01).
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Angchaisuksiri P, Pingsuthiwong S, Aryuchai K, Busabaratana M, Sura T, Atichartakarn V, Sritara P. Prevalence of the G1691A mutation in the factor V gene (factor V Leiden) and the G21210A prothrombin gene mutation in the Thai population. Am J Hematol. 2000;65:119–122. [DOI] [PubMed]
- 2.Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, van der Velden PA, Reitsma PH. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64–67. [DOI] [PubMed]
- 3.Bjorkman A, Burtscher IM, Svensson PJ, Hillarp A, Besjakov J, Benoni G. Factor V Leiden and the prothrombin 20210A gene mutation and osteonecrosis of the knee. Arch Orthop Trauma Surg. 2005;125:51–55. [DOI] [PubMed]
- 4.Bjorkman A, Svensson PJ, Hillarp A, Burtscher IM, Rünow A, Benoni G. Factor V Leiden and prothrombin gene mutation: risk factors for osteonecrosis of the femoral head in adults. Clin Orthop Relat Res. 2004;425:168–172. [DOI] [PubMed]
- 5.Chang JD, Lee SH, Oh SY, Wi YH, Lee JS. The risk factors associated with alcohol-induced osteonecrosis of the femoral head [in Korean]. J Korean Orthop Assoc. 2004;39:692–699.
- 6.den Heijer M, Koster T, Blom HJ, Bos GM, Briet E, Reitsma PH, Vandenbroucke JP, Rosendaal FR. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med. 1996;334:759–762. [DOI] [PubMed]
- 7.De Stefano V, Chiusolo P, Paciaroni K, Leone G. Epidemiology of factor V Leiden: clinical implications. Semin Thromb Hemost. 1998;24:367–379. [DOI] [PubMed]
- 8.Gardeniers JWM. A new international classification of osteonecrosis of the ARCO Committee on terminology and classification. J Jpn Orthop Assoc. 1992;66:18–20.
- 9.Glueck CJ, Freiberg RA, Fontaine RN, Tracy T, Wang P. Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res. 2001;386:19–33. [DOI] [PubMed]
- 10.Glueck CJ, Freiberg RA, Tracy T, Stroop D, Wang P. Thrombophilia and hypofibrinolysis: pathophysiologies of osteonecrosis. Clin Orthop Relat Res. 1997;334:43–56. [DOI] [PubMed]
- 11.Han CD, Choe WS, Yoo JH. Effect of polyethylene wear on osteolysis in cementless primary total hip arthroplasty: minimal 5-year follow-up study. J Arthroplasty. 1999;14:714–723. [DOI] [PubMed]
- 12.Hirota Y, Hotokebuchi T, Sugioka Y. Idiopathic osteonecrosis of the femoral head: nationwide epidemiologic studies in Japan. In: Urbiniak JR, Jones JP Jr, eds. Osteonecrosis: Etiology, Diagnosis, Treatment. Rosemont IL: American Academy of Orthopaedic Surgeons; 1997:51–58.
- 13.Hong SH, Song J, Kim JQ. Genetic variation of the methylenetetrahydrofolate reductase and cystathionine beta-synthase genes in Korean patients with coronary artery disease and a new polymorphism in intron 7. Mol Cell Probes. 2001;15:119–123. [DOI] [PubMed]
- 14.Hsu LA, Ko YL, Wang SM, Chang CJ, Hsu TS, Chiang CW, Lee YS. The C677T mutation of the methylenetetrahydrofolate reductase gene is not associated with the risk of coronary artery disease or venous thrombosis among Chinese in Taiwan. Hum Hered. 2001;51:41–45. [DOI] [PubMed]
- 15.Jones LC, Mont MA, Le TB, Petri M, Hungerford DS, Wang P, Glueck CJ. Procoagulants and osteonecrosis. J Rheumatol. 2003;30:783–791. [PubMed]
- 16.Jun ZJ, Ping T, Lei Y, Li L, Ming SY, Jing W. Prevalence of factor V Leiden and prothrombin G20210A mutations in Chinese patients with deep vein thrombosis and pulmonary embolism. Clin Lab Haematol. 2006;28:111–116. [DOI] [PubMed]
- 17.Kim YH, Kim VE. Uncemented porous-coated anatomic total hip replacement: results at six years in a consecutive series. J Bone Joint Surg Br. 1993;75:6–13. [DOI] [PubMed]
- 18.Kim YW, Yoon KY, Park S, Shim YS, Cho HI, Park SS. Absence of factor V Leiden mutation in Koreans. Thromb Res. 1997;86:181–182. [DOI] [PubMed]
- 19.Lee JS, Koo KH, Ha YC, Koh KK, Kim SJ, Kim JR, Song HR, Cho SH. Role of thrombotic and fibrinolytic disorders in osteonecrosis of the femoral head. Clin Orthop Relat Res. 2003;417:270–276. [DOI] [PubMed]
- 20.Lu Y, Zhao Y, Liu G, Wang X, Liu Z, Chen B, Hui R. Factor V gene G1691A mutation, prothrombin gene G20210A mutation, and MTHFR gene C677T mutation are not risk factors for pulmonary thromboembolism in Chinese population. Thromb Res. 2002;106:7–12. [DOI] [PubMed]
- 21.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215–1218. [DOI] [PMC free article] [PubMed]
- 22.Salvati EA, Della Valle AG, Westrich GH, Rana AJ, Specht L, Weksler BB, Wang P, Glueck CJ. The John Charnley Award: heritable thrombophilia and development of thromboembolic disease after total hip arthroplasty. Clin Orthop Relat Res. 2005;441:40–55. [DOI] [PubMed]
- 23.Wisotzkey JD, Bell T, Monk JS. Simultaneous polymerase chain reaction restriction fragment length polymorphism identification of the factor V Leiden allele and the prothrombin 20210A mutation. Diagn Mol Pathol. 1998;7:180–183. [DOI] [PubMed]
- 24.Yi P, Pogribny IP, James SJ. Multiplex PCR for simultaneous detection of 677 C→T and 1298 A→C polymorphisms in methylenetetrahydrofolate reductase gene for population studies of cancer risk. Cancer Lett. 2002;181:209–213. [DOI] [PubMed]
- 25.Zalavras C, Dailiana Z, Elisaf M, Bairaktari E, Vlachogiannopoulos P, Katsaraki A, Malizos KN. Potential aetiological factors concerning the development of osteonecrosis of the femoral head. Eur J Clin Invest. 2000;30:215–221. [DOI] [PubMed]
- 26.Zalavras CG, Malizos KN, Dokou E, Vartholomatos G. The 677C→T mutation of the methylene-tetrahydrofolate reductase gene in the pathogenesis of osteonecrosis of the femoral head. Haematologica. 2002;87:111–112. [PubMed]
- 27.Zalavras CG, Vartholomatos G, Dokou E, Malizos KN. Factor V Leiden and prothrombin gene mutations in femoral head osteonecrosis. Thromb Haemost. 2002;87:1079–1080. [PubMed]
- 28.Zalavras CG, Vartholomatos G, Dokou E, Malizos KN. Genetic background of osteonecrosis. Associated with thrombophilic mutations? Clin Orthop Relat Res. 2004;422:251–255. [DOI] [PubMed]