Abstract
Although the effect of being overweight on the long- and short-term outcome of THA remains unclear, the majority of orthopaedic surgeons believe being overweight negatively influences the longevity of a hip implant. We asked whether complications and long-term survival of cemented THA differed in overweight patients (body mass index [BMI] > 25 kg/m2) and obese patients (BMI > 30 kg/m2) compared with normal-weight patients (BMI < 25 kg/m2). We retrospectively analyzed 411 consecutive patients (489 THAs) treated with cemented THA between 1974 and 1993. Except for cardiovascular comorbidity, we observed no differences in demographics among these weight groups. We found no differences in the number of intraoperative or postoperative complications. The survival rates for the three BMI groups were similar. The 10-year survival for any revision was 94.9% (95% confidence interval, 91.6%–98.2%), 90.4% (95% confidence interval, 85.6%–95.2%), and 91% (95% confidence interval, 81.2%–100%) for normal-weight, overweight, and obese patients, respectively. Cox regression analysis showed BMI and weight had no major influence on survival rates. The differences in mean Harris hip score at final followup were 4.8 between normal-weight and overweight patients and 7.1 between normal-weight and obese patients. Being overweight and obesity had no influence on perioperative complication rates in this cohort and did not negatively influence the long-term survival of cemented THA.
Level of Evidence: Level III, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Whether being overweight influences the fate of a THA is still debated. One study suggests obese patients are more likely to undergo THA for osteoarthritis (OA) of the hip than control patients with lower body mass index (BMI) [7]. Therefore, it is important for the orthopaedic surgeon who is planning the joint arthroplasty to know the effect of obesity on the fate of THA [7, 11, 22]. Although being overweight or obese have a negative influence on health and mobility, it is not certain whether they have a negative influence on the short- and long-term results after THA as well [4, 6, 19, 21].
The assumption that being overweight or obese negatively influences the long-term survival of THA could preclude some obese patients from having joint arthroplasty. Recently, the Wall Street Journal mentioned more orthopaedic surgeons refuse to perform THA in obese patients because of the fear of complications [15]. A large international survey of orthopaedic surgeons performed in 12 European countries revealed 80.9% believe the long-term outcome of THA is impaired by being overweight [20]. Several short-term outcome studies, summarized in two reviews [4, 19], however, failed to show a negative influence of obesity on the short-term results of THA.
We asked whether obesity influences the long-term survival, clinical outcomes scores, and perioperative complication rates. We also asked whether BMI and body weight were risk factors for revision.
Materials and Methods
We retrospectively reviewed the medical records of 411 consecutive patients (489 hips) who underwent primary THA between 1974 and 1993. We divided our patients into three groups based on body mass index (BMI) at the time of surgery: (1) patients with a normal body weight (BMI < 25 kg/m2); (2) patients who were overweight (BMI > 25 kg/m2); and (3) patients who were morbidly obese (BMI > 30 kg/m2). One hundred sixty-three patients (201 hips [41%]) had a normal body weight. One hundred forty-two patients (172 hips [35%]) had a BMI greater than 25 kg/m2 and 35 (42 hips [9%]) of these patients had a BMI greater than 30 kg/m2. For 106 patients (116 hips [24%]), no BMI (weight and/or height) was documented preoperatively. To avoid selection bias, these patients were included in the overall (survival) analysis. During followup, 164 patients (184 hips) died after a minimum followup of 1 year (mean, 11.6 years; range, 1–29.3 years) and an additional 37 patients (50 hips) were lost to followup after a minimum followup of 0.1 year (mean, 6.8 years; range, 0.1–15.6 years). These patients are included in the survival analysis and radiographic analysis until their last outpatient clinic contact. Of these patients lost to followup, two had a BMI greater than 30 kg/m2, eight had a BMI greater than 25 kg/m2, and 12 had a normal BMI; for 16 patients, no BMI was documented.
Sample size power analysis was performed assuming a 10-year survival rate of 95% in normal-weight individuals. We assumed a difference of 10% survival rate in overweight patients was of clinical importance. When using a power of 0.8 and an alpha of 0.05, a sample size of 159 hips is needed per group. Our number of patients with a BMI greater or less than 25 kg/m2 therefore seems sufficient.
For maximum followup, 210 patients (255 hips) were available. The minimum followup in these 210 patients was 10 years (mean, 14.9 years; range, 10–28.1 years). We then compared long-term survivorship, functional outcome, and perioperative complication rate. The average age at the time of surgery was 67 years (range, 22–88 years). One hundred seventeen (24%) of these patients were male (Table 1). The indication for THA was idiopathic OA in 235 hips (48%), acetabular dysplasia in 165 hips (34%), rheumatoid arthritis in eight (2%), avascular necrosis in 30 (6%), posttraumatic in 23 (5%), and other causes in 28 (4%). Apart from cardiologic comorbidity, which occurred more often in overweight and obese patients (Fisher’s exact test, p = 0.028 for BMI > 30 kg/m2 versus BMI < 30 kg/m2 and p = 0.044 for BMI > 25 kg/m2 versus BMI < 25 kg/m2), we observed no differences between the patients who were obese or overweight and the normal-weight patients (Table 1). The average BMI of all patients was 25.3 kg/m2 (range, 17.9–41.1 kg/m2).
Table 1.
Demographic data per BMI group shown in number and percentage
| Demographics and comorbidity | BMI < 25 kg/m2 (n = 201 hips) | BMI > 25 kg/m2 (n = 172 hips) | p Value* | BMI > 30 kg/m2 (n = 42 hips) | p Value* |
|---|---|---|---|---|---|
| Age (years)‡ | 65.0 (21–83) | 65.7 (22–87) | 0.50 | 64.0 (49–79) | 0.56 |
| Percent idiopathic osteoarthritis | 90 (44.8%) | 82 (42.7%) | 0.46 | 23 (54.8%) | 0.22 |
| Female | 152 (75.6%) | 134 (69.8%) | 0.63 | 30 (71.4%) | 0.84 |
| Comorbidity | |||||
| Central nervous system | 14 (7.0%) | 17 (8.9%) | 0.35 | 5 (11.9%) | 0.33 |
| Respiratory | 11 (5.5%) | 10 (5.2%) | 1.0 | 5 (11.9%) | 0.16 |
| Cardiovascular§ | 43 (21.4%) | 51 (26.6%) | 0.07 | 16 (38.1%) | 0.03 |
| Diabetes | 8 (4.0%) | 9 (4.7%) | 0.62 | 4 (9.5%) | 0.12 |
* p values show comparison with the group with a BMI of less than 25 kg/m2; ‡age is given as an average, with range in parentheses; age was compared using a t test; §cardiovascular comorbidity is higher (p < 0.05) in the group with a BMI of greater than 30 kg/m2; for all the other demographic data, no differences were found using a Fisher’s exact test; BMI = body mass index.
The same prosthetic implant and surgical procedure were used in all patients. All patients were placed in a supine position and all had an anterolateral approach and a cemented Weber Rotation THA System (Allopro, Baar, Switzerland) implanted [5]. This system consists of a wrought CoNiCrMo alloy stem (Protasul® 10; Sulzer AG, Winterthur, Switzerland) with a cylindrical neck (the trunnion) made of a cast CoCrMo alloy (Protasul® 2) composite welded to the stem, which is grit-blasted with glass particles. The 32-mm head was made from Protasul® 2 or Al2O3 ceramic (Biolox®; Feldmühle, Plochingen, Germany) and placed on a Protasul® 2 cylinder. The stem and the nonhighly crosslinked polyethylene Weber socket were cemented using low-viscosity Sulfix® (Sulzer AG) cement. Until the 1980s, we used two types of cups, a flat type and a hemispheric type. Because of the inferior results of the flat type, their use was discontinued. In this study, 112 flat type and 377 hemispheric type sockets were used. The percentages of flat cups used were not different among the weight groups.
We (DH, RKM, FHRdM) obtained Harris hip scores (HHS) for patients whose THA was not revised at final followup.
We (DH, FHRdM) performed a radiographic analysis using the weightbearing pelvic and lateral radiographs taken at the latest followup. Loosening of the stem was ranked according to Harris et al. [8] and loosening of the cup according to Hodgkinson et al. [9]. For both components, loosening was scored as definitive, probable, possible, or no loosening. Loosening was scored by comparing the radiographs at last followup with previous radiographs.
Complications were retrieved from the clinical charts. We noted the presence of hematoma when patients underwent exploratory surgery for suspected hematoma. Early infection was defined as requiring antibiotic treatment and/or débridement within 3 months after the operation.
A survival analysis was performed using the Life Table Method using revision for aseptic loosening, revision for any reason, and radiographic loosening (definitive loosening) as end points. We performed survivorship analysis for the acetabular and femoral component separately and for both components combined. Because all patients were seen annually or biannually, all could be included in the survival analysis until their last followup. Equality of the survival curves for the normal-weight, overweight, and obese patients were compared using a log rank test. Differences in HHS among the three study groups were evaluated using analysis of variance. A difference greater than 4 points was considered clinically important [10]. We also compared BMI as a continuous variable with the HHS at maximum followup by means of Pearson correlation analysis to explore the overall influence of BMI on outcome. Differences in loosening between the normal-weight, overweight, and obese patients were evaluated using Fisher’s exact test. Differences in perioperative and postoperative complications were compared using Fisher’s exact test. Cox regression analysis was performed for survival of the implant (any revision) with weight and BMI as risk factors.
Results
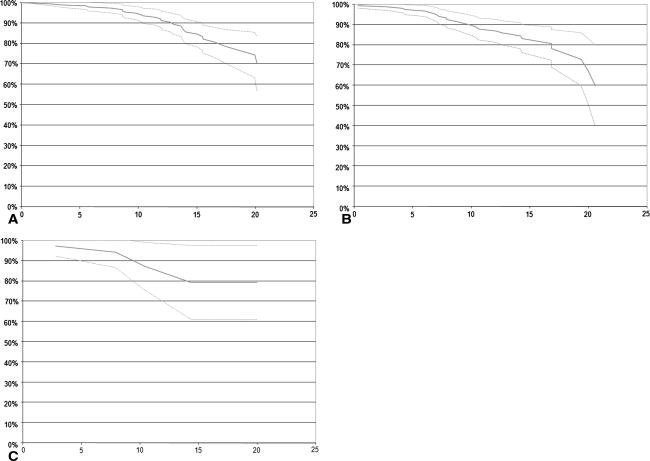
We observed no differences between the survival rates for normal-weight patients and overweight patients and morbidly obese and normal-weight patients for all end points using a log rank test (Table 2; Fig. 1). Fifty four patients (64 hips) underwent revision surgery, of which five hips were revised for septic loosening, 54 for aseptic loosening of at least one of the components, and five for other reasons (periprosthetic fractures and heterotopic ossifications). The rate of infection causing septic loosening was similar in patients with a BMI of between 25 kg/m2 and 30 kg/m2 (n = 4) and with a normal body weight (n = 1) (p = 0.13).
Table 2.
Survival rates
| Number at risk and revisions | All | BMI < 25 kg/m2 | BMI > 25 kg/m2 | BMI > 30 kg/m2 |
|---|---|---|---|---|
| Number at risk | ||||
| At start | 489 | 201 | 172 | 42 |
| At 10 years | 336 | 161 | 122 | 30 |
| At 15 years | 181 | 92 | 69 | 14 |
| At 20 years | 49 | 29 | 17 | 4 |
| Any revision | ||||
| At 10 years | 92.4 (89.8–95.0) | 94.9 (91.6–98.2) | 90.4 (85.6–95.2) | 91.0 (81.2–100) |
| At 15 years | 83.7 (79.4–88.0) | 85.9 (80.0–91.8) | 83.1 (76.2–90.0) | 79.5 (61.5–97.4) |
| At 20 years | 72.6 (64.5–96.4) | 75.6 (65.5–85.6) | 68.3 (53.0–83.6) | 79.5 (61.5–97.4) |
| Aseptic stem loosening | ||||
| At 10 years | 95.1 (92.9–97.2) | 96.6 (93.9–99.3) | 94.2 (90.3–98.1) | 91.0 (81.2–100) |
| At 15 years | 89.3 (85.7–92.9) | 91.4 (86.6–96.2) | 87.5 (81.1–93.9) | 79.5 (61.5–97.4) |
| At 20 years | 84.1 (78.1–90.0) | 85.2 (76.4–94.0) | 82.7 (73.8–91.6) | 79.5 (61.5–97.4) |
| Aseptic cup loosening | ||||
| At 10 years | 96.9 (95.1–98.6) | 97.7 (94.4–100) | 97.2 (94.5–99.9) | 97.1 (91.4–100) |
| At 15 years | 90.0 (86.4–93.5) | 89.6 (84.3–94.9) | 91.5 (85.9–97.1) | 84.9 (67.5–100) |
| At 20 years | 79.9 (72.3–98.5) | 79.4 (69.7–89.0) | 80.0 (66.4–93.6) | 84.9 (67.5–100) |
| Aseptic loosening, both components | ||||
| At 10 years | 94.0 (91.6–96.4) | 96.0 (93.1–98.9) | 92.8 (88.5–97.1) | 91.0 (81.2–100) |
| At 15 years | 85.9 (81.8–90.0) | 86.7 (80.8–92.6) | 86.1 (79.4–93.0) | 79.5 (61.5–97.4) |
| At 20 years | 74.5 (66.3–82.7) | 85.2 (76.4–94.0) | 70.8 (55.1–86.5) | 79.5 (61.5–97.4) |
| Radiographic stem loosening | ||||
| At 10 years | 94.9 (92.7–97.1) | 96.5 (93.7–99.3) | 94.0 (89.9–98.1) | 91.0 (81.2–100) |
| At 15 years | 88.9 (85.1–92.7) | 91.1 (86.1–96.1) | 86.7 (79.8–93.6) | 78.7 (59.8–97.6) |
| At 20 years | 78.1 (70.7–85.5) | 78.9 (68.3–98.5) | 77.5 (65.8–98.5) | 63.0 (31.5–98.5) |
| Radiographic cup loosening | ||||
| At 10 years | 96.8 (95.0–98.6) | 97.6 (95.2–99.9) | 97.1 (94.3–99.9) | 97.1 (91.4–100) |
| At 15 years | 89.3 (85.5–93.1) | 84.9 (79.4–90.4) | 90.8 (84.7–96.9) | 84.2 (65.6–100) |
| At 20 years | 76.6 (68.5–84.7) | 76.2 (65.8–86.6) | 76.1 (61.6–98.5) | 67.3 (34.2–100) |
| Radiographic loosening, both components | ||||
| At 10 years | 93.4 (91.0–95.8) | 95.9 (93.5–98.3) | 92.6 (88.2–97.4) | 91.0 (81.2–100) |
| At 15 years | 85.1 (80.8–89.4) | 85.6 (81.3–89.9) | 85.4 (78.4–92.4) | 78.7 (59.8–97.6) |
| At 20 years | 67.4 (58.4–76.4) | 69.6 (60.6–78.6) | 63.5 (46.9–80.1) | 50.0 (16.1–83.8) |
Values are expressed as percentages, with 95% confidence intervals in parentheses; BMI = body mass index.
Fig. 1A–C.
Survival rates are shown for patients with (A) a BMI less than 25 kg/m2, (B) a BMI greater than 25 kg/m2, and (C) a BMI greater than 30 kg/m2. The x-axis shows years and the y-axis shows survival rates. The solid line represents survival rate and the dotted lines represent the 95% confidence intervals.
Patients with a BMI greater than 30 kg/m2 had lower (p = 0.02) HHS than patients with a BMI less than 25 kg/m2 and patients with a BMI greater than 25 kg/m2 had lower (p = 0.02) HHS than patients with a BMI less than 25 kg/m2 (Table 3). The differences in average HHS between the three groups were greater than 4 points, indicating these differences were clinically relevant. Body mass index showed a poor correlation (rho = −0.17; p = 0.024) with HHS.
Table 3.
Average Harris hip score per BMI group
| BMI < 25 kg/m2 | BMI > 25 kg/m2 | BMI > 30 kg/m2 |
|---|---|---|
| 91.6 (89.3–93.9) | 86.8 (83.5–90.1)* | 83.7 (74.5–92.3)* |
Values are expressed as averages, with 95% confidence intervals in parentheses; * difference with group with a BMI of less than 25 kg/m2 (p = 0.02); BMI = body mass index.
Several local and systemic complications occurred, which were similarly distributed among the normal-weight, overweight, and obese patients (Table 4). We observed no differences in the rates of radiographic loosening among the normal-weight versus overweight patients (p = 0.30) and normal-weight versus obese patients (p = 0.47) (Table 5).
Table 4.
Complications per BMI group
| Complication | All (n = 489 hips) | BMI < 25 kg/m2 (n = 201 hips) | BMI > 25 kg/m2 (n = 172 hips) | p Value* | BMI > 30 kg/m2 (n = 42 hips) | p Value* |
|---|---|---|---|---|---|---|
| Venous thromboembolism | 2 (0.4%) | 1 (0.5%) | 0 | 0 | ||
| Cardiac | 6 (1.2%) | 1 (0.5%) | 4 (2.3%) | 1.0 | 2 (4.8%) | 1.0 |
| Respiratory | 3 (0.6%) | 1 (0.5%) | 1 (0.6%) | 1.0 | 1 (2.4%) | 0.31 |
| Abdominal | 4 (0.8%) | 2 (1.0%) | 1 (0.6%) | 1.0 | 0 | |
| Other systemic (including urinary tract infection) | 18 (3.7%) | 8 (4.0%) | 9 (5.2%) | 0.62 | 1 (2.4%) | 1.0 |
| Hematoma | 10 (2.0%) | 6 (3.0%) | 2 (1.2%) | 0.30 | 2 (4.8%) | 0.63 |
| Early infection | 5 (0.8%) | 2 (1.0%) | 3 (1.7%) | 0.67 | 0 | |
| Intraoperative complication | 24 (4.9%) | 11 (5.5%) | 11 (6.4%) | 0.67 | 2 (4.8%) | 1.0 |
| Any complication | 68 (13.9%) | 30 (14.9%) | 28 (16.3%) | 0.78 | 6 (14.3%) | 1.0 |
* p values are given for the comparison with the group with a BMI of less than 25 kg/m2 (Fisher exact test); BMI = body mass index.
Table 5.
Radiographic analysis of the unrevised hips
| Component | Definitive loosening | Probable loosening | Possible loosening | |||
|---|---|---|---|---|---|---|
| Number of hips | Time until loosening (years)* | Number of hips | Time until loosening (years)* | Number of hips | Time until loosening (years)* | |
| Acetabular† | 2 | 18.3 (18.2–18.3) | 2 | 16.2 (13.9–18.4) | 15 | 15.9 (9.0–23.0) |
| Femoral‡ | 6 | 18.9 (15.9–22.6) | 1 | 23.2 | 11 | 17.9 (14.0–22.8) |
Body mass index and body weight were not risk factors for revision (Exp[B] = 1.00 [95% confidence interval, 0.93–1.08] and Exp[B] = 1.01 [95% confidence interval, 0.99–1.03], respectively).
Discussion
The influence of being overweight on the long- and short-term outcome of THA is controversial in the literature but the majority of orthopaedic surgeons believe being overweight negatively influences the longevity of a hip implant [20]. Because the issue is controversial, we asked whether obesity influences the long-term survival, clinical outcomes scores, and perioperative complication rates, and whether BMI and body weight were risk factors for revision.
We note several limitations of our study. First, we did not study wear. It could be hypothesized that more body weight causes more wear. Although it can be expected that excessive wear may influence the rate of revision, we did not see a difference in revision rates between the weight groups [2]. Second, we studied only patients with cemented THA. Our analysis may not be valid for uncemented THA. One study of 300 patients with the cementless PM prosthesis suggested obesity negatively influenced medium-term survival, showing a twofold increase in loosening/revision rate in obese patients [6]. Another recent study suggested no difference in the outcome of uncemented THA in obese versus normal-weight patients, although a high revision rate for the acetabular component was present [13].
Our data suggest BMI and weight do not influence the long-term survival of cemented THA. We also found no differences in the incidence of THA-related complications for the overweight patients undergoing THA. Cardiovascular comorbidity was more common in the obese patients; however, we observed no differences in perioperative cardiac complications.
The percentage of overweight and obese individuals in our study is lower than those reported in American studies. In a study including 1071 American patients undergoing THA, 36% of the patients had a BMI greater than 30 kg/m2 [16]. In The Netherlands, the annual incidence of obesity (BMI > 30 kg/m2) gradually inclined from 5% in 1981 to 7% in 1993 and 10% in 2005 [3]. In our study, 9% had a BMI greater than 30 kg/m2. For the overweight patients (BMI > 25 kg/m2), these percentages were 33% in 1981 and 37% in 1993 and 35% in our study. Because OA is more common in overweight patients, we believe these percentages indicate our patient group is comparable to the average Dutch population [7]. This also indicates absence of a selection bias. All patients were operated on in our hospital regardless of their weight. Another major difference between our Dutch population and the American population is extreme obesity (BMI > 40 kg/m2) was low in our country before 1993. We had only two patients who had a BMI greater than 40 kg/m2 (neither had revision and had HHS of 87 and 90). This low number of patients with a BMI greater than 40 kg/m2 means our study does not supply an answer for the long-term fate of THAs in these extremes.
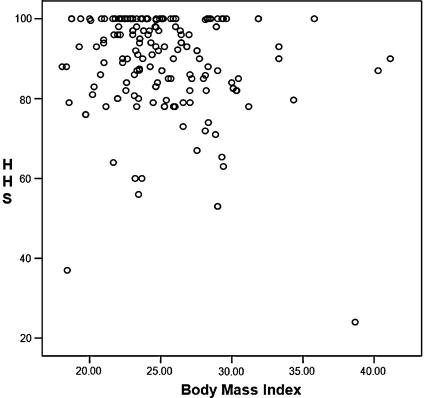
Several publications report on the short-term results of THA in the obese in which the HHS after surgery are compared between obese and normal-weight patients. The literature contains controversial data suggesting either similar or worse outcomes for obese patients undergoing THA. Two large studies reported lower HHS in obese patients after short-term followup [1, 14]. Both showed lower HHS with an average difference of 5 points, but neither compared the preoperative HHS among the different groups. The clinical relevance of these small differences in the postoperative HHS without a comparison of the preoperative HHS is debatable, especially because other studies showed no differences in postoperative HHS between the several weight groups [18]. Another study suggested the level of activity is lower, which continues to be so after THA [12]. The same problem occurs in our study because no preoperative HHS was available for analysis. If patients who are more obese have initial lower HHS and similar improvement as normal-weight patients after the arthroplasty, the same difference remains. Although our data suggest differences between the average HHS in the weight groups, the differences between the mean HHS were small (4.8 and 7.1). However; the only study on the responsiveness and discriminative ability of the HHS showed a difference of 4 points is enough to be clinically relevant, indicating our measured differences are clinically relevant [10]. However, the correlation of HHS with BMI as a continuous variable was poor (rho = −0.17), but the content validity of the HHS is poor, eg, a large ceiling effect is visible, which could influence the correlation coefficient measured (Fig. 2).
Fig. 2.
A scatterplot shows HHS versus BMI. The ceiling effect of the HHS can be seen.
In a review of patient characteristics affecting the outcome of THA, a body weight greater than 70 kg was mentioned as a factor that negatively influences the outcome of THA [23]. They suggest weight alone is a much stronger predictor for the outcome than BMI because height has no influence on the prosthesis. In our series, neither body weight nor BMI influenced outcome.
One study stated patients who underwent bariatric surgery before having THA had an excellent outcome, although the average postoperative BMI of 29 kg/m2 still indicated overweight. The main question we would ask is whether the outcome would have been worse if no bariatric surgery was performed [17].
We do not intend to suggest being overweight has no risks. We believe it is important to motivate overweight patients to lose weight. Being overweight could increase the rate of OA and has an increased risk for several nonorthopaedic morbidities [7]. However, should a (cemented) THA be necessary in an overweight or obese patient, the arguments that survival is shorter in obese patients and that obese patients have a higher risk of perioperative complications do not seem valid.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution either has waived or does not require approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aderinto J, Brenkel IJ, Chan P. Weight change following total hip replacement: a comparison of obese and non-obese patients. Surgeon. 2005;3:269–272, 305. [DOI] [PubMed]
- 2.Bordini B, Stea S, De Clerico M, Strazzari S, Sasdelli A, Toni A. Factors affecting aseptic loosening of 4750 total hip arthroplasties: multivariate survival analysis. BMC Musculoskelet Disord. 2007;8:69. [DOI] [PMC free article] [PubMed]
- 3.Central Bureau of Statistics, The Netherlands. Available at: http://www.cbs.nl. Accessed January 2007.
- 4.Crawford RW, Murray DW. Total hip replacement: indications for surgery and risk factors for failure. Ann Rheum Dis. 1997;56:455–457. [DOI] [PMC free article] [PubMed]
- 5.de Jong PT, van der Vis HM, de Man FH, Marti RK. Weber rotation total hip replacement: a prospective 5- to 20-year followup study. Clin Orthop Relat Res. 2004;419:107–114. [DOI] [PubMed]
- 6.Dickob M, Martini T. The cementless PM hip arthroplasty: four-to-seven-year results. J Bone Joint Surg Br. 1996;78:195–199. [PubMed]
- 7.Flugsrud GB, Nordsletten L, Espehaug B, Havelin LI, Engeland A, Meyer HE. The impact of body mass index on later total hip arthroplasty for primary osteoarthritis: a cohort study in 1.2 million persons. Arthritis Rheum. 2006;54:802–807. [DOI] [PubMed]
- 8.Harris WH, McCarthy JC Jr, O’Neill DA. Femoral component loosening using contemporary techniques of femoral cement fixation. J Bone Joint Surg Am. 1982;64:1063–1067. [PubMed]
- 9.Hodgkinson JP, Shelley P, Wroblewski BM. The correlation between the roentgenographic appearance and operative findings at the bone-cement junction of the socket in Charnley low friction arthroplasties. Clin Orthop Relat Res. 1988;228:105–109. [PubMed]
- 10.Hoeksma HL, van den Ende CH, Ronday HK, Heering A, Breedveld FC. Comparison of the responsiveness of the Harris Hip Score with generic measures for hip function in osteoarthritis of the hip. Ann Rheum Dis. 2003;62:935–938. [DOI] [PMC free article] [PubMed]
- 11.Karlson EW, Mandl LA, Aweh GN, Sangha O, Liang MH, Grodstein F. Total hip replacement due to osteoarthritis: the importance of age, obesity, and other modifiable risk factors. Am J Med. 2003;114:93–98. [DOI] [PubMed]
- 12.McClung CD, Zahiri CA, Higa JK, Amstutz HC, Schmalzried TP. Relationship between body mass index and activity in hip or knee arthroplasty patients. J Orthop Res. 2000;18:35–39. [DOI] [PubMed]
- 13.McLaughlin JR, Lee KR. The outcome of total hip replacement in obese and non-obese patients at 10- to 18-years. J Bone Joint Surg Br. 2006;88:1286–1292. [DOI] [PubMed]
- 14.Moran M, Walmsley P, Gray A, Brenkel IJ. Does body mass index affect the early outcome of primary total hip arthroplasty? J Arthroplasty. 2005;20:866–869. [DOI] [PubMed]
- 15.Naik G. Surgeons’ weighty dilemma; wary of extra risk, work, some doctors won’t replace knees, hips of obese patients. Wall Street Journal. February 28, 2006:B1, B8. [PubMed]
- 16.Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(suppl 3):46–50. [DOI] [PubMed]
- 17.Parvizi J, Trousdale RT, Sarr MG. Total joint arthroplasty in patients surgically treated for morbid obesity. J Arthroplasty. 2000;15:1003–1008. [DOI] [PubMed]
- 18.Stickles B, Phillips L, Brox WT, Owens B, Lanzer WL. Defining the relationship between obesity and total joint arthroplasty. Obes Res. 2001;9:219–223. [DOI] [PubMed]
- 19.Stukenborg-Colsman C, Ostermeier S, Windhagen H. [What effect does of obesity have on the outcome of total hip and knee arthroplasty: review of the literature] [in German]. Orthopade. 2005;34:664–667. [DOI] [PubMed]
- 20.Stürmer T, Günther KP, Brenner H. Obesity, overweight and patterns of osteoarthritis: the Ulm Osteoarthritis Study. J Clin Epidemiol. 2000;53:307–313. [DOI] [PubMed]
- 21.Sutherland CJ, Wilde AH, Borden LS, Marks KE. A ten-year follow-up of one hundred consecutive Muller curved-stem total hip-replacement arthroplasties. J Bone Joint Surg Am. 1982;64:970–982. [PubMed]
- 22.Wendelboe AM, Hegmann KT, Biggs JJ, Cox CM, Portmann AJ, Gildea JH, Gren LH, Lyon JL. Relationships between body mass indices and surgical replacements of knee and hip joints. Am J Prev Med. 2003;25:290–295. [DOI] [PubMed]
- 23.Young NL, Cheah D, Waddell JP, Wright JG. Patient characteristics that affect the outcome of total hip arthroplasty: a review. Can J Surg. 1998;41:188–195. [PMC free article] [PubMed]